Use of Spirulina platensis and Curcuma longa as Nutraceuticals in Poultry
Abstract
1. Introduction
1.1. Spirulina spp.
1.2. Curcuma spp.
2. Growth-Promoting Effects on Broiler Chicken
2.1. Growth-Promoting Effects of Spirulina
- Levels of spirulina: The concentration or inclusion level of spirulina in diets can vary among studies. Different levels of supplementation may have different effects on broiler performance.
- Broiler hybrids: Different broiler strains or hybrids may respond differently to spirulina supplementation. Genetic variations among broiler breeds can influence their ability to utilize nutrients and respond to dietary interventions.
- Broiler age: The age of broilers at the time of spirulina supplementation can influence the outcomes. The physiological and metabolic status of broilers change as they grow, which can affect their response to dietary interventions.
- Housing conditions: Variances in housing conditions, such as temperature, humidity, and ventilation, can impact broiler performance. Environmental stressors may interact with spirulina supplementation and influence the results.
- Feed preparation: The processing and formulation of broiler diets, including the method of spirulina incorporation, can affect the availability and digestibility of nutrients. Differences in feed preparation techniques among studies may contribute to variations in the results.
- Administration method: The way spirulina is administered to broilers can vary. It could be mixed directly into the diet, offered as a separate supplement, or administered via drinking water. The mode of administration may influence the interaction between spirulina and the birds’ digestive system.
2.2. Growth-Promoting Effects of Curcuma
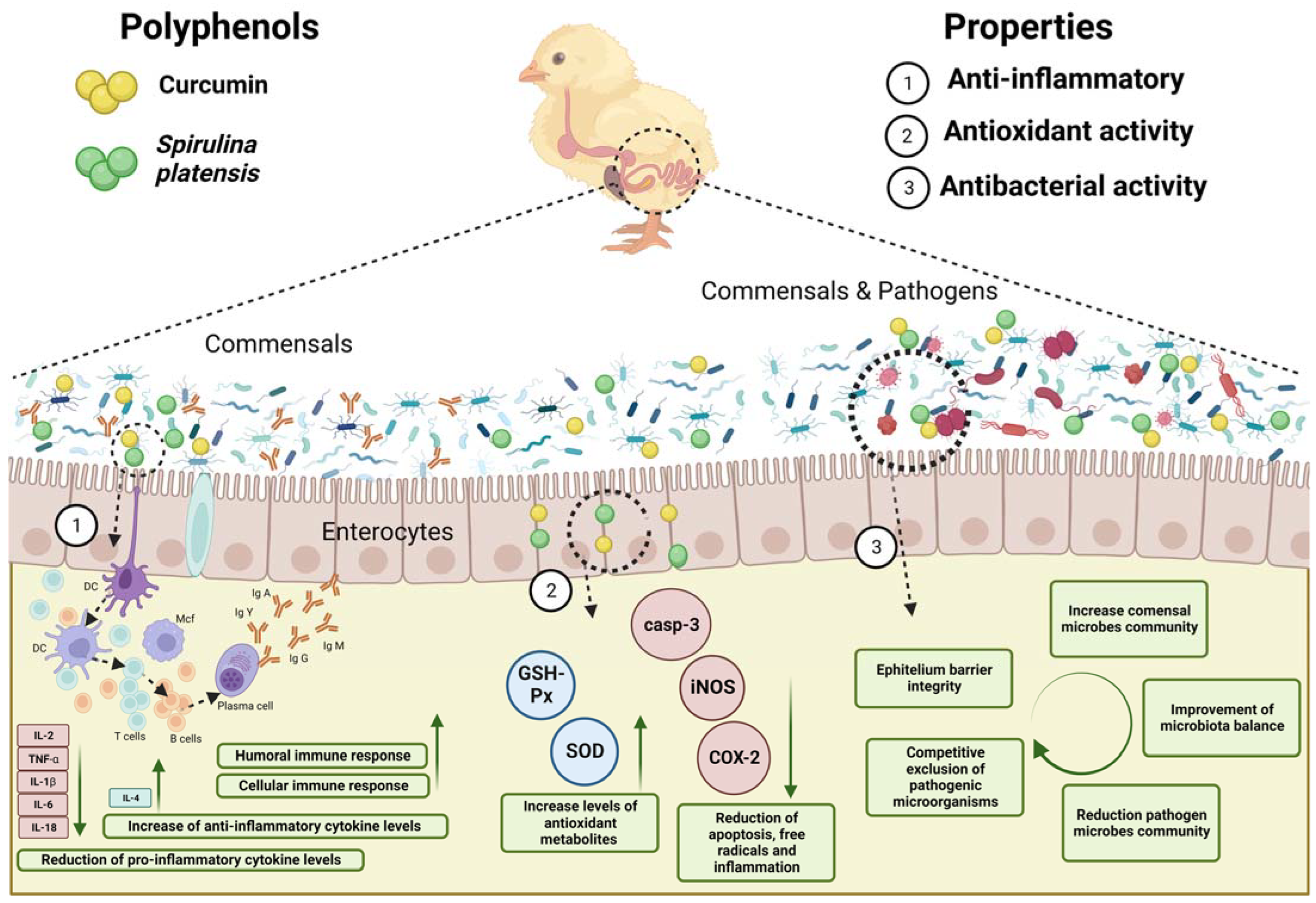
3. Intestinal Morphology, Microbiota Modulation, and Immunomodulation
3.1. Effects of Spirulina on Intestinal Morphology, Microbiota Modulation, and Immunomodulation
3.2. Effects of Curcuma on Intestinal Morphology, Microbiota Modulation, and Immunomodulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Bonos, E.; Kasapidou, E.; Kargopoulos, A.; Karampampas, A.; Nikolakakis, I.; Christaki, E.; Nikolakakis, I. Spirulina as a functional ingredient in broiler chicken diets. S. Afr. J. Anim. Sci. 2016, 46, 94–102. [Google Scholar] [CrossRef]
- Dhama, K.; Saminathan, M.; Amarpal, J.S.; Tiwari, R.; Sunkara, L.T.; Malik, Y.S.; Singh, R.K. Effect of Immunomodulation and Immunomodulatory Agents on Health with some Bioactive Principles, Modes of Action and Potent Biomedical Applications. Int. J. Pharmacol. 2015, 11, 253–290. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef]
- Waheed Janabi, A.H.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-rich foods (FRF): A promising nutraceutical approach against lifespan-shortening diseases. Iran. J. Basic Med. Sci. 2020, 23, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Cencic, A.; Chingwaru, W. The Role of Functional Foods, Nutraceuticals, and Food Supplements in Intestinal Health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef]
- Kalra, E.K. Nutraceutical-definition and introduction. AAPS Pharm. Sci. 2003, 5, 25. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R. The role of exogenous enzymes in promoting growth and improving nutrient digestibility in poultry. Iran. J. Vet. Res. 2018, 19, 157–164. [Google Scholar]
- Elgeddawy, S.A.; Shaheen, H.M.; El-Sayed, Y.S.; Abd Elaziz, M.; Darwish, A.; Samak, D.; Betiha, G.E.; Mady, R.A.; Bin-Jumah, M.; Allam, A.A.; et al. Effects of the dietary inclusion of a probiotic or prebiotic on florfenicol pharmacokinetic profile in broiler chicken. J. Anim. Physiol. Anim. Nutr. 2020, 104, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Ghoreyshi, S.M.; Omri, B.; Chalghoumi, R.; Bouyeh, M.; Seidavi, A.; Dadashbeiki, M.; Lucarini, M.; Durazzo, A.; van den Hoven, R.; Santini, A. Effects of Dietary Supplementation of L-Carnitine and Excess Lysine-Methionine on Growth Performance, Carcass Characteristics, and Immunity Markers of Broiler Chicken. Animals 2019, 9, 362. [Google Scholar] [CrossRef]
- Khatun, A.; Chowdhury, S.D.; Roy, B.C.; Dey, B.; Haque, A.; Chandran, B. Comparative effects of inorganic and three forms of organic trace minerals on growth performance, carcass traits, immunity, and profitability of broilers. J. Adv. Vet. Anim. Res. 2019, 6, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Xie, M.; Wu, Y.; Hou, S. Effect of Supplemental Cyanocobalamin on the Growth Performance and Hematological Indicators of the White Pekin Ducks from Hatch to Day 21. Animals 2019, 9, 633. [Google Scholar] [CrossRef]
- Moghaddam, H.S.; Emadi, M. The effect of threonine and vitamin A on immune system in broiler chickens. Int. J. Adv. Biol. Biomed. Res. 2014, 26, 756–763. [Google Scholar]
- Horváth, M.; Babinszky, L. Impact of selected antioxidant vitamins (Vitamin A, E and C) and micro minerals (Zn, Se) on the antioxidant status and performance under high environmental temperature in poultry. A review. Acta Agr. Scand. Sect. A Anim. Sci. 2019, 68, 152–160. [Google Scholar] [CrossRef]
- Ravindran, V. Poultry feed availability and nutrition in developing countries. FAO Poult. Dev. Rev. 2013, 2, 60–63. [Google Scholar]
- Rinttilä, T.; Apajalahti, J. Intestinal microbiota and metabolites—Implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013, 22, 647–658. [Google Scholar] [CrossRef]
