1. Introduction
Maize (
Zea mays L.) is a food source of fundamental importance for mankind, classified as one of the “big three”, along with wheat and rice [
1,
2,
3]. Moreover, apart from direct consumption from humans, maize is a key element in feed production, covering almost all farmed animals, and also pets [
4]. During the last two decades, maize has been utilized for multiple other uses, such as biofuel, that led to the development of additional varieties/hybrids that meet with these requirements [
5].
During storage, maize is attacked by an extremely wide variety of insect pests, which are similar to those that infest other grains and related amylaceous commodities, such as wheat, barley, rice, etc., as well as flour, semolina, bran, etc. [
6,
7]. Nevertheless, there is one single species that can develop only on maize during storage and not on other grains: the larger grain borer,
Prostephanus truncatus (Horn) (Coleoptera: Bostrychidae) [
8,
9,
10]. Several attempts to test the development of this species in other major grains apart from maize have failed, which is a characteristic that is postulated to occur due to the increased kernel size of maize as compared to that of other species [
10,
11]. The geographical distribution of
P. truncatus constitutes one of the most interesting paradigms of poor phytosanitary measures at the post-harvest stages of durable agricultural commodities and has been thoroughly previously reviewed [
10]. This species was present only in Meso-America [
12], but after its accidental introduction in sub-Saharan Africa during the late 1970s or early 1980s [
13,
14], it has been established in more than 20 African countries so far, while it expands to other areas as well that, until recently, were not considered as suitable for
P. truncatus development [
10]. A recent geographical predictive model illustrated that the potential range expansion of
P. truncatus is enormous, indicating new, high-risk areas worldwide where the pest is not currently found, such as regions in the tropics of the western hemisphere and Asia [
15].
Even from its initial detection in Africa, it became evident that the control of
P. truncatus was problematic, as this species seems to have a natural tolerance to active ingredients that were effective for other stored-product insect species that coexist in the same commodity, such as the maize weevil,
Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). For instance, it has been reported that the organophosphorous compound chlorpyrifos-methyl was not effective for the control of this species, but it could control with success
S. zeamais [
16]. In this context, different “cocktails” of active ingredients have been used to control both species, which is a strategy that resulted in commercial formulations that are now registered for this purpose in Africa [
10]. More recent studies have shown that inert materials, such as diatomaceous earths (DEs), were less effective for the control of
P. truncatus as compared to other species [
17]. Nevertheless, there are studies that show that DEs are extremely effective for the control of this species at relatively low concentrations [
18,
19]. Newer active ingredients, such as the bacterial insecticide spinosad, have been found to be very effective in the case of
P. truncatus control [
20,
21], while other studies have shown that the progeny production of this species could not be totally avoided in spinosad-treated maize [
20]. On the other hand, insect growth regulators (IGRs) (juvenile hormone analogues, chitin synthesis inhibitors) sufficiently suppressed
P. truncatus progeny production when applied on maize kernels at doses higher than 5 ppm [
22]. The insect growth regulator S-Methoprene, which is registered for direct application on the grains in many countries, does not affect the adults of
P. truncatus or the relative lesser grain borer,
Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae), but it has a detrimental effect on progeny production capacity, as newly-hatched larvae are exposed to the insecticide prior to boring into the kernel [
10,
23].
Based on the above references and considering that different experimental protocols were tested each time (e.g., different insect strains, temperatures, maize hybrids, concentrations of insecticides etc.), the data from one study may not be comparable to other studies as well. In this regard, and considering the importance of this species, it is essential to evaluate a wide range of insecticides that are currently in use in stored product protection, in experimental protocols that should be conducted in parallel, i.e., with comparable biotic and abiotic conditions. Therefore, the aim of the present study was to evaluate the efficacy of several contact insecticides for the control of P. truncatus under controlled laboratory conditions.
2. Materials and Methods
2.1. Insects and Maize
The P. truncatus population used in the present study was originally collected in Tanzania in 2005 and since then, was continuously reared in the Laboratory of Entomology and Agricultural Zoology of the Department of Agriculture, Crop production and Rural Environment of the University of Thessaly, on maize at 25 ± 0.5 °C, 55 ± 5% relative humidity (RH) and continuous darkness. Adults of P. truncatus (<2 weeks old) were collected through sieving (sieve opening diameter: 1 mm) and used for experimentation.
Free of insects maize kernels, provided by a local retailer, were used in the bioassays. Before the beginning of the trials, the grains moisture content was 13.5 ± 0.2% and their bulk density was 87.3 kg/hl, as determined by a Multitest moisture meter (Multitest, Gode SAS, Le Catelet, France).
2.2. Inert Dusts and Insecticides
Three inert dusts were evaluated, namely a diatomaceous earth (DE) formulation, a zeolite and a kaolin. The zeolite contained 85% clinoptilolite and originated from natural deposits (Olympos S.A.–Industrial Minerals, Assiros, Greece), whereas the DE tested was a commercial dust DE formulation (Silicid, CAS number: 61790-53-2; Detia Garda GmbH, Laudenbach, Germany).
Three residual insecticides were also tested, i.e., the pyrethroid deltamethrin, the bacterial insecticide spinosad and the juvenile hormone analogue S-Methoprene. The deltamethrin formulation tested was SEGURO 2.5 EC (deltamethrin 2.5% w/v, Sharda Cropchem Espana S.L., Murcia, Spain), and it was chosen as it is one commonly used active ingredient against a wide spectrum of insect pests, whereas it is also registered as a grain protectant. The spinosad formulation used in the tests contained 48 g of active ingredient (AI) per liter, which was prepared as a suspension concentrate (SC) and provided by Dow AgroSciences (Laser 480 SC, spinosad 48% w/v, Dow AgroSciences Export-SAS, Valbonne, France). Finally, the S-Methoprene evaluated was Diacon IGR (33.6% w/o, Dow Agrosciences Ltd., Cambridge, UK).
