Accumulation of Stinging Nettle Bioactive Compounds as a Response to Controlled Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
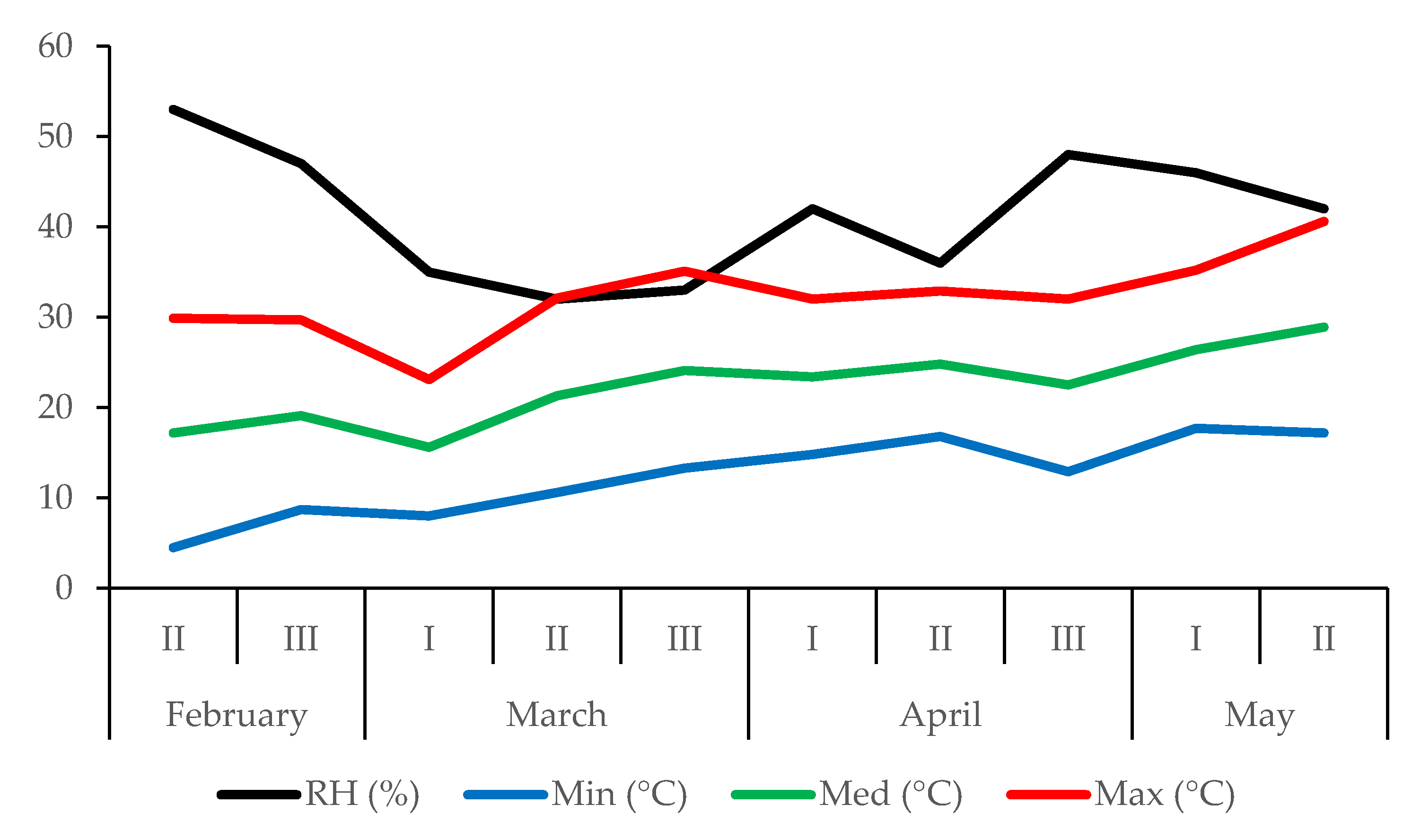
2.2. Cultivating Conditions
2.3. Determination of Physico-Chemical Properties
2.4. Determination of the Ascorbic Acid Content
2.5. Determination of Total Phenolic and Individual Phenolic Compounds
2.6. Determination of Pigment Compounds
2.7. Determination of Antioxidant Capacity
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Da Silva, E.C.; de Albuquerque, M.B.; de Azevedo Neto, A.D.; da Silva Junior, C.D. Drought and Its Consequences to Plants—From Individual to Ecosystem. In Responses of Organisms to Water Stress; Akinci, S., Ed.; InTechOpen Limited: London, UK, 2013. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Habib-Ur-Rahman, M.; Ahmad, A.; Raza, A.; Hasnain, M.U.; Alharby, H.F.; Alzahrani, Y.M.; Bamagoos, A.A.; Hakeem, K.R.; Ahmad, S.; Nasim, W.; et al. Impact of climate change on agricultural production; Issues, challenges, and opportunities in Asia. Front. Plant Sci. 2022, 13, 925548. [Google Scholar] [CrossRef]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Alterations of Endogenous Hormonal Levels in Plants under Drought and Salinity. Am. J. Plant Sci. 2016, 7, 1357–1371. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The Impact of Drought in Plant Metabolism: How to Exploit Tolerance Mechanisms to Increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Bárzana, G.; Carvajal, M. Genetic regulation of water and nutrient transport in water stress tolerance in roots. J. Biotechnol. 2020, 324, 134–142. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, M.; Gu, W.; Chen, Z.; Gu, Y.; Pei, L.; Tian, R. Effect of drought on photosynthesis, total antioxidant capacity, bioactive component accumulation, and the transcriptome of Atractylodes lancea. BMC Plant. Biol. 2021, 21, 293. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought Stress Causes a Reduction in the Biosynthesis of Ascorbic Acid in Soybean Plants. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress Enhances the Synthesis of Secondary Plant Products: The Impact of Stress-Related Over-Reduction on the Accumulation of Natural Products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Lynch, J.; Cain, M.; Frame, D.; Pierrehumbert, R. Agriculture’s Contribution to Climate Change and Role in Mitigation Is Distinct from Predominantly Fossil CO2-Emitting Sectors. Front. Sustain. Food Syst. 2021, 4, 2020. [Google Scholar] [CrossRef]
- Ali Al Meselmani, M. Nutrient Solution for Hydroponics. In Recent Research and Advances in Soilless Culture; Turan, M., Argin, S., Yildirim, E., Güneş, A., Eds.; IntechOpen Limited: London, UK, 2022. [Google Scholar] [CrossRef]
- Stegelmeier, A.A.; Rose, D.M.; Joris, B.R.; Glick, B.R. The Use of PGPB to Promote Plant Hydroponic Growth. Plants 2022, 11, 2783. [Google Scholar] [CrossRef]
- Opačić, N.; Radman, S.; Fabek Uher, S.; Benko, B.; Voća, S.; Šic Žlabur, J. Nettle Cultivation Practices—From Open Field to Modern Hydroponics: A Case Study of Specialized Metabolites. Plants 2022, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef] [PubMed]
- De Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Pardossi, A.; Remorini, D.; Guidi, L. Antioxidant and photosynthetic response of a purple-leaved and a green-leaved cultivar of sweet basil (Ocimum basilicum) to boron excess. Environ. Exp. Bot. 2013, 85, 64–75. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Kleinwächter, M.; Paulsen, J.; Schnug, E.; Selmar, D. Stress-induced changes of bioactive compounds in Tropaeolum majus L. Ind. Crops Prod. 2014, 60, 349–359. [Google Scholar] [CrossRef]