- Sugiharto, S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agr. Sci. 2016, 15, 99–111. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Ropy, A.; Abdel-Razik, A.H. Effect of dietary sodium butyrate supplementation on growth, blood biochemistry, haematology and histomorphometry of intestine and immune organs of Japanese quail. Animal 2019, 13, 1234–1244. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Alagawany, M.; Elwan, H.A.M.; Fathi, M.A.; Farag, M.R. Effect of Sodium Butyrate on Intestinal Health of Poultry—A Review. Ann. Anim. Sci. 2020, 20, 29–41. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Sharif, S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008, 9, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Soomro, R.N.; Abd El-Hack, M.E.; Shah, S.S.; Taha, A.E.; Alagawany, M.; Swelum, A.A.; Hussein, E.O.S.; Ba-Aawdh, H.A.; Saadeldin, I.; El-Edel, M.A.; et al. Impact of restricting feed and probiotic supplementation on growth performance, mortality and carcass traits of meat-type quails. Anim. Sci. J. 2019, 90, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Alagawany, M.; Chaudhry, M.T.; Saeed, M.; Ahmad, E.A.M.; El-Sayed, A.A. Does the gradual increase in dietary zinc oxide supplementation can affect egg quality, serum indices, and productive performance of laying hens? Trop. Anim. Health Prod. 2020, 52, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El Baz, F.K.; El-Baroty, G.S. Spirulina species as a source of carotenoids and a-tocopherol and its anticarcinoma factors. Biotechnology 2003, 2, 222–240. [Google Scholar] [CrossRef]
- Jamil, A.; Akanda, M.; Rahman, M.; Hossain, M.; Islam, M. Prebiotic competence of spirulina on the production performance of broiler chickens. J. Adv. Vet. Anim. Res. 2015, 2, 304. [Google Scholar] [CrossRef]
- Velten, S.; Neumann, C.; Bleyer, M.; Gruber-Dujardin, E.; Hanuszewska, M.; Przybylska-Gornowicz, B.; Liebert, F. Effects of 50 Percent Substitution of Soybean Meal by Alternative Proteins from Hermetia illucens or Spirulina platensis in Meat-Type Chicken Diets with Graded Amino Acid Supply. Anim. Sci. 2018, 8, 119–136. [Google Scholar] [CrossRef]
- Sugiharto, S.; Yudiarti, T.; Isroli, I.; Widiastuti, E. Effect of feeding duration of Spirulina platensis on growth performance, haematological parameters, intestinal microbial population and carcass traits of broiler chicks. SA J. An. Sci. 2018, 48, 98. [Google Scholar] [CrossRef]
- Rasool, M.; Sabina, E.P.; Lavanya, B. Anti-inflammatory Effect of Spirulina fusiformis on Adjuvant-Induced Arthritis in Mice. Biol. Pharm. Bull. 2006, 29, 2483–2487. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.-J. Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina: Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and medical applications of spirulina microalgae. Mini Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.G.; Richmond, W.; Fu, P.C. Enzymatic Determination of Total Serum Cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Mcdonald, R.E.; Hultin, H.O. Some Characteristics of the Enzymic Lipid Peroxidation System in the Microsomal Fraction of Flounder Skeletal Muscle. J. Food Sci. 1987, 52, 15–21. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.J. [241 Total antioxidant status in plasma and body fluids. Methods Enzym. 1994, 234, 279–293. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Mei, X.; Xu, D.; Xu, S.; Lv, J. Protective effects of polysacchride of Spirulina platensis and Sargassum thunbeergii on vascular of alloxan induced diabetic rats. Zhongguo Zhong Yao Za Zhi 2005, 30, 211–215. (In Chinese) [Google Scholar] [PubMed]
- Ferreira-Hermosillo, A.; Torres-Durán, P.; Shamosh-Halabe, S.; Juarez-Oropeza, M. Biological effects of Spirulina and current research on its antioxidant activity. Rev. Int. Cienc. Tecnol. Biomed. Toctli RICTB 2011, 2, 1. [Google Scholar]
- Anbarasan, V.; Kumar, V.K.; Kumar, P.S.; Venkatachalam, T. In vitro evaluation of antioxidant activity of blue green algae Spirulina platensis. Int. J. Pharm. Sci. Res. 2011, 2, 2616. [Google Scholar]
- Khan, Z.; Bhadouria, P.; Bisen, P. Nutritional and Therapeutic Potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Tirkey, N.; Pilkhwal, S.; Chopra, K. Renoprotective Effect of Spirulina fusiformis on Cisplatin-Induced Oxidative Stress and Renal Dysfunction in Rats. Ren. Fail. 2006, 28, 247–254. [Google Scholar] [CrossRef]
- Minkova, K.M.; Tchernov, A.A.; Tchorbadjieva, M.I.; Fournadjieva, S.T.; Antova, R.E.; Busheva, M.C.H. Purification of C-phycocyanin from Spirulina (Arthrospira) fusiformis. J. Biotechnol. 2003, 102, 55–59. [Google Scholar] [CrossRef]
- Safari, R.; Raftani Amiri, Z.; Esmaeilzadeh Kenari, R. Antioxidant and antibacterial activities of C-phycocyanin from common name Spirulina platensis. Iran. J. Fish. Sci. 2020, 19, 1911–1927. [Google Scholar]
- Xalxo, R.K.; Sao, S.; Sahu, P.K. Effect of antibacterial properties of cyanobacterial Spirulina platensis. Int. J. Pharm. Life Sci. 2013, 4, 2950–2956. [Google Scholar]
- Romay, C.h.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory and Neuroprotective Effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef]