2.3. First Series of Bioassays: Inert Dusts
In a first series of bioassays, the efficacy of the three inert dusts against adults of P. truncatus was evaluated. Two hundred grams of maize kernels were introduced in glass 1 L jars (Bormioli Luigi S.p.A., Parma, Italy) and dusted with the three insert dust formulations at three dose rates, i.e., 200, 500 and 1000 mg/kg (ppm), using separate jars for each inert dust formulation. Jars were afterwards sealed and shaken manually for 1 min to homogenously distribute the inert dusts in the entire maize mass. An additional series of maize-filled jars was left untreated and served as control.
Twenty grams of treated maize kernels were transferred in plastic, cylindrical vials (Rotilabo®-sample tins with snap-on lid, 3 cm diameter, 8 cm height, Carl Roth Gmbh and Co. Kg, Karlsruhe, Germany). Afterwards, 10 adults were inserted in each vial using a fine brush. There were three replicates for each treatment, whereas the whole procedure was repeated three times (three vial replicates per treatment × three experiment replicates = 9 replicates in total for each treatment). During the bioassay, all vials were kept at dark, 30 ± 0.5 °C and 55 ± 5% RH. All measurements were performed with a precision balance (Precisa 40SM-200A, Pag Oerlikon A.G., Zurich, Switzerland). Adult mortality was measured after 7, 14, 21 and 28 d of exposure, whereas dead individuals were removed from the vials. After the final mortality count (Day 28), all adults (dead or alive) were removed, and the vials were kept for an additional time interval of 65 d at the aforementioned conditions. The number and weight of the insect-infested and the uninfested kernels was initially planned to be counted for each vial separately 65 d after the final mortality count. However, 28 d after the final mortality count, a high number of progeny was noted in the vials with the inert dust-treated maize, which led to mold development. Moreover, adults in these treatments started piercing through the plastic vials trying to escape. As a result, the incubation period for progeny production for the inert dust treatments was terminated 28 d after the final mortality count.
The percentage of infested kernels (PiK%) was calculated according to the following formula:
where NiK and NuK stand for the number of infested and uninfested kernels, respectively.
2.4. Second Series of Bioassays: Residual Insecticides
In the second series of bioassays, we evaluated the efficacy of the three residual insecticides against P. truncatus adults. Specifically, we investigated the insecticidal effect of deltamethrin and spinosad at two dose rates, i.e., 0.5 and 1 ppm, and the effect of S-Methoprene applied at 1 and 5 ppm. These doses were selected because they are the respective label doses for the application of these insecticidal formulations on stored cereals as grain protectants.