- Shafiq, S.; Akram, N.A.; Ashraf, M. Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci. Hortic. 2015, 185, 68–75. [Google Scholar] [CrossRef]
- Shan, C.; Zhao, X. Effects of lanthanum on the ascorbate and glutathione metabolism of Vigna radiata seedlings under salt stress. Biol. Plant 2014, 58, 595–599. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Rahman, M.D.; Narayan, O. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Šola, I.; Davosir, D.; Kokić, E.; Zekirovski, J. Effect of Hot- and Cold-Water Treatment on Broccoli Bioactive Compounds, Oxidative Stress Parameters and Biological Effects of Their Extracts. Plants 2023, 12, 1135. [Google Scholar] [CrossRef]
- Gmižić, D.; Pinterić, M.; Lazarus, M.; Šola, I. High Growing Temperature Changes Nutritional Value of Broccoli (Brassica oleracea L. convar. botrytis (L.) Alef. var. cymosa Duch.) Seedlings. Foods 2023, 12, 582. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Radman, S.; Fabek Uher, S.; Opačić, N.; Benko, B.; Galić, A.; Samirić, P.; Voća, S. Plant Response to Mechanically-Induced Stress: A Case Study on Specialized Metabolites of Leafy Vegetables. Plants 2021, 10, 2650. [Google Scholar] [CrossRef]
- Dujmović, M.; Opačić, N.; Radman, S.; Fabek Uher, S.; Petek, M.; Čoga, L.; Galić, A.; Dobričević, N.; Toth, N.; Božidar, B.; et al. Nutrient Solution Management—Innovative Agricultural Practice for Higher Nutrient Quality of Stinging Nettle. In Proceedings of the Book of Abstracts of the 10th International Congress of Food Technologists, Biotechnologists and Nutritionists, Zagreb, Croatia, 30 November–2 December 2022; pp. 18–19. [Google Scholar]
- Dujmović, M.; Opačić, N.; Radman, S.; Fabek Uher, S.; Voća, S.; Galić, K.; Kurek, M.; Ščetar, M.; Šic Žlabur, J. Functional and Nutritional Properties, Packaging Possibilities and Potential Use of Stinging Nettle—Case Study. In Proceedings of the 4th International Congress on Food Safety and Quality “One Health”, Dubrovnik, Croatia, 9–12 November 2022; Volume 73, p. 32. [Google Scholar]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, P.; Ieri, F.; Vignolini, P.; Bacci, L.; Baronti, S.; Romani, A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. J. Agric. Food Chem. 2008, 19, 9127–9132. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Vitkauskaitė, M.; Paulauskienė, A.; Černiauskienė, J. Wild Stinging Nettle (Urtica dioica L.) Leaves and Roots Chemical Composition and Phenols Extraction. Plants 2023, 12, 309. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Kruk, V.; Pedisić, S.; Çınar, F.; Bursać Kovačević, D.; Žutić, I.; Dragović-Uzelac, V. Accelerated Solvent Extraction as a Green Tool for the Recovery of Polyphenols and Pigments from Wild Nettle Leaves. Processes 2020, 8, 803. [Google Scholar] [CrossRef]
- Król, A.; Amarowicz, R.; Weidner, S. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant. 2014, 36, 1491–1499. [Google Scholar] [CrossRef]
- Moayedinezhad, A.; Mohammadparast, B.; Salekdeh, G.H.; Mohseni Fard, E.; Nejatian, M.A. Effects of drought stress on total phenolics, phenolic acids, polyamines and some organic acids in two important Iranian grapevine cultivars. J. Plant Process Funct. 2020, 8, 19–26. [Google Scholar]
- HunterLab. L a b Color Scale. Available online: https://www.hunterlab.com/en/ (accessed on 9 May 2023).
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2002. [Google Scholar]
- Ough, C.S.; Amerine, M.A. Methods for Analysis of Musts and Wines, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Otles, S.; Yalcin, B. Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci. World J. 2012, 2012, 564367. [Google Scholar] [CrossRef]