- Amer, S.A.; Al-Khalaifah, H.S.; Gouda, A.; Osman, A.; Goda, N.I.A.; Mohammed, H.A.; Darwish, M.I.M.; Hassan, A.M.; Mohamed, S.K.A. Potential Effects of Anthocyanin-Rich Roselle (Hibiscus sabdariffa L.) Extract on the Growth, Intestinal Histomorphology, Blood Biochemical Parameters, and the Immune Status of Broiler Chickens. Antioxidants 2022, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Hoseinifar, S.H.; Hung, T.Q.; Lumsangkul, C.; Jaturasitha, S.; El-Haroun, E.; Paolucci, M. Dietary inclusion of chestnut (Castanea sativa) polyphenols to Nile tilapia reared in biofloc technology: Impacts on growth, immunity, and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 2020, 105, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Olaizola, M. Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomol. Eng. 2003, 20, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Alagawany, M.; Farag, M.R.; Elnesr, S.S. Beneficial impacts of bee pollen in animal production, reproduction and health. J. Anim. Physiol. Anim. Nutr. 2019, 103, 477–484. [Google Scholar] [CrossRef]
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed. Sci. Technol. 2019, 250, 32–40. [Google Scholar] [CrossRef]
- Lan, Y.; Verstegen, M.W.A.; Tamminga, S.; Williams, B.A. The role of the commensal gut microbial community in broiler chickens. World’s Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Artis, D. Commensal bacteria at the interface of host metabolism and the immune system. Nat. Immunol. 2013, 14, 676–684. [Google Scholar] [CrossRef]
- Adil, S.; Magray, S. Impact and manipulation of gut microflora in poultry: A review. J. Anim. Vet. Adv. 2012, 11, 873–877. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Alyemni, A.H.; Dafalla, Y.M.; Khan, R.U. The effect of phytogenics on growth traits, blood biochemical and intestinal histology in broiler chickens exposed to Clostridium perfringens challenge. J. Appl. Anim. Res. 2017, 46, 691–695. [Google Scholar] [CrossRef]
- Olnood, C.G.; Beski, S.S.M.; Choct, M.; Iji, P.A. Novel probiotics: Their effects on growth performance, gut development, microbial community and activity of broiler chickens. Anim. Nutr. 2015, 1, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Choct, M. Managing gut health through nutrition. Br. Poult. Sci. 2009, 50, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.L. The Role of Probiotics in the Poultry Industry. Int. J. Mol. Sci. 2009, 10, 3531–3546. [Google Scholar] [CrossRef]
- Hussein, E.O.S.; Ahmed, S.H.; Abudabos, A.M.; Suliman, G.M.; Abd El-Hack, M.E.; Swelum, A.A.; NAlowaimer, A. Ameliorative Effects of Antibiotic-, Probiotic- and Phytobiotic-Supplemented Diets on the Performance, Intestinal Health, Carcass Traits, and Meat Quality of Clostridium perfringens -Infected Broilers. Animals 2020, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.S.; Stahl, W.H. Turmeric—Chemistry, technology, and quality. Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301. [Google Scholar] [CrossRef] [PubMed]
- Fallah, R.; Mirzaei, E. Effect of dietary inclusion of turmeric and thyme powders on performance, blood parameters and immune system of broiler chickens. J. Livest. Sci. 2016, 7, 180–186. [Google Scholar]
- Holt, P.R.; Katz, S.; Kirshoff, R. Curcumin Therapy in Inflammatory Bowel Disease: A Pilot Study. Dig. Dis. Sci. 2005, 50, 2191–2193. [Google Scholar] [CrossRef]
- Mondal, M.; Yeasmin, T.; Karim, R.; Siddiqui, M.N.; Nabi, S.R.; Sayed, M.; Siddiky, N.A. Effect of dietary supplementation of turmeric (Curcuma longa) powder on the growth performance and carcass traits of broiler chicks. SAARC J. Agric. 2015, 13, 188–199. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Javdani, M.; Nikousefat, Z.; Selvaggi, M.; Tufarelli, V.; Laudadio, V. The use of Turmeric (Curcuma longa) in poultry feed. World’s Poult. Sci. J. 2012, 68, 97–103. [Google Scholar] [CrossRef]
- Araújo, C.; Leon, L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo. Cruz. 2001, 96, 723–728. [Google Scholar] [CrossRef]
- Anantkawlas, M. A study of turmeric processing and its export from India. Res. Front. 2014, 2, 51–56. [Google Scholar]
- Kafi, A.; Uddin, M.N.; Uddin, M.J.; Khan, M.M.H.; Haque, M.E. Effect of Dietary Supplementation of Turmeric (Curcuma longa), Ginger (Zingiber officinale) and their Combination as Feed Additives on Feed Intake, Growth Performance and Economics of Broiler. Int. J. Poult. Sci. 2017, 16, 257–265. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Huang, H.; Zhou, L.; Li, W.; Zhou, H.; Hou, G.; Liu, J.; Hu, L. Effects of Dietary Supplementation with Turmeric Rhizome Extract on Growth Performance, Carcass Characteristics, Antioxidant Capability, and Meat Quality of Wenchang Broiler Chickens. Ital. J. Anim. Sci. 2015, 14, 3. [Google Scholar] [CrossRef]
- Qasem, M.; Alhajj, M.; Jer El Nabi, A.; Al-Mufarrej, S. Effects of dietary supplement of turmeric powder (Curcuma longa) on blood biochemistry parameters and antioxidant activity in chickens. SA J. An. Sci. 2016, 46, 204. [Google Scholar] [CrossRef]
- Sun, M.; Su, X.; Ding, B.; He, X.; Liu, X.; Yu, A.; Lou, H.; Zhai, G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine 2012, 7, 1085–1100. [Google Scholar] [CrossRef]
- Kurkure, N.; Pawar, S.; Kognole, S.; Bhandarkar, A.; Ganorkar, A.; Kalorey, D. Ameliorative effect of turmeric (Curcuma longa) in induced aflatoxicosis in cockerels. Ind. J. Vet. Pathol. 2000, 24, 26–28. [Google Scholar]
- Gouda, M.M.; Prabhakar Bhandary, Y. Natural antibiotic effect of turmeric in poultry management. Int. J. Poul. Fish Sci. 2018, 2, 1–2. [Google Scholar] [CrossRef]
- Dono, N.D. Turmeric (Curcuma longa Linn.) Supplementation as an Alternative to Antibiotics in Poultry Diets. Indones Bull. Anim. Vet. Sci. 2014, 23, 41–49. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.; Ramos, L.; Zúñiga Paredes, J.; Carlosama-Yépez, M.; Moreno, C.; Ruales, P. Effects of turmeric rhizome powder and curcumin in poultry production. A review. J. Anim. Feed Sci. 2017, 26, 293–302. [Google Scholar] [CrossRef]