A similar experimental protocol to the one described above was followed. Briefly, one hundred grams of maize kernels were placed in glass 1 L jars. Spraying solutions were prepared by diluting the appropriate amounts of the insecticidal formulations in distilled water. Spraying was conducted on a tray, on which maize kernels were spread in a thin layer, using a Kyoto BD-183 K airbrush (Grapho-tech, Japan) and a total volume rate of 1 mL of spraying solution per 100 g maize. Afterwards, kernels were placed back into the jars and manually shaken, as described previously, to achieve homogenous distribution of the insecticide in the maize kernels. Twenty grams of maize kernels together with ten P. truncatus adults were placed in the plastic, cylindrical vials, as described above. The whole experimental procedure was repeated three times, preparing new lots of insecticide-treated maize kernels every time, whereas there were three vial replicates for each treatment, as described for the inert dust bioassay. Vials were kept at the same conditions described above, whereas adult mortality was measured after 7, 14, 21 and 28 d. After the final mortality was measured, all adults were removed, and vials were maintained for an additional period of 65 d at the aforementioned conditions. After this period, the number of offspring and the infestation patterns on the kernels (number and weight of infested and uninfested kernels) were determined for each vial, separately.
2.5. Statistical Analysis
All analyses were performed using the JMP® Software (version 7.0) (SAS Institute Inc., Cary, NC, USA). Prior to analysis, all data were checked for normality using Shapiro–Wilk test at α < 0.05. The Kruskal–Wallis test was used for the statistical comparison of the mortality rates among the inert dusts or residual insecticides at 7, 14, 21 and 28 d of exposure within each dose. The same analysis was also performed for the comparison of progeny production and the infestation patterns on the kernels among the inert dusts or residual insecticides within each application dose. When differences were detected among treatments, the Dunn’s test for multiple comparisons was performed for post-hoc testing.
3. Results
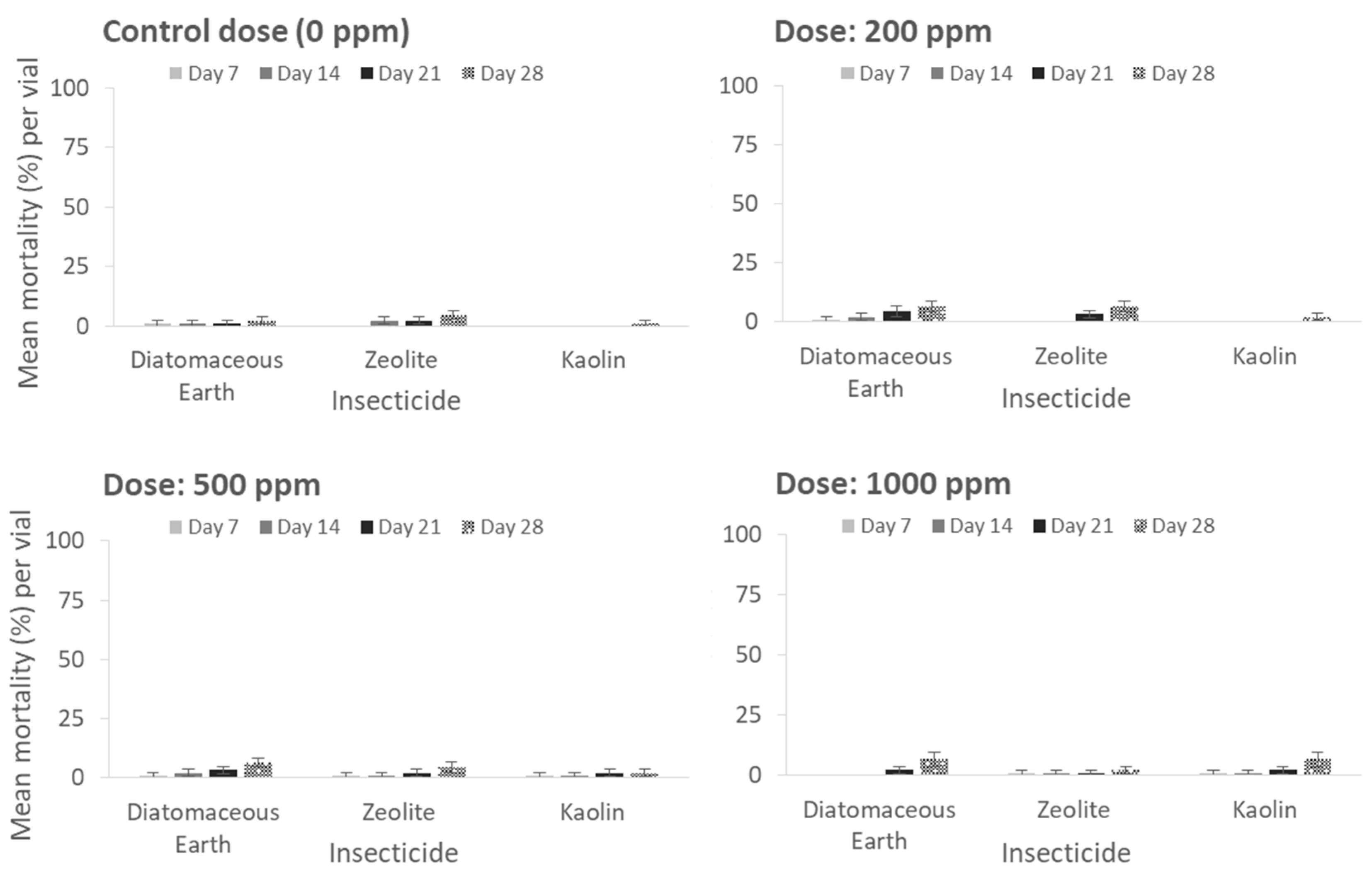
The mortality of
P. truncatus adults was significantly affected by the insecticidal formulation only in the case of the residual insecticides. Furthermore, the differences observed among the treatments after 7, 14 and 21 days of exposure were found to be comparable to those observed after 28 days, as indicated by the Kruskal–Wallis test. Hence, the data related to these analyses are not referenced within the context of this study. Control adult mortality, expressed as mean percentage of dead adults for each inert dust application and evaluation interval, was in all cases low and did not exceed 10% even after 28 d of exposure (x
2 = 2.6, df = 2,
p = 0.27) (
Figure 1). Low mortality levels were also recorded among the three inert dusts within the same application rates even after 28 d of exposure (at 28 d-exposure for 200 ppm: x
2 = 2.85, df = 2,
p = 0.23; 500 ppm: x
2 = 3.30, df = 2,
p = 0.19; 1000 ppm: x
2 = 1.73, df = 2,
p = 0.41) (
Figure 1).
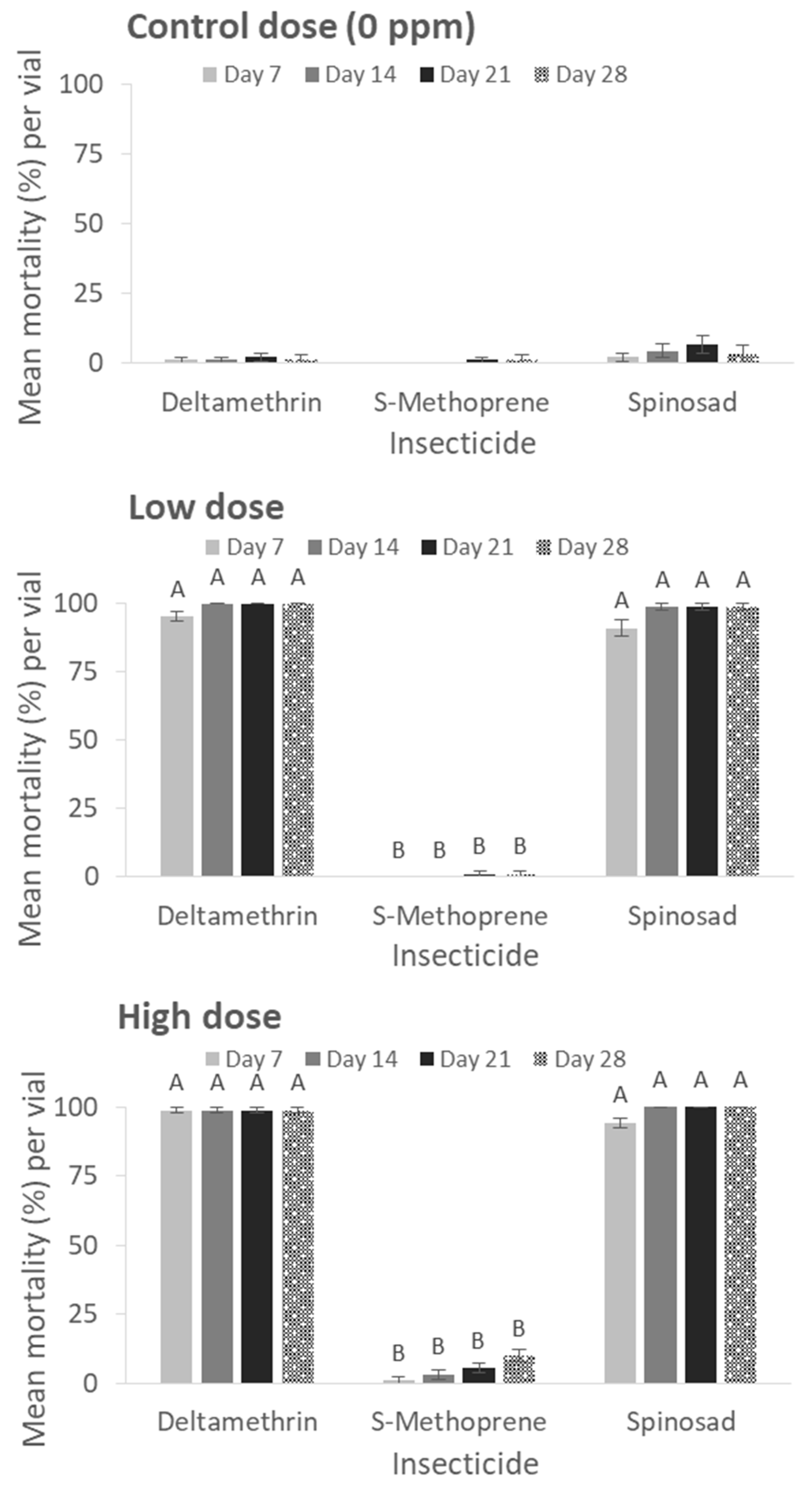
In contrast, high mortality levels that exceeded 95% were recorded for deltamethrin and spinosad already after 7 d of exposure at the lowest application rate (0.5 ppm) and reached complete control (100%) after 14 d of exposure for both insecticidal formulations (deltamethrin, spinosad) and all application rates (
Figure 2). The application of S-Methoprene did not result in high mortality rates, irrespective of the application rate and the evaluation interval (at 28 d exposure for 0 ppm: x
2 = 3.27, df = 2,
p = 0.19; low dose: x
2 = 24.0, df = 2,
p < 0.01; high dose: x
2 = 23.41, df = 2,
p < 0.01) (
Figure 2).