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll-letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Diplock, A.T.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- SAS®/STAT 9.4; SAS Institute Inc.: Cary, NC, USA, 2018.
- Ngoune Liliane, T.; Shelton Charles, M. Factors Affecting Yield of Crops. In Agronomy—Climate Change and Food Security; Amanullah, D., Ed.; InTechOpen Limited: London, UK, 2020. [Google Scholar] [CrossRef]
- Santini, M.; Noce, S.; Antonelli, M.; Caporaso, L. Complex drought patterns robustly explain global yield loss for major crops. Sci. Rep. 2022, 12, 5792. [Google Scholar] [CrossRef]
- Najla, S.; Sanoubar, R.; Murshed, R. Morphological and biochemical changes in two parsley varieties upon water stress. Physiol. Mol. Biol. Plants 2012, 18, 133–139. [Google Scholar] [CrossRef]
- Orimoloye Israel, R. Agricultural Drought and Its Potential Impacts: Enabling Decision-Support for Food Security in Vulnerable Region. Front. Sustain. Food Syst. 2022, 6, 2022. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Lu, X.; Zhang, M.; Jin, J. Effect of Drought on Agronomic Traits of Rice and Wheat: A Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 839. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. In Climate Change and Plant Abiotic Stress Tolerance, 1st ed.; Tuteja, N., Gill, S.S., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2014. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef]
- Yang, M.; Ding, S. Algorithm for appearance simulation of plant diseases based on symptom classification. Front. Plant Sci. 2022, 13, 2022. [Google Scholar] [CrossRef] [PubMed]
- Veazie, P.; Cockson, P.; Henry, J.; Perkins-Veazie, P.; Whipker, B. Characterization of Nutrient Disorders and Impacts on Chlorophyll and Anthocyanin Concentration of Brassica rapa var. Chinensis. Agriculture 2020, 10, 461. [Google Scholar] [CrossRef]
- Yu, X.; Guo, L.; Jiang, G.; Song, Y.; Muminov, M.A. Advances of organic products over conventional productions with respect to nutritional quality and food security. Acta Ecol. Sin. 2018, 38, 53–60. [Google Scholar] [CrossRef]
- Baafi, E.; Gracen, V.E.; Manu-Aduening, J.; Blay, E.T.; Ofori, K.; Carey, E.E. Genetic control of dry matter, starch and sugar content in sweetpotato. Acta Agric. Scand. B Soil Plant Sci. 2017, 67, 110–118. [Google Scholar] [CrossRef]
- Gao, K.; Yu, Y.F.; Xia, Z.T.; Yang, G.; Xing, Z.L.; Qi, L.T.; Ling, L.Z. Response of height, dry matter accumulation and partitioning of oat (Avena sativa L.) to planting density and nitrogen in Horqin Sandy Land. Sci. Rep. 2019, 9, 7961. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.; Debella, A. Review on dry matter production and partitioning as affected by different environmental conditions. Int. J. Adv. Res. Biol. Sci. 2020, 7, 37–46. [Google Scholar] [CrossRef]
- Mogren, L.M.; Beacham, A.M.; Reade, J.P.H.; Monaghan, J.M. Moderate water stress prevents the postharvest decline of ascorbic acid in spinach (Spinacia oleracea L.) but not in spinach beet (Beta vulgaris L.). J. Sci. Food Agric. 2015, 96, 2976–2980. [Google Scholar] [CrossRef]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Nutritional, Biophysical and Physiological Characteristics of Wild Rocket Genotypes As Affected by Soilless Cultivation System, Salinity Level of Nutrient Solution and Growing Period. Front. Plant Sci. 2017, 8, 300. [Google Scholar] [CrossRef]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How Do Plants Respond to Combined Drought and Salinity Stress?—A Systematic Review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef]