- Prakash, P.; Radha; Kumar, M.; Kumari, N.; Prakash, S.; Rathour, S.; Thakur, M.; Jamwal, R.; Janjua, S.; Ali, M.; et al. Therapeutic Uses of Wild Plants by Rural Inhabitants of Maraog Region in District Shimla, Himachal Pradesh, India. Horticulturae 2021, 7, 343. [Google Scholar] [CrossRef]
- Prakash, P.; Radha; Kumar, M.; Pundir, A.; Puri, S.; Prakash, S.; Kumari, N.; Thakur, M.; Rathour, S.; Jamwal, R.; et al. Documentation of Commonly Used Ethnoveterinary Medicines from Wild Plants of the High Mountains in Shimla District, Himachal Pradesh, India. Horticulturae 2021, 7, 351. [Google Scholar] [CrossRef]
- Park, S.-S.; Kim, J.-M.; Kim, E.-J.; Kim, H.-S.; An, B.-K.; Kang, C.-W. Effects of Dietary Turmeric Powder on Laying Performance and Egg Qualities in Laying Hens. Korean J. Poult. Sci. 2012, 39, 27–32. [Google Scholar] [CrossRef]
- Kosti, D.; Dahiya, D.S.; Dalal, R.; Tewatia, B.S.; Vijayalakshmy, K. Role of turmeric supplementation on production, physical and biochemical parameters in laying hens. World’s Poult. Sci. J. 2020, 76, 625–637. [Google Scholar] [CrossRef]
- Chandran, D. Veterinary Phytomedicine in India: A Review. Int. J. Sci. Res. Sci. Tech. 2021, 8, 598–605. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A.; et al. Garlic (Allium sativum L.) Bioactives and Its Role in Alleviating Oral Pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. BioMed Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
- Tajodini, M.; Saeedi, H.R.; Moghbeli, P. Use of black pepper, cinnamon and turmeric as feed additives in the poultry industry. World’s Poult. Sci. J. 2015, 71, 175–183. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Hussain, M.B.; Sultan, M.T.; Arshad, M.S.; Waheed, M.; Shariati, M.A.; Plygun, S.; Hashempur, M.H. Biochemistry, Safety, Pharmacological Activities, and Clinical Applications of Turmeric: A Mechanistic Review. Evid. Based Complement. Altern. Med. 2020, 2020, 7656919. [Google Scholar] [CrossRef]
- Gull, N.; Arshad, F.; Naikoo, G.A.; Hassan, I.U.; Pedram, M.Z.; Ahmad, A.; Aljabali, A.A.A.; Mishra, V.; Satija, S.; Charbe, N.; et al. Recent Advances in Anticancer Activity of Novel Plant Extracts and Compounds from Curcuma longa in Hepatocellular Carcinoma. J. Gastrointest. Cancer 2022, 14. [Google Scholar] [CrossRef]
- Vafaeipour, Z.; Razavi, B.M.; Hosseinzadeh, H. Effects of turmeric (Curcuma longa) and its constituent (curcumin) on the metabolic syndrome: An updated review. J. Integr. Med. 2022, 20, 193–203. [Google Scholar] [CrossRef]
- Boroumand, N.; Samarghandian, S.; Hashemy, S.I. Immunomodulatory, anti-inflammatory, and antioxidant effects of curcumin. J. Herbmed. Pharmacol. 2018, 7, 211–219. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Tanvir, E.M.; Hossen, M.S.; Hossain, M.F.; Afroz, R.; Gan, S.H.; Khalil, M.I.; Karim, N. Antioxidant Properties of Popular Turmeric (Curcuma longa) Varieties from Bangladesh. J. Food Qual. 2017, 2017, 8471785. [Google Scholar] [CrossRef]
- Remadevi, R.; Surendran, E.; Kimura, T. (Eds.) Turmeric in Traditional Medicine. Turmeric: The Genus Curcuma; CRC Press: Boca Raton, FL, USA, 2007; pp. 409–436. ISBN 9780849370342. [Google Scholar]
- Huang, M.-T.; Ma, W.; Lu, Y.-P.; Chang, R.L.; Fisher, C.; Manchand, P.S.; Newmark, H.L.; Conney, A.H.; You, M. Effects of curcumin, demethoxycurcumin, bisdemethoxycurcumin and tetrahydrocurcumin on 12-O-tetradecanoylphorbol-13-acetateinduced tumor promotion. Carcinogenesis 1995, 16, 2493–2497. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Maekawa, T.; Hidaka, K.; Bando, H.; Takeda, Y.; Yamaguchi, H. Chemical Studies on Antioxidant Mechanism of Curcumin: Analysis of Oxidative Coupling Products from Curcumin and Linoleate. J. Agric. Food Chem. 2001, 49, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, K. Dietary spices in health and diseases (II). Indian J. Physiol. Pharmacol. 2008, 52, 327–354. [Google Scholar]
- Gowda, N.K.S.; Ledoux, D.R.; Rottinghaus, G.E.; Bermudez, A.J.; Chen, Y.C. Efficacy of Turmeric (Curcuma longa), Containing a Known Level of Curcumin, and a Hydrated Sodium Calcium Aluminosilicate to Ameliorate the Adverse Effects of Aflatoxin in Broiler Chicks. Poult. Sci. 2008, 87, 1125–1130. [Google Scholar] [CrossRef]
- Kumari, P.; Gupta, M.; Ranjan, R.; Singh, K.; Yadava, R. Curcuma longa as feed additive in broiler birds and its patho-physiological effects. Indian J. Exp. Biol. 2007, 45, 272–277. [Google Scholar]
- Mehala, C.; Moorthy, M. Production performance of broilers fed with Aloe vera and Curcuma longa (Turmeric). Int. J. Poult. Sci. 2008, 7, 852–856. [Google Scholar] [CrossRef]
- Oke, O.E. Evaluation of physiological response and performance by supplementation of Curcuma longa in broiler feed under hot humid tropical climate. Trop. Anim. Health Prod. 2018, 50, 1071–1077. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.; Khan, Z.; Chakraverty, N. Soluble curcumin: A promising oral supplement for health management. J. Appl. Pharm. Sci. 2011, 1, 1–7. [Google Scholar]
- Zhang, J.; Hou, X.; Ahmad, H.; Zhang, H.; Zhang, L.; Wang, T. Assessment of free radicals scavenging activity of seven natural pigments and protective effects in AAPH-challenged chicken erythrocytes. Food Chem. 2014, 145, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.S.; Sekhara, N.C.; Satyanarayana, M.N.; Srinivasan, M. Effect of Curcumin on Serum and Liver Cholesterol Levels in the Rat. J. Nutr. 1970, 100, 1307–1315. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Free Radical Reactions of Curcumin in Membrane Models. Free Radic. Biol. Med. 1997, 23, 838–843. [Google Scholar] [CrossRef]