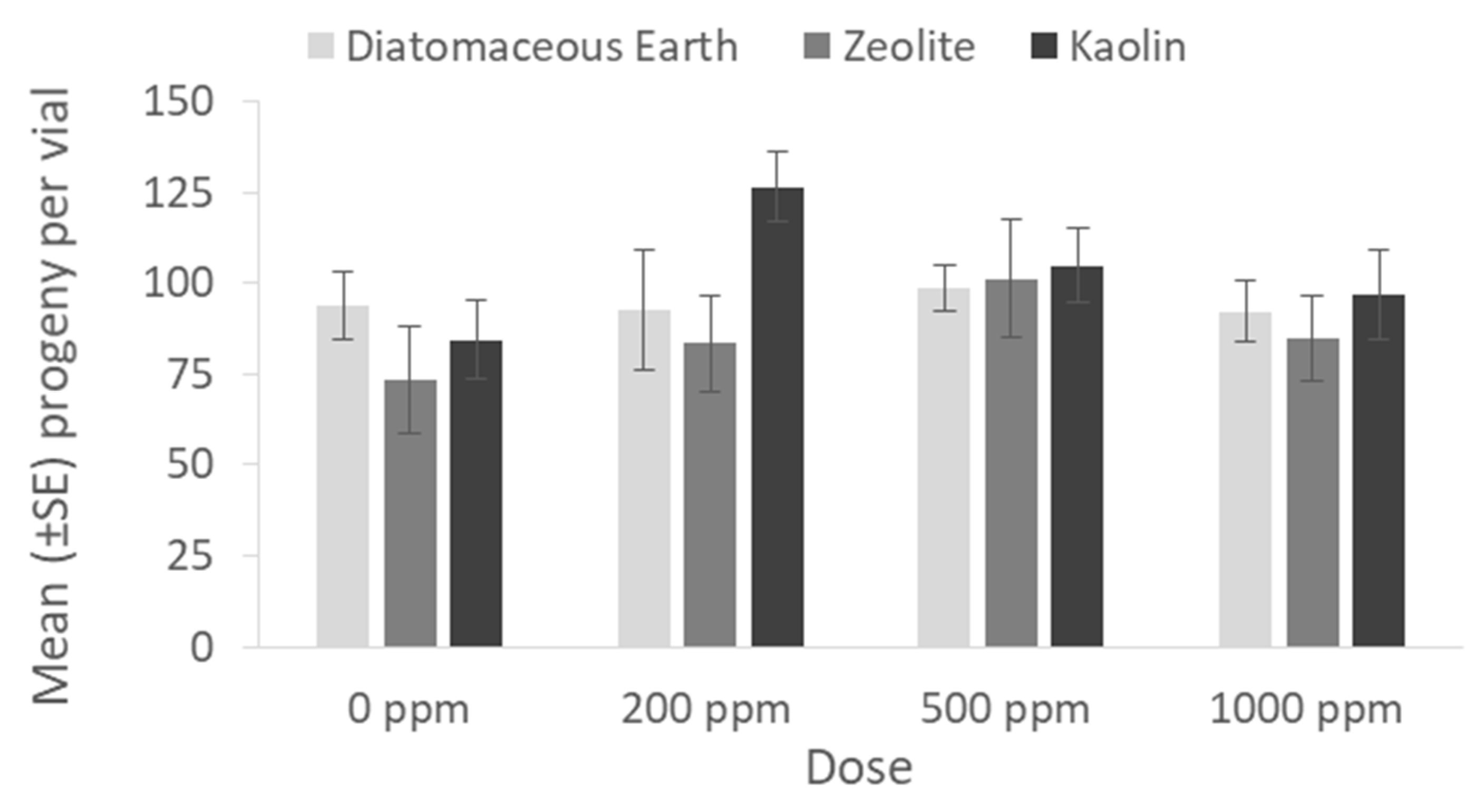
The number of progeny for each application rate of the inert dusts and the residual insecticides tested is presented in
Figure 3 and
Figure 4, respectively. The application of inert dusts did not affect the population growth of
P. truncatus, as adult emergence was in all cases high for the inert dust-treated maize and similar to the untreated control (0 ppm: x
2 = 1.45, df = 2,
p = 0.48; 200 ppm: x
2 = 4.87, df = 2,
p = 0.08; 500 ppm: x
2 = 0.31, df = 2,
p = 0.85; 1000 ppm: x
2 = 0.08, df = 2,
p = 0.95) (
Figure 3). On the contrary, the application of deltamethrin and spinosad suppressed progeny production to almost zero individuals (0 ppm: x
2 = 14.29, df = 2,
p < 0.01; low dose: x
2 = 22.29, df = 2,
p < 0.01; high dose: x
2 = 11.42, df = 2,
p < 0.01) (
Figure 4).