- Livingston, N.J.; Spittlehouse, D.L. 10—Carbon Isotope Fractionation in Tree Rings in Relation to the Growing Season Water Balance. In Stable Isotopes and Plant Carbon-Water Relations; Ehleringer, J.R., Hall, A.E., Farquhar, G.D., Eds.; Academic Press Inc.: San Diego, CA, USA, 1993; pp. 141–153. [Google Scholar] [CrossRef]
- Radman, S.; Javornik, M.; Žutić, I.; Opačić, N.; Benko, B. Impact of different nutrient solution composition on stinging nettle growth and mineral content. Acta Hortic. 2021, 1320, 157–166. [Google Scholar] [CrossRef]
- Opačić, N.; Dujmović, M.; Šic Žlabur, J.; Fabek Uher, S.; Benko, B.; Toth, N.; Čoga, L.; Petek, M.; Voća, S.; Radman, S. Floating Hydroponics as a Sustainable Agricultural Practice in Nettle Cultivation—Successful Management of Nutrient Solution. In Proceedings of the CASEE Conference 2022, Prague, Czech Republic, 22–24 June 2022. [Google Scholar]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Horticulturae Ann. 2010, 22, 9–13. [Google Scholar] [CrossRef]
- Westwood, C.T.; Mulcock, H. Nutritional evaluation of five species of forage brassica. Proc. J. N. Z. Grassl. 2012, 74, 31–38. [Google Scholar] [CrossRef]
- Priecina, L.; Karklina, D. Composition of Major Organic Acids in Vegetables and Species. In Proceedings of the CBU International Conference on Innovation, Technology Transfer and Education, Prague, Czech Republic, 25–27 March 2015. [Google Scholar] [CrossRef]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The Multiple Roles of Ascorbate in the Abiotic Stress Response of Plants: Antioxidant, Cofactor, and Regulator. Front. Plant Sci. 2021, 12, 2021. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Ma, Y.H.; Ma, F.W.; Wang, Y.H.; Zhang, J.K. The responses of the enzymes related with ascorbate–glutathione cycle during drought stress in apple leaves. Acta Physiol. Plant. 2011, 33, 173–180. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Orsák, M.; Kotikova, Z.; Hnilička, F.; Lachman, J.; Stanovič, R. Effect of drought and waterlogging on hydrophilic antioxidants and their activity in potato tubers. Plant Soil Environ. 2020, 66, 128–134. [Google Scholar] [CrossRef]
- Dere, S.; Kusvuran, S.; Dasgan, H.Y. Does drought increase the antioxidant nutrient capacity of tomatoes? Int. J. Food Sci. Technol. 2022, 57, 6633–6645. [Google Scholar] [CrossRef]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of drying methods on chemical composition and antioxidant activity of underutilized stinging nettle leaves. Heliyon 2020, 6, e03938. [Google Scholar] [CrossRef]
- Paulauskienė, A.; Tarasevičienė, Ž.; Laukagalis, V. Influence of Harvesting Time on the Chemical Composition of Wild Stinging Nettle (Urtica dioica L.). Plants 2021, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.E.A.; Ueda, Y.; Imahori, Y.; Ayaki, M. l-ascorbic acid metabolism in spinach (Spinacia oleracea L.) during postharvest storage in light and dark. Postharvest Biol. Technol. 2003, 28, 47–57. [Google Scholar] [CrossRef]
- Lawal, O.O.; Essien, N.C.; Essien, N.M.; Ochalla, F. Vitamin C content of some processed green leafy vegetables. Euro. J. Exp. Bio. 2015, 5, 110–112. [Google Scholar]
- Hailemariam, G.A.; Wudineh, T.A. Effect of Cooking Methods on Ascorbic Acid Destruction of Green Leafy Vegetables. J. Food Qual. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Institute of Medicine. Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. Available online: https://pubmed.ncbi.nlm.nih.gov/25077263/ (accessed on 13 March 2023).