- Cleary, K.; McFeeters, R.F. Effects of Oxygen and Turmeric on the Formation of Oxidative Aldehydes in Fresh-Pack Dill Pickles. J. Agric. Food Chem. 2006, 54, 3421–3427. [Google Scholar] [CrossRef]
- Singh, R.K.; Rai, D.; Yadav, D.; Bhargava, A.; Balzarini, J.; De Clercq, E. Synthesis, antibacterial and antiviral properties of curcumin bioconjugates bearing dipeptide, fatty acids and folic acid. Eur. J. Med. Chem. 2010, 45, 1078–1086. [Google Scholar] [CrossRef]
- Akosile, O.A.; Majekodunmi, B.C.; Sogunle, O.M.; Baloyi, J.J.; Fushai, F.; Bhebhe, E.; Oke, O.E. Research Note: Responses of broiler chickens to in ovo feeding with clove and cinnamon extract under hot-humid environments. Poult. Sci. 2023, 102, 102391. [Google Scholar] [CrossRef]
- Pieper, G.M.; Jordan, M.; Dondlinger, L.A.; Adams, M.B.; Roza, A.M. Peroxidative Stress in Diabetic Blood Vessels: Reversal by Pancreatic Islet Transplantation. Diabetes 1995, 44, 884–889. [Google Scholar] [CrossRef]
- Lovkova, M.Y.; Buzuk, G.N.; Sokolova, S.M.; Klimenteva, N.I. Chemical Features of Medicinal Plants (Review). Appl. Bioch. Microbiol. 2001, 37, 229–237. [Google Scholar] [CrossRef]
- Seo, K.-I.; Choi, M.-S.; Jung, U.J.; Kim, H.-J.; Yeo, J.; Jeon, S.-M.; Lee, M.-K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008, 52, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Golian, A.; Kermanshahi, H.; Reza Bassami, M. Effects of curcumin or nanocurcumin on blood biochemical parameters, intestinal morphology and microbial population of broiler chickens reared under normal and cold stress conditions. J. Appl. Anim. Res. 2017, 46, 200–209. [Google Scholar] [CrossRef]
- Attia, A.Y.; Al-Harthi, A.M.; Hassan, S.S. Turmeric (Curcuma longa Linn.) as a phytogenic growth promoter alternative for antibiotic and comparable to mannan oligosaccharides for broiler chicks. Rev. Mex. Cienc. Pecu. 2017, 8, 11. [Google Scholar] [CrossRef]
- Attia, Y.; Bakhashwain, A.; Bertu, N. Utilisation of thyme powder (Thyme vulgaris L.) as a growth promoter alternative to antibiotics for broiler chickens raised in a hot climate. Eur. Poult. Sci. Arch. Für Geflügelkunde 2018, 82, 1–15. [Google Scholar] [CrossRef]
- Sugiharto, S. Alleviation of heat stress in broiler chicken using turmeric (Curcuma longa)—A short review. J. Anim. Behav. Biometeorol. 2020, 8, 215–222. [Google Scholar] [CrossRef]
- Ekine, O.; Udoudo, E.; George, O. Influence of turmeric (Curcuma longa) as feed additive on the performance, serum enzymes and lipid profile of broiler chickens. Niger. J. Anim. Sci. 2020, 22, 57–63. [Google Scholar]
- Ürüşan, H.; Bölükbaşi, C. The Influence of Turmeric Powder (Curcuma longa) on Fatty Acid Composition and Shelf Life of Broiler Chicken Meat. Alınteri. Zirai. Bilimler. Dergisi. 2020, 35, 29–35. [Google Scholar] [CrossRef]
- Imoru, A.; Onibi, G.; Osho, I. Nutritional and biochemical compositions of turmeric (Curcuma longa Linn) Rhizome powder–A promising animal feed additive. Int. J. Sci. Eng. Res. 2018, 9, 424–429. [Google Scholar]
- Ikpeama, A.; Onwuka, G.; Nwankwo, C. Nutritional composition of Tumeric (Curcuma longa) and its antimicrobial properties. Int. J. Sci. Eng. Res. 2014, 5, 1085–1089. [Google Scholar]
- Hajati, H.; Zaghari, M. Spirulina Platensis in Poultry Nutrition; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019; ISBN 1-5275-2128-1. [Google Scholar]
- Park, J.H.; Lee, S.I.; Kim, I.H. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult. Sci. 2018, 97, 2451–2459. [Google Scholar] [CrossRef]
- Shanmugapriya, B.; Saravana Babu, S. Supplementary effect of Spirulina platensis on performance, hematology and carcass yield of broiler chicken. Indian Streams Res. J. 2014, 4, 1–7. [Google Scholar]
- Bondar, A.; Macari, V.; Rudic, V.; Pistol, G.; Putin, V.; Rotaru, A.; Chiriac, T.; Solcan, G.; Solcan, C. Effects of ZooBioR2 Product as Feed Supplement in Laying Hens on the Morphofunctional State of Intestinal Mucosa. Arq. Bras. Med. Vet. Zootec. 2022, 74, 626–632. [Google Scholar] [CrossRef]
- Kharde, S.; Shirbhate, R.; Bahiram, K.; Nipane, S. Effect of Spirulina supplementation on growth performance of broilers. Indian J. Vet. Res. 2012, 21, 66–69. [Google Scholar]
- Kaoud, H.A. Effect of Spirulina platensis as a dietary supplement on broiler performance in comparison with prebiotics. Sci. J. Appl. Res. 2015, 1, 44–48. [Google Scholar]
- Bellof, G.; Alarcon, S.C. Effect of Spirulina platensis in the organic broiler production. Archiv. für. Geflügelkunde 2013, 77, 73–80. [Google Scholar]
- Toyomizu, M.; Sato, K.; Taroda, H.; Kato, T.; Akiba, Y. Effects of dietary Spirulina on meat colour in muscle of broiler chickens. Br. Poult. Sci. 2001, 42, 197–202. [Google Scholar] [CrossRef]
- Altmann, B.A.; Neumann, C.; Velten, S.; Liebert, F.; Mörlein, D. Meat Quality Derived from High Inclusion of a Micro-Alga or Insect Meal as an Alternative Protein Source in Poultry Diets: A Pilot Study. Foods 2018, 7, 34. [Google Scholar] [CrossRef]
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of Dietary Microalgae on Growth Performance, Profiles of Amino and Fatty Acids, Antioxidant Status, and Meat Quality of Broiler Chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef]
- Nouzarian, R.; Tabeidian, S.; Toghyani, M.; Ghalamkari, G.; Toghyani, M. Effect of turmeric powder on performance, carcass traits, humoral immune responses, and serum metabolites in broiler chickens. J. Anim. Feed Sci. 2011, 20, 389–400. [Google Scholar] [CrossRef]