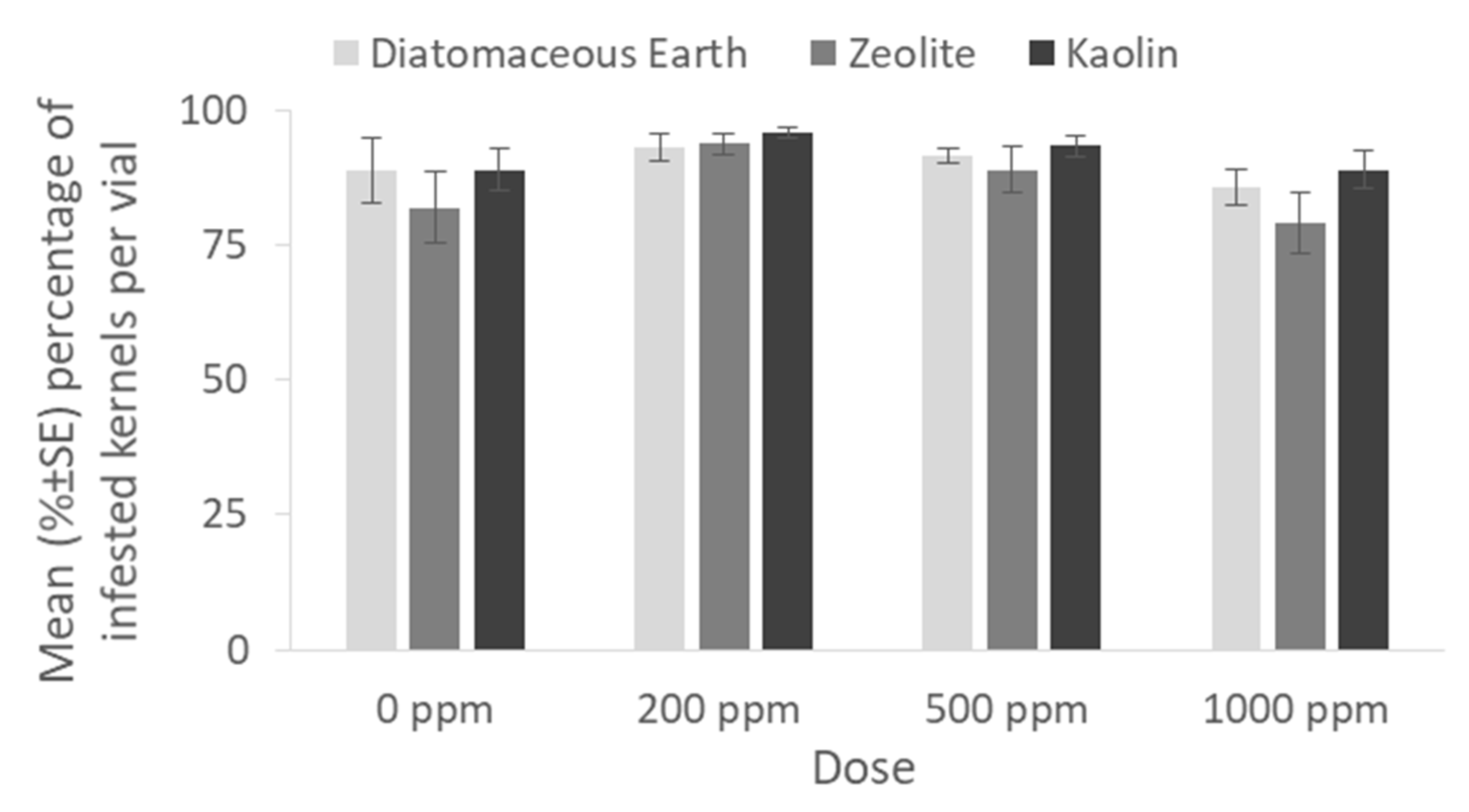
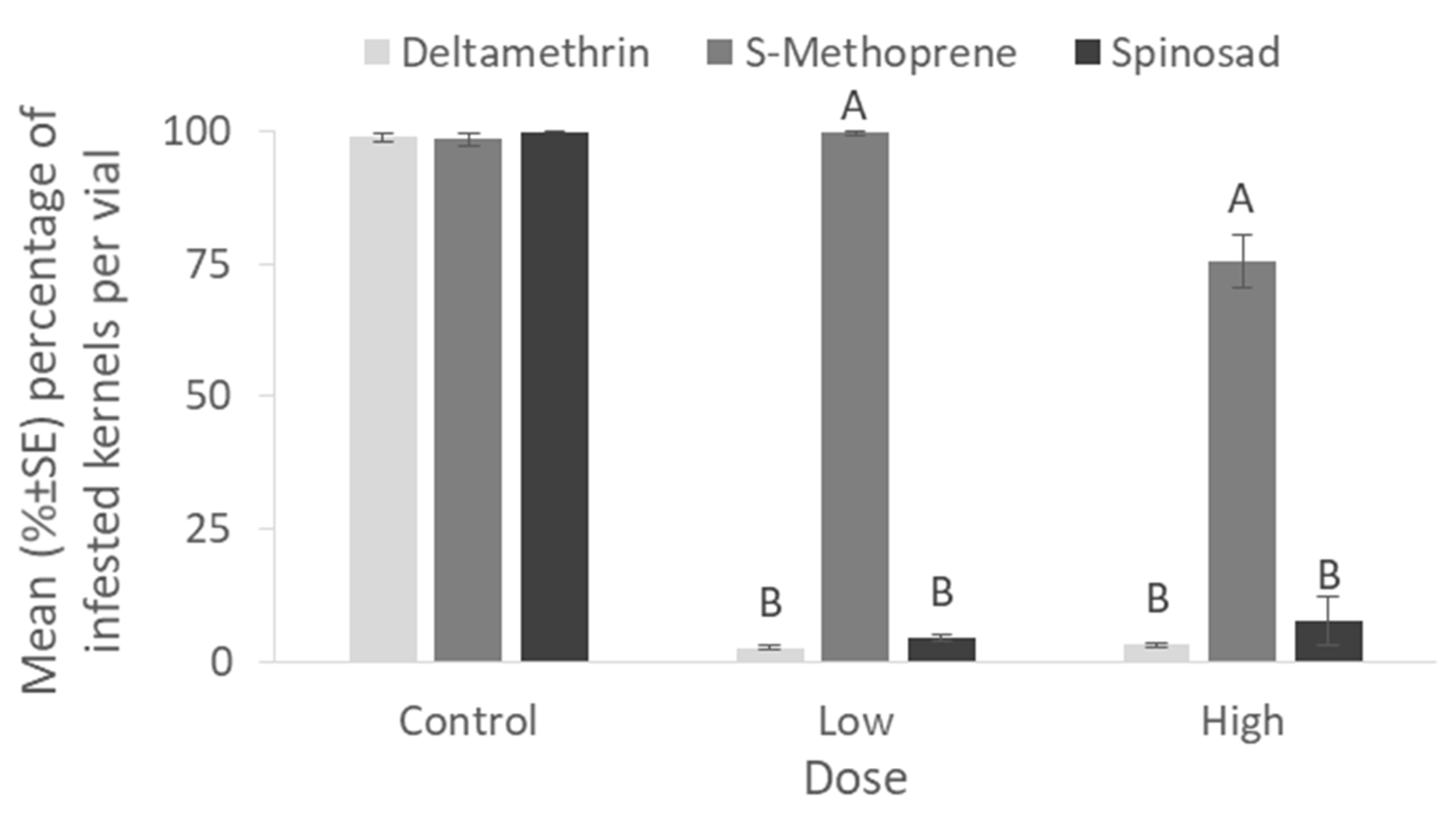
The percentage of infested by
P. truncatus maize kernels after exposure to inert dusts or residual insecticides is represented in
Figure 5 and
Figure 6, respectively. Over 90% of the kernels were found to be damaged in all inert dusts and doses tested (0 ppm: x
2 = 0.45, df = 2,
p = 0.79; 200 ppm: x
2 = 0.32, df = 2,
p = 0.84; 500 ppm: x
2 = 1.21, df = 2,
p = 0.54; 1000 ppm: x
2 = 3.9, df = 2,
p = 0.14) (
Figure 5). On the other hand, less than 10% of the kernels were damaged with the application of even the lower dose of 0.5 ppm of deltamethrin and spinosad, while significantly higher percentages were observed in the case of S-Methoprene for both doses (0 ppm: x