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate–deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, Z.; Esmaielpour, B.; Estaji, A. Ameliorative effects of ascorbic acid on tolerance to drought stress on pepper (Capsicum annuum L.) plants. Physiol. Mol. Biol. Plants 2020, 26, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology—Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen Limited: London, UK, 2022. [Google Scholar] [CrossRef]
- Albergaria, E.A.; Oliveira, A.F.M.; Albuquerque, U.P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. S. Afr. J. Bot. 2020, 131, 12–17. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kadioglu, A.; Turgut, R. Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rosc.) Eichler. Can. J. Plant Sci. 1999, 80, 373–378. [Google Scholar] [CrossRef]
- Aninbon, C.; Jogloy, S.; Vorasoot, N.; Nuchadomrong, S.; Senawong, T.; Holbrook, C.; Patanothai, A. Effect of mid season drought on phenolic compounds in peanut genotypes with different levels of resistance to drought. Field Crops Res. 2016, 187, 127–134. [Google Scholar] [CrossRef]
- Puente-Garza, C.A.; Meza-Miranda, C.; Ochoa-Martínez, D.; García-Lara, S. Effect of in vitro drought stress on phenolic acids, flavonols, saponins, and antioxidant activity in Agave salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech. f. Phytochem 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Espadas, J.L.; Castaño, E.; Marina, M.L.; Rodríguez, L.C.; Plaza, M. Phenolic compounds increase their concentration in Carica papaya leaves under drought stress. Acta Physiol. Plant. 2019, 41, 180. [Google Scholar] [CrossRef]
- Talbi, S.; Rojas, J.A.; Sahrawy, M.; Rodríguez-Serrano, M.; Cárdenas, K.E.; Debouba, M.; Sandalio, L.M. Effect of drought on growth, photosynthesis and total antioxidant capacity of the saharan plant Oudeneya africana. Environ. Exp. Bot. 2020, 176, 104099. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Malin, V.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Profile, Antioxidant Capacity and Antimicrobial Activity of Nettle Leaves Extracts Obtained by Advanced Extraction Techniques. Molecules 2021, 26, 6153. [Google Scholar] [CrossRef] [PubMed]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Elez Garofulić, I.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive Compounds in Wild Nettle (Urtica dioica L.) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habitat Variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Ince, A.E.; Sahin, S.; Sumnu, G. Comparison of microwave and ultrasound-assisted extraction techniques for leaching of phenolic compounds from nettle. J. Food Sci. Technol. 2014, 10, 2776–2782. [Google Scholar] [CrossRef] [PubMed]