- Arslan, M.; Haq, A.; Ashraf, M.; Iqbal, J.; Mund, M.D. Effect of turmeric (Curcuma longa) supplementation on growth performance, immune response, carcass characteristics and cholesterol profile in broilers. Veterinaria 2017, 66, 16–20. [Google Scholar]
- Shohe, A.; Vidyarthi, V.; Zuyie, R. Performance of broiler chicken on diet supplemented with Turmeric powder (Curcuma longa). Livest. Res. Int. 2019, 7, 77–82. [Google Scholar]
- Rahayu, W.; Tjiptasurasa, S.; Indriyani, D. Curcuma xanthorriza. J. Far. Indones. 2015, 6, 2–4. [Google Scholar]
- Durrani, F.; Ismail, M.; Sultan, A.; Suhail, S.; Chand, N.; Durrani, Z. Effect of different levels of feed added turmeric (Curcuma longa) on the performance of broiler chicks. J. Agric. Biol. Sci. 2006, 1, 9–11. [Google Scholar]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Alshehri, M.A.; Sayed, S.; Shukry, M.; El-Sayed, Y.S. The Impact of Curcumin on Growth Performance, Growth-Related Gene Expression, Oxidative Stress, and Immunological Biomarkers in Broiler Chickens at Different Stocking Densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef] [PubMed]
- Al-Sultan, S.I. The Effect of Curcuma longa (tumeric) on Overall Performance of Broiler Chickens. Int. J. Poult. Sci. 2003, 2, 351–353. [Google Scholar] [CrossRef]
- Daneshyar, M.; Ghandkanlo, M.; Bayeghra, F.; Farhangpajhoh, F.; Aghaei, M. Effects of dietary turmeric supplementation on plasma lipoproteins, meat quality and fatty acid composition in broilers. SA J. An. Sci. 2011, 41, 420–428. [Google Scholar] [CrossRef]
- Laganá, C.; Saldanha, E.S.P.B.; Sartori, J.R.; Turco, P.H.N.; Gonzales, E.; Luciano, R.L.; Zanatta, G.; Fascina, V.B. Turmeric on Poultry Production: A Review. Agric. Sci. 2019, 10, 1592–1601. [Google Scholar] [CrossRef]
- Hosseini Vashan, S.J.; Golian, A.; Yaghobfar, A.; Zarban, A.; Afzali, N.; Esmaeilinasab, P. Antioxidant status, immune system, blood metabolites and carcass characteristic of broiler chickens fed turmeric rhizome powder under heat stress. Afr. J. Biotechnol. 2012, 11, 16118–16125. [Google Scholar] [CrossRef]
- Emadi, M.; Kermanshahi, H. Effect of Turmeric Rhizome Powder on Performance and Carcass Characteristics of Broiler Chickens. Int. J. Poult. Sci. 2006, 5, 1069–1072. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Lieb, D.C.; Cole, B.K.; Taylor-Fishwick, D.A.; Chakrabarti, S.K.; Nadler, J.L. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid. Res. 2011, 50, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S.; Isroli, I.; Widiastuti, E.; Prabowo, N.S. Effect of turmeric extract on blood parameters, feed efficiency and abdominal fat content in broilers. J. Indones. Trop. Anim. Agric. 2011, 36, 21–26. [Google Scholar] [CrossRef][Green Version]
- Yarru, L.P.; Settivari, R.S.; Gowda, N.K.S.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G.E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 2009, 88, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Eevuri, T.; Putturu, R. Use of certain herbal preparations in broiler feeds—A review. Vet. World 2013, 6, 172–179. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, G.; Wang, Y.; Wu, C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2019, 98, 422–429. [Google Scholar] [CrossRef]
- Kichu, M.; Nizamuddin; Zuyie, R.; Rutsa, M.C.; Savino, N.; Singh, R. Influence of Turmeric Based Diet on the Performance of Broiler Chicken. Asian J. Anim. Sci. 2023, 17, 21–30. [Google Scholar] [CrossRef]
- Moustafa, E.S.; Alsanie, W.F.; Gaber, A.; Kamel, N.N.; Alaqil, A.A.; Abbas, A.O. Blue-Green Algae (Spirulina platensis) Alleviates the Negative Impact of Heat Stress on Broiler Production Performance and Redox Status. Animals 2021, 11, 1243. [Google Scholar] [CrossRef]
- Omar, A.E.; Al-Khalaifah, H.S.; Osman, A.; Gouda, A.; Shalaby, S.I.; Roushdy, E.M.; Abdo, S.A.; Ali, S.A.; Hassan, A.M.; Amer, S.A. Modulating the Growth, Antioxidant Activity, and Immunoexpression of Proinflammatory Cytokines and Apoptotic Proteins in Broiler Chickens by Adding Dietary Spirulina platensis Phycocyanin. Antioxidants 2022, 11, 991. [Google Scholar] [CrossRef]
- Fathi, M.A. Effect of dietary supplementation of algae meal (Spirulina platensis) as growth promoter on performance of broiler chickens. Egypt Poult. Sci. 2018, 38, 375–389. [Google Scholar]
- Evans, A.M.; Smith, D.L.; Moritz, J.S. Effects of algae incorporation into broiler starter diet formulations on nutrient digestibility and 3 to 21 d bird performance. J. Appl. Poult. Res. 2015, 24, 206–214. [Google Scholar] [CrossRef]
- Tavernari, F.D.E.C.; Roza, L.F.; Surek, D.; Sordi, C.; Silva, M.L.B.D.; Albino, L.F.T.; Migliorini, M.J.; Paiano, D.; Boiago, M.M. Apparent metabolisable energy and amino acid digestibility of microalgae Spirulina platensis as an ingredient in broiler chicken diets. Br. Poult. Sci. 2018, 59, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Swelum, A.A.; Salama, A.; Al-Ghadi, M.Q.; Qattan, S.Y.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Alhimaidi, A.R.; Almutairi, B.O.; Ammari, A.A.; et al. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020, 19, 1046–1056. [Google Scholar] [CrossRef]