2 = 2.25, df = 2,
p = 0.32; low dose: x
2 = 20.21, df = 2,
p < 0.01; high dose: x
2 = 17.59, df = 2,
p < 0.01) (
Figure 6).
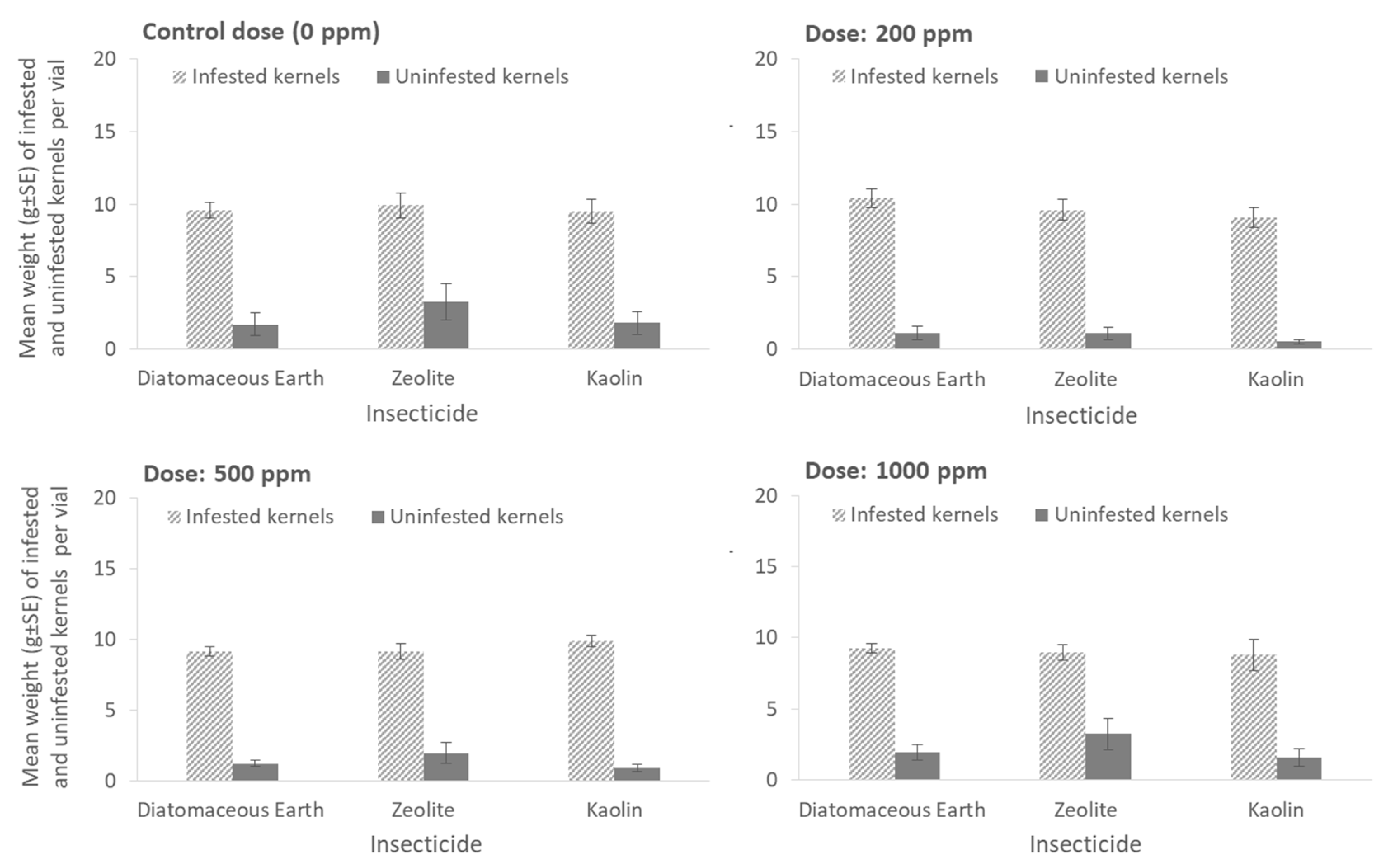
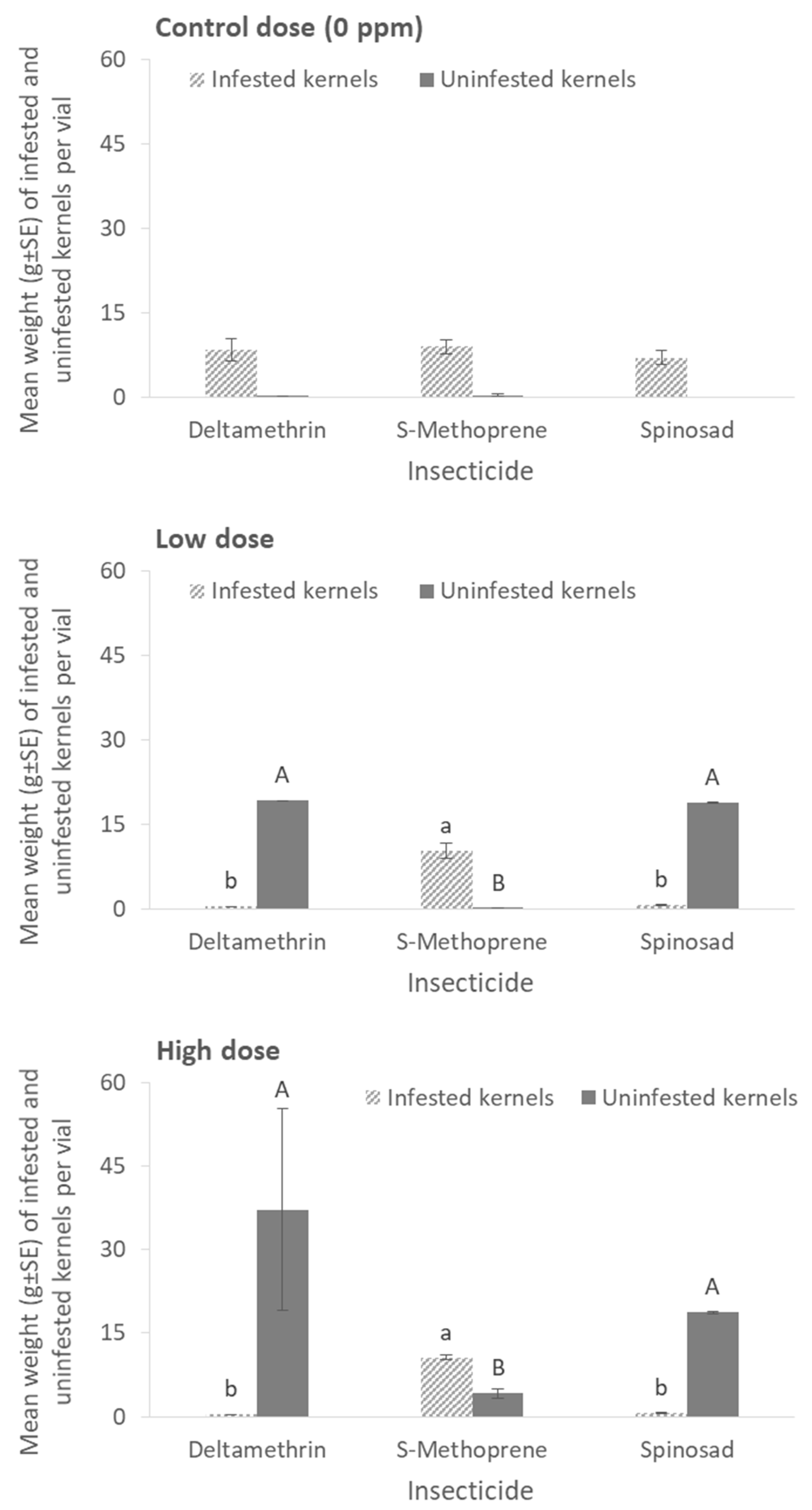
Last but not least, the weights of infested and uninfested kernels in each vial after exposure to inert dusts or residual insecticides are presented in
Figure 7 and
Figure 8, respectively. Most of the kernels treated with the inert dusts were infested and thus were heavier than the uninfested ones within the same vial, while no differences were found for the weight of infested or uninfested kernels among the insecticides at all doses tested (for the weight of infested kernels at 0 ppm: x
2 = 0.39, df = 2,
p = 0.82; 200 ppm: x
2 = 0.72, df = 2,
p = 0.42; 500 ppm: x
2 = 1.82, df = 2,
p = 0.40; 1000 ppm: x
2 = 0.12, df = 2,
p = 0.93; for the weight of uninfested kernels at 0 ppm: x
2 = 0.25, df = 2,
p = 0.87; 200 ppm: x
2 = 0.46, df = 2,
p = 0.79; 500 ppm: x
2 = 1.09, df = 2,
p = 0.57; 1000 ppm: x
2 = 3.65, df = 2,
p = 0.16) (
Figure 7).
Deltamethrin and spinosad provided the best grain protection, since the weight of infested kernels was extremely low (<0.6 g per vial) in all cases tested (for the infested kernels at 0 ppm: x
2 = 0.51, df = 2,
p = 0.77; low dose: x
2 = 18.60, df = 2,
p < 0.01; high dose: x
2 = 18.60, df = 2,
p < 0.01; for the uninfested kernels at 0 ppm: x
2 = 3.49, df = 2,
p = 0.17; low dose: x
2 = 18.13, df = 2,
p < 0.01; high dose: x
2 = 19.01, df = 2,
p < 0.01) (
Figure 8).