- Francišković, M.; Gonzalez-Pérez, R.; Orčić, D.; Sánchez de Medina, F.; Martínez-Augustin, O.; Svirčev, E.; Simin, N.; Mimica-Dukić, N. Chemical Composition and Immuno-Modulatory Effects of Urtica dioica L. (Stinging Nettle) Extracts. Phytother. Res. 2017, 31, 1183–1191. [Google Scholar] [CrossRef]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2015, 96, 2952–2962. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species. Arch. Pharm. Res. 2004, 11, 1114–1119. [Google Scholar] [CrossRef]
- Zhang, M.; Bu, T.; Liu, S.; Kim, S. Optimization of Caffeic Acid Extraction from Dendropanax morbifera Leaves Using Response Surface Methodology and Determination of Polyphenols and Antioxidant Properties. Horticulturae 2021, 7, 491. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; uz Zaman, Q.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective Roles and Mechanisms of Caffeic Acid in Counter Plant Stress: A Mini Review. Pak. J. Agric. Sci. 2019, 32, 8–19. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Ferulic Acid: An Antioxidant Found Naturally in Plant Cell Walls and Feruloyl Esterases Involved in its Release and Their Applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, V.; Guleria, P. Naringin: Biosynthesis and Pharmaceutical Applications. Indian J. Pharm. Sci. 2019, 81, 988–999. [Google Scholar] [CrossRef]
- Chaouachi, L.; Marín-Sanz, M.; Kthiri, Z.; Boukef, S.; Harbaoui, K.; Barro, F.; Karmous, C. The opportunity of using durum wheat landraces to tolerate drought stress: Screening morpho-physiological components. AoB PLANTS 2023, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shojaie, B.; Mostajeran, A.; Ghanadian, M. Flavonoid dynamic responses to different drought conditions: Amount, type, and localization of flavonols in roots and shoots of Arabidopsis thaliana L. Turk. J. Biol. 2016, 40, 612–622. [Google Scholar] [CrossRef]