- Alwaleed, E.A.; El-Sheekh, M.; Abdel-Daim, M.M.; Saber, H. Effects of Spirulina platensis and Amphora coffeaeformis as dietary supplements on blood biochemical parameters, intestinal microbial population, and productive performance in broiler chickens. Environ. Sci. Pollut. Res. Int. 2021, 28, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Aladaileh, S.H.; Khafaga, A.F.; Abd El-Hack, M.E.; Al-Gabri, N.A.; Abukhalil, M.H.; Alfwuaires, M.A.; Bin-Jumah, M.; Alkahtani, S.; Abdel-Daim, M.M.; Aleya, L.; et al. Spirulina platensis ameliorates the sub chronic toxicities of lead in rabbits via anti-oxidative, anti- inflammatory, and immune stimulatory properties. Sci. Total Environ. 2020, 701, 134879. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M.; Abd El-Hac, M.E.; Dhama, K. Nutritional and Healthical Aspects of Spirulina (Arthrospira) for Poultry, Animals and Human. Int. J. Pharm. 2015, 12, 36–51. [Google Scholar] [CrossRef]
- Kaushik, P.; Chauhan, A. In vitro antibacterial activity of laboratory grown culture of Spirulina platensis. Indian J. Microbiol. 2008, 48, 348–352. [Google Scholar] [CrossRef]
- Mirzaie, S.; Zirak-Khattab, F.; Hosseini, S.A.; Donyaei-Darian, H. Effects of dietary Spirulina on antioxidant status, lipid profile, immune response and performance characteristics of broiler chickens reared under high ambient temperature. Asian Australas. J. Anim. Sci. 2018, 31, 556–563. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Garlich, J.D.; Kidd, M.T. Dietary Spirulina Platensis Enhances Humoral and Cell-Mediated Immune Functions in Chickens. Immunopharmacol. Immunotoxicol. 1996, 18, 465–476. [Google Scholar] [CrossRef]
- Bhat, V.B.; Madyastha, K.M. C-Phycocyanin: A Potent Peroxyl Radical Scavenger in Vivo and in Vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef]
- Piñero Estrada, J.E.; Bermejo Bescós, P.; Villar del Fresno, A.M. Antioxidant activity of different fractions of Spirulina platensis protean extract. Farmaco 2001, 56, 497–500. [Google Scholar] [CrossRef]
- Bermejo, P.; Piñero, E.; Villar, Á.M. Iron-chelating ability and antioxidant properties of phycocyanin isolated from a protean extract of Spirulina platensis. Food Chem. 2008, 110, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Beheiry, R.R.; Abdel Fattah, D.M.; Roushdy, E.M.; Hassan, F.A.M.; Ismail, T.A.; Zaitoun, N.M.A.; Abo-Elmaaty, A.M.A.; Metwally, A.E. Effects of different feeding regimens with protease supplementation on growth, amino acid digestibility, economic efficiency, blood biochemical parameters, and intestinal histology in broiler chickens. BMC Vet. Res. 2021, 17, 283. [Google Scholar] [CrossRef]
- Dinarello, C. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Chen, J.-C.; Yang, S.-Y.; Wang, M.-F.; Liu, T.-C.; Chan, Y.-C. Expression of COX-2 and NMDA receptor genes at the cochlea and midbrain in salicylate-induced tinnitus: COX-2 and NMDA Receptor Gene Expression in a Tinnitus Model. Laryngoscope 2011, 121, 361–364. [Google Scholar] [CrossRef]
- Romay, C.; Ledón, N.; González, R. Further studies on anti-inflammatory activity of phycocyanin in some animal models of inflammation. Inflamm. Res. 1998, 47, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Naser, M.A.F.; Abdel-Wareth, A.A.A.; Saleh, A.A.; Elsayed, S.A.M.; Abdel Fattah, D.M.; Metwally, A.E. Effect of dietary supplementation of alpha-galactosidase on the growth performance, ileal digestibility, intestinal morphology, and biochemical parameters in broiler chickens. BMC Vet. Res. 2020, 16, 144. [Google Scholar] [CrossRef]
- Amer, S.A.; Tolba, S.A.; AlSadek, D.M.M.; Abdel Fattah, D.M.; Hassan, A.M.; Metwally, A.E. Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Vet. Res. 2021, 17, 312. [Google Scholar] [CrossRef]
- Khan, S.; Mobashar, M.; Mahsood, F.K.; Javaid, S.; Abdel-Wareth, A.A.; Ammanullah, H.; Mahmood, A. Spirulina inclusion levels in a broiler ration: Evaluation of growth performance, gut integrity, and immunity. Trop. Anim. Health Prod. 2020, 52, 3233–3240. [Google Scholar] [CrossRef]
- Saini, M.K.; Vaish, V.; Sanyal, S.N. Role of cytokines and Jak3/Stat3 signaling in the 1, 2-Dimethylhydrazine Dihydrochloride-induced rat model of colon carcinogenesis. Eur. J. Cancer Prev. 2013, 22, 215–228. [Google Scholar] [CrossRef]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Reddy, M.N.; Reddanna, P.; Madyastha, K.M. Selective Inhibition of Cyclooxygenase-2 by C-Phycocyanin, a Biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 2000, 277, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.E.; Chen, Y.; Ho, E.A.; Martinez, S.A.; Davies, N.M. Pharmacological effects of a C-phycocyanin-based multicomponent nutraceutical in an in-vitro canine chondrocyte model of osteoarthritis. Can. J. Vet. Res. 2015, 79, 241–249. [Google Scholar] [PubMed]
- Shih, C.-M.; Cheng, S.-N.; Wong, C.-S.; Kuo, Y.-L.; Chou, T.-C. Antiinflammatory and Antihyperalgesic Activity of C-Phycocyanin. Anesth. Analg. 2009, 108, 1303–1310. [Google Scholar] [CrossRef]
- Emadi, M.; Kermanshah, H.; Maroufyan, E. Effect of Varying Levels of Turmeric Rhizome Powder on Some Blood Parameters of Broiler Chickens Fed Corn-Soybean Meal Based Diets. Int. J. Poult. Sci. 2007, 6, 345–348. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. BioFactors 2013, 39, 69–77. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Li, Y.; Wang, J.; Liu, Y.; Luo, X.; Lei, W.; Xie, L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020, 101, 1079–1084. [Google Scholar] [CrossRef]
- Raheem, M.A.; Jiangang, H.; Yin, D.; Xue, M.; ur Rehman, K.; Rahim, M.A.; Fu, D.; Song, X.; Tu, J.; Khan, I.M.; et al. Response of lymphatic tissues to natural feed additives, curcumin (Curcuma longa) and black cumin seeds (Nigella sativa), in broilers against Pasteurella multocida. Poult. Sci. 2021, 100, 101005. [Google Scholar] [CrossRef]