4. Discussion
In the current study, we evaluated six formulations of insecticides with different modes of action that are currently in use in stored product protection. Our findings indicated extreme differences among the various formulations tested, which underlines the value of the simultaneous evaluation of a wide range of insecticides under similar conditions, especially temperature and relative humidity, but also using the same insect strain and maize hybrid. In an earlier study, it was found that spinosad was less effective against
P. truncatus at lower temperatures [
24]. In contrast, a commercial DE formulation was shown to be more effective at 20 °C than at 30 °C [
18]. Additional published works clearly illustrate that the performance of
P. truncatus can be considerably affected by the strain, as certain strains can develop faster than others [
25].
Interestingly, none of the inert materials tested here was found to be effective for the control of
P. truncatus despite the fact that an earlier study showed that DEs can be highly effective against the adults of this species [
18]. In fact, there was no effect of the inert materials on the exposed adults, as mortality was similar to that of the untreated maize, or even if there were some differences, these can be considered as biologically meaningless. Most of the studies available focus on the mode of action of DEs, but we assume that also zeolite and kaolin have a similar mode of action. The low susceptibility of
P. truncatus adults to the inert materials tested is also evident from the increased progeny production in the treated substrate regardless of the exposure interval of the parental adults. Based on the available literature, DE is regarded as one of the most promising alternative insecticides and has been shown to be effective for a wide range of stored-product insect species, such as the sawtoothed beetle,
Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae), the confused flour beetle,
Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae), and the rice weevil,
Sitophilus oryzae (L.) (Coleoptera: Curculionidae) [
26,
27,
28,
29].
In contrast, the results of a series of field bioassays in Africa demonstrated a reduced mortality of
P. truncatus on DE-treated maize [
30]. Although we are unaware of the causes of this ineffectiveness, we estimate that this can be attributed to the fact that the interaction of the particles of the inert materials with the external surface of the maize kernel may result in poor particle retention and thus reduced efficacy [
31]. For instance, it has been shown that a commercial DE formulation was less effective for the control of
S. oryzae on maize as compared with rice [
27]. Similar results have been also reported for the application of DE on maize and wheat against adults of
T. confusum [
28].
In addition to the above, the negligible efficacy of the inert materials against
P. truncatus could be partially due to the way that the specific species infest the grain, given that the presence of dust is beneficial for the development of its larvae in their initial instars [
10]. Hodges reported that
P. truncatus prefers dusty environments and produces high amounts of dust during the infestation [
12], which is a characteristic that is also confirmed by the study of Sakka and Athanassiou [
32]. This is in absolute agreement with the current findings given that the production of frass recorded here was very high and corresponded to almost 50% of the initial weight of the maize kernels used. The increased amounts of dust, apart from the infestation
per se, are likely to negatively interact with the inert material particles through a “dilution” of the killing agent. Finally, insect movement is a critical factor that affects the susceptibility of stored-product insects to inert materials, which may explain the reduced susceptibility reported here, given that
P. truncatus adults are more slow-moving than other major stored-product beetle species [
10]. Higher dose rates of the inert materials could result in increased mortality, although it is generally regarded that doses above 1500–2000 ppm (e.g., 1.5–2.0 g/kg of grain) may not be desirable for health and environmental reasons [
33]. However, one potential approach to address the low effectiveness of the inert dusts tested in this study involves the combination of these materials with other residual insecticides. Indeed, this solution holds great promise not only in terms of using insecticides in lower concentrations but also in the integration of multiple modes of action against insect pests [
34,
35,
36]. Additional investigation toward this direction has the potential to reveal novel control methods against
P. truncatus, which are socially suitable, ecologically consistent and economically practicable [
34].
In contrast with the data above, in the second series of bioassays, we have found that deltamethrin and spinosad were highly effective against
P. truncatus, as they were able to kill 95% of the exposed individuals at their low dose (0.5 ppm), causing an impressive suppression in the percentage of offspring emergence as compared with the untreated maize. Conversely, S-Methoprene did cause some low adult mortality, but it reduced progeny production as well. Previous studies provided similar results for the efficacy of certain IGRs for the control of this species [
22,
37], but most of these data are focused on the effectiveness of IGRs that are categorized in the group of chitin biosynthesis inhibitors, and there are disproportionally few data for S-Methoprene, which is a juvenile hormone analogue. This active ingredient has been extensively evaluated with very good results for the control of
R. dominica, a relative species with
P. truncatus, even if a part of the grain mass is treated [
23,
38,
39]. Despite the fact that after exposure to S-Methoprene, the majority of the exposed adults were still active, we estimate that the reduction in the progeny production may have occurred through reduced oviposition, as it has been mentioned already for other IGRs as well [
40].
Even if insect mortality is an apparently important indicator for the efficacy of a given grain protectant, the infestation patterns could also provide valuable data to quantify the damage that is caused by stored-product insects in the treated substrate. Thus, we consider it essential that when possible, these parameters should be evaluated in parallel with efficacy indicators, as even if some insecticides act faster than others, the infestation patterns may be disproportionally high. In this context, any conclusions regarding the effectiveness of a given grain protectant may be misleading if changes in the kernel characteristics are not recorded. Taking into account the grain damage as the primary indicator of efficacy, S-Methoprene and spinosad appeared to be more effective than deltamethrin, which may be related with the activity of
P. truncatus larvae in the treated substrate. This signifies the non-analogous correlation between parental adult morality and progeny production feeding and that progeny production suppression may be more important than the mortality of the initial colonizers. Consequently, the three active ingredients tested in the second series of bioassays, from the most to the least effective one, can be classified as spinosad > S-Methoprene > deltamethrin. This observation stands in accordance with previous works with
P. truncatus [
10,
20,
41] and can be used as a stepping stone toward the utilization of selective insecticides with favorable ecotoxicological characteristics, such as spinosad, for the protection of stored products.