- Hardo Panintingjati Brotosudarmo, T.; Limantara, L.; Dwi Chandra, R.; Heriyanto, A. Chloroplast Pigments: Structure, Function, Assembly and Characterization. In Plant Growth and Regulation—Alterations to Sustain Unfavorable Conditions; Ratnadewi, D., Hamim, Eds.; InTechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic Variation in Growth and Physiological Response to Drought Stress and Re-Watering Reveals the Critical Role of Recovery in Drought Adaptation in Maize Seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Song, J.; Zhao, N.; Ding, J. Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. PeerJ 2020, 8, e10046. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of Drought Stress on Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities in Lettuce Seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Ghotbi-Ravandi, A.A.; Shahbazi, M.; Shariati, M.; Mulo, P. Effects of Mild and Severe Drought Stress on Photosynthetic Efficiency in Tolerant and Susceptible Barley (Hordeum vulgare L.) Genotypes. J. Agron. Crop Sci. 2014, 200, 403–415. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and Photosynthesis. Subcell Biochem. 2016, 79, 111–139. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]

| Standard | Calibration Curve Equation | R2 Value |
|---|---|---|
| Caffeic acid | y = 13159.9x + 12112.7 | 0.9998 |
| Coumaric acid | y = 2551.11x + 2349.01 | 0.9999 |
| Ellagic acid | y = 33829.1x − 6862.97 | 1.0000 |
| Ferulic acid | y = 27461.5x − 90059.3 | 0.9870 |
| Naringin | y = 3941.28x − 30329.7 | 0.9914 |
| Treatments | L* | a* | b* | C* | h° |
|---|---|---|---|---|---|
| 24 h | 41.34 ± 1.45 | −14.65 ± 0.56 | 23.17 b ± 1.85 | 27.41 b ± 1.85 | 122.35 a ± 1.13 |
| 48 h | 41.77 ± 1.68 | −13.91 ± 1.00 | 22.29 b ± 2.07 | 26.28 b ± 2.26 | 121.99 a ± 0.99 |
| 96 h | 44.22 ± 0.98 | −15.62 ± 0.32 | 27.69 a ± 0.44 | 31.79 a ± 0.49 | 119.44 b ± 0.44 |
| ANOVA | NS | NS | 0.0137 | 0.0169 | 0.0148 |
| LSD | 2.8008 | 1.3804 | 3.2414 | 3.4151 | 1.8092 |
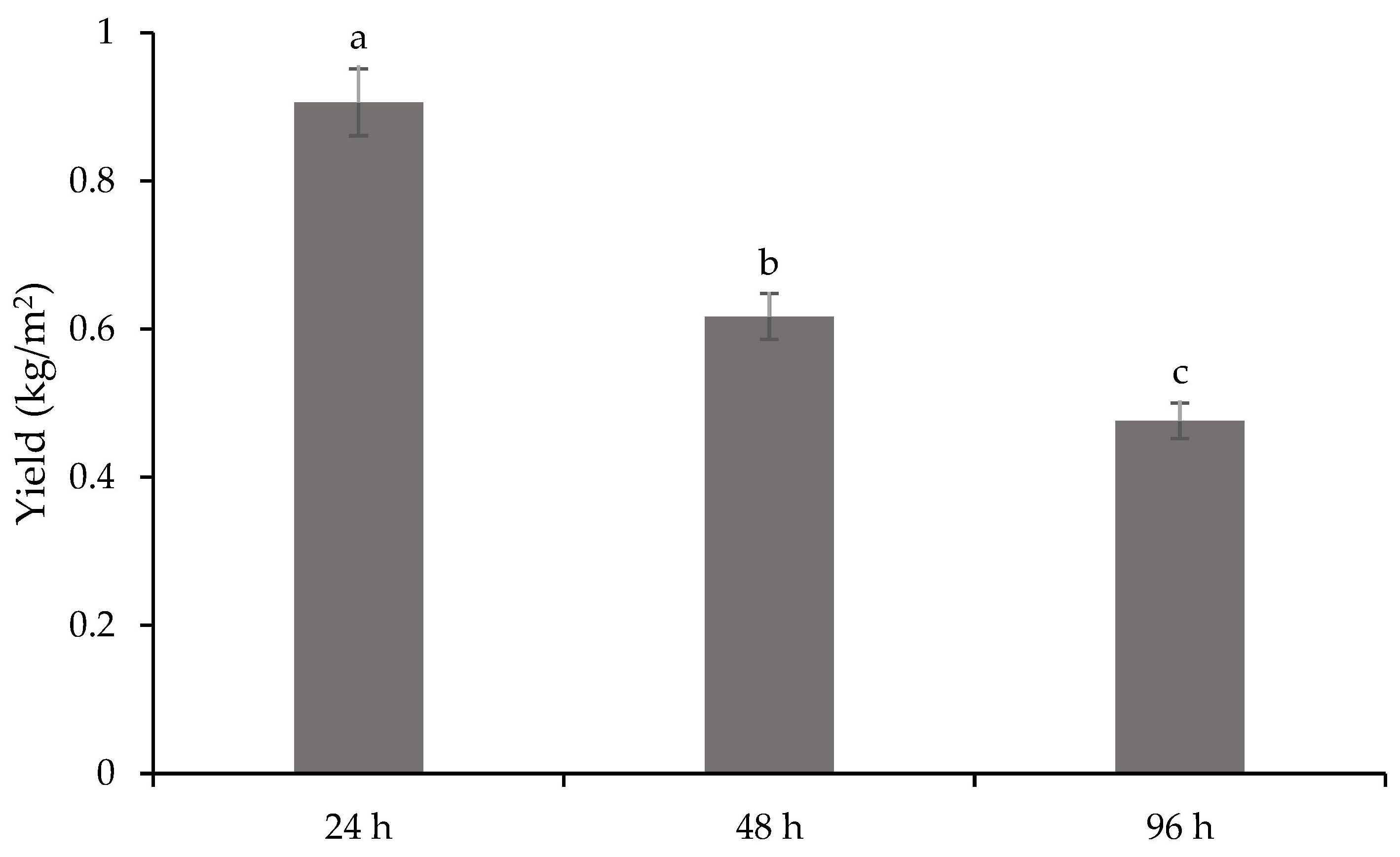
| Treatments | DM (%) | TA (%) |
|---|---|---|
| 24 h | 18.85 b ± 0.43 | 0.29 ± 0.03 |
| 48 h | 21.50 a ± 0.56 | 0.24 ± 0.01 |
| 96 h | 21.28 a ± 0.64 | 0.26 ± 0.06 |
| ANOVA | 0.0018 | NS |
| LSD | 1.0957 | 0.0769 |
| Treatments | AsA (mg/100 g fw) | TPC (mg GAE/100 g fw) | TNFC (mg GAE/100 g fw) | TFC (mg CTH/100 g fw) |
|---|---|---|---|---|
| 24 h | 73.01 c ± 2.63 | 328.06 b ± 0.51 | 166.01 c ± 0.90 | 162.05 a ± 0.40 |
| 48 h | 79.52 b ± 3.60 | 400.21 a ± 1.41 | 237.33 a ± 1.35 | 162.88 a ± 2.76 |
| 96 h | 96.80 a ± 2.48 | 308.45 c ± 0.52 | 173.61 b ± 0.56 | 134.84 b ± 0.63 |