- Attia, T.; Elbanna, H.; Zahran, M. Effect of Dietary Supplementation of BAM-100® on Growth Performance, Haematological and Biochemical Parameters in Broiler Chickens. J. Curr. Vet. Res. 2020, 2, 55–60. [Google Scholar] [CrossRef]
- Rajput, N.; Muhammad, N.; Yan, R.; Zhong, X.; Wang, T. Effect of Dietary Supplementation of Curcumin on Growth Performance, Intestinal Morphology and Nutrients Utilization of Broiler Chicks. J. Poult. Sci. 2013, 50, 44–52. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Sieo, C.C.; Abdullah, N.; Tan, W.S.; Ho, Y.W. Influence of β-glucanase-producing Lactobacillus strains on intestinal characteristics and feed passage rate of broiler chickens. Poult. Sci. 2005, 84, 734–741. [Google Scholar] [CrossRef]
- Saleena, L.A.K.; Chandran, D.; Rayirath, G.; Shanavas, A.; Rajalingam, S.; Vishvanathan, M.; Sharun, K.; Dhama, K. Development of Low-calorie Functional Yoghurt by Incorporating Mannitol Producing Lactic Acid Bacteria (Leuconostoc pseudomesenteroides) in the Standard Yoghurt Culture. J. Pure Appl. Microbiol. 2022, 16, 729–736. [Google Scholar] [CrossRef]
- Camay, R.M. Visceral Characteristics of Broiler Chickens (Gallus gallus domesticus) Supplemented with Turmeric Rhizome Powder (Curcuma longa). Universal. J. Agr. Res. 2023, 11, 300–305. [Google Scholar] [CrossRef]
- Naderi, M.; Akbari, M.R.; Asadi-Khoshoei, E.; Khaksar, K.; Khajali, F. Effects of Dietary Inclusion of Turmeric (Curcuma longa) and Cinnamon (Cinnamomum verum) Powders on Performance, Organs Relative Weight and Some Immune System Parameters in Broiler Chickens. Poult. Sci. J. 2014, 2, 153–163. [Google Scholar] [CrossRef]
- Yusuf, M.S.; Hassan, M.A.; Abdel-Daim, M.M.; El nabtiti, A.S.; Ahmed, A.M.; Moawed, S.A.; El-Sayed, A.K.; Cui, H. Value Added by Spirulina platensis in Two Different Diets on Growth Performance, Gut Microbiota, and Meat Quality of Japanese Quails. Vet. World 2016, 9, 1287–1293. [Google Scholar] [CrossRef]

| Drug Form | Dose | Effect | Source |
|---|---|---|---|
| Turmeric powder | 1000 g of turmeric/kg | Enhanced feed utilization and improved weight gain | [118] |
| Spirulina platensis | 1% | Increased body weight, decreased feed consumption ratios, improved blood parameters | [124] |
| Curcumin | 200 mg/kg | Enhanced bird growth performance, behavioral patterns, and immunity | [137] |
| Turmeric powder | 0.6 g/kg | Improved broiler performance index and net profit per bird | [148] |
| Spirulina platensis | 0.5–1% | Improved broiler production performance and balancing of the redox status | [149] |
| Spirulina platensis phycocyanin | 0, 0.25, 0.5, 0.75, and 1 g kg–1 diet | Enhanced growth-promoting, antioxidant, and anti-inflammatory properties | [150] |
| Spirulina plantesis | 0.7–0.9 g/kg | Improved growth performance, blood parameters, and biochemical changes in serum and microbial load | [151] |
| Drug Form | Dose | Effect | Source |
|---|---|---|---|
| Spirulinaplatensis | 1% | Decreased the numbers of coliform in the ileum and the caecum | [34] |
| Turmeric powder | 1% | Increased the abundance of Lactobacillus spp. in the chicken intestines | [83] |
| Spirulina platensis powder | 10 g/kg | Increased the levels of beneficial lactic acid bacteria | [155] |
| Spirulinaplatensis | 1–2 g/kg | Increased the Lactobacillus spp. count | [189] |
| Drug Form | Dose | Effect | Source |
|---|---|---|---|
| Turmeric rhizome extract | 300 mg/kg | Enhanced antioxidant capability, growth performance, and breast muscle weight ratio and reduced abdominal fat ratio | [73] |
| Turmeric rhizome extract | 50 and 100 mg/kg curcumin | Improved antioxidant capability, high growth performance, increased breast muscle weight ratio, reduction in the abdominal fat ratio | [104] |
| Curcuma spp. | Increased anti-inflammatory activity and weight index of lymphoid | [117] | |
| Curcuma longa (Turmeric) | 0.5 and 1.0% | Increased both erythrocytic and total leukocytic counts in addition to bursa, thymus, and spleen weight | [138] |
| Curcumin powder | 2.000 mg/kg | Significantly decreased absolute and relative abdominal fat weight and markedly decreased concentrations of plasma low-density lipoprotein cholesterol and plasma and hepatic triglyceride | [147] |
| Curcumin | Enhanced anti-inflammatory properties | [177] | |
| Curcuma longa powder | 1% | Immune enhancer | [181] |
| Spirulina powder | 2 g Spirulina/kg feed | Improved gut integrity and immunity in broiler production | [171] |
| Spirulina platensis phycocyanin | 0, 0.25, 0.5, 0.75, and 1 g kg–1 diet | Enhanced antioxidant and anti-inflammatory properties | [150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondar, A.; Horodincu, L.; Solcan, G.; Solcan, C. Use of Spirulina platensis and Curcuma longa as Nutraceuticals in Poultry. Agriculture 2023, 13, 1553. https://doi.org/10.3390/agriculture13081553
Bondar A, Horodincu L, Solcan G, Solcan C. Use of Spirulina platensis and Curcuma longa as Nutraceuticals in Poultry. Agriculture. 2023; 13(8):1553. https://doi.org/10.3390/agriculture13081553
Chicago/Turabian StyleBondar, Adrian, Loredana Horodincu, Gheorghe Solcan, and Carmen Solcan. 2023. "Use of Spirulina platensis and Curcuma longa as Nutraceuticals in Poultry" Agriculture 13, no. 8: 1553. https://doi.org/10.3390/agriculture13081553
APA StyleBondar, A., Horodincu, L., Solcan, G., & Solcan, C. (2023). Use of Spirulina platensis and Curcuma longa as Nutraceuticals in Poultry. Agriculture, 13(8), 1553. https://doi.org/10.3390/agriculture13081553