| ANOVA | 0.0002 | ≤0.0001 | ≤0.0001 | ≤0.0001 |
| LSD | 5.8844 | 1.8354 | 1.9781 | 3.2987 |
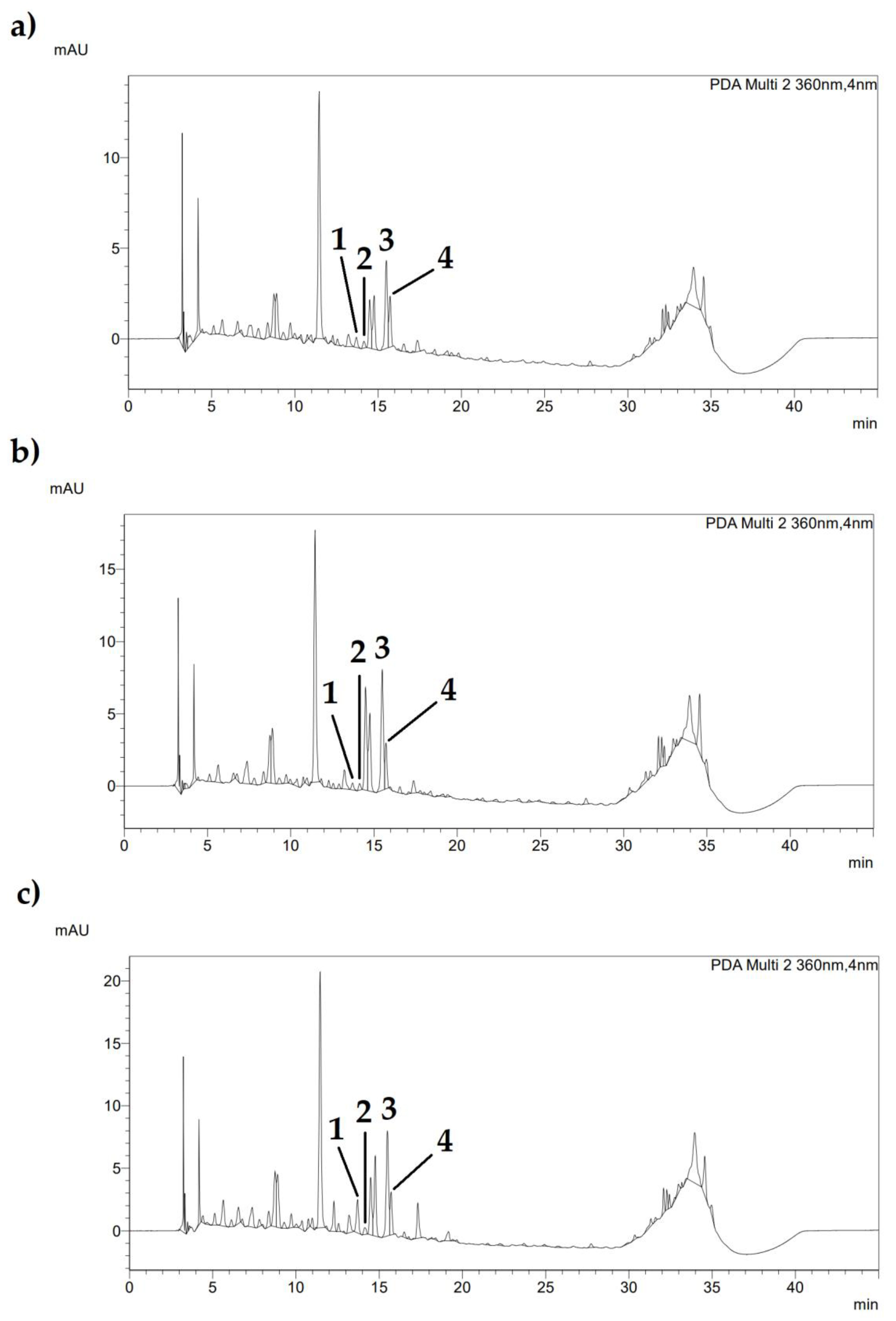
| Treatments | Caffeic Acid (mg/L) | Coumaric Acid (mg/L) | Ellagic Acid (mg/L) | Ferulic Acid (mg/L) | Naringin (mg/L) |
|---|---|---|---|---|---|
| 24 h | nd | 1.15 b ± 0.10 | 0.79 ± 0.18 | 4.19 ± 0.05 | 7.88 ± 0.01 |
| 48 h | nd | 0.61 b ± 0.32 | 1.23 ± 0.47 | 4.22 ± 0.03 | 7.89 ± 0.07 |
| 96 h | nd | 6.81 a ± 1.94 | 1.52 ± 0.22 | 4.23 ± 0.07 | 7.96 ± 0.01 |
| ANOVA | - | 0.0010 | NS | NS | NS |
| LSD | - | 2.2702 | 0.6293 | 0.102 | 0.0793 |
| Treatments | Chl_a (mg/g) | Chl_b (mg/g) | TCh (mg/g) | TCa (mg/g) |
|---|---|---|---|---|
| 24 h | 0.85 a ± 0.01 | 0.50 a ± 0.02 | 1.34 a ± 0.03 | 0.23 a ± 0.01 |
| 48 h | 0.64 c ± 0.01 | 0.26 c ± 0.01 | 0.90 c ± 0.01 | 0.23 b ± 0.01 |
| 96 h | 0.71 b ± 0.01 | 0.42 b ± 0.01 | 1.13 b ± 0.01 | 0.22 c ± 0.01 |
| ANOVA | ≤0.0001 | ≤0.0001 | ≤0.0001 | 0.0004 |
| LSD | 0.0143 | 0.0242 | 0.0387 | 0.0036 |
| Treatments | ABTS (µmol TE/L) | FRAP (µmol TE/L) |
|---|---|---|
| 24 h | 2435.07 a ± 4.23 | 3114.24 c ± 51.31 |
| 48 h | 2444.83 a ± 4.57 | 3773.49 a ± 39.22 |
| 96 h | 2410.72 b ± 12.78 | 3220.97 b ± 40.84 |
| ANOVA | 0.0058 | ≤0.0001 |
| LSD | 16.4 | 88.134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dujmović, M.; Opačić, N.; Radman, S.; Fabek Uher, S.; Voća, S.; Šic Žlabur, J. Accumulation of Stinging Nettle Bioactive Compounds as a Response to Controlled Drought Stress. Agriculture 2023, 13, 1358. https://doi.org/10.3390/agriculture13071358
Dujmović M, Opačić N, Radman S, Fabek Uher S, Voća S, Šic Žlabur J. Accumulation of Stinging Nettle Bioactive Compounds as a Response to Controlled Drought Stress. Agriculture. 2023; 13(7):1358. https://doi.org/10.3390/agriculture13071358
Chicago/Turabian StyleDujmović, Mia, Nevena Opačić, Sanja Radman, Sanja Fabek Uher, Sandra Voća, and Jana Šic Žlabur. 2023. "Accumulation of Stinging Nettle Bioactive Compounds as a Response to Controlled Drought Stress" Agriculture 13, no. 7: 1358. https://doi.org/10.3390/agriculture13071358
APA StyleDujmović, M., Opačić, N., Radman, S., Fabek Uher, S., Voća, S., & Šic Žlabur, J. (2023). Accumulation of Stinging Nettle Bioactive Compounds as a Response to Controlled Drought Stress. Agriculture, 13(7), 1358. https://doi.org/10.3390/agriculture13071358













