Nematicidal and Insecticidal Activity of Proteases from Carica papaya and Ananas comosus
Abstract
1. Introduction
2. Material and Methods
2.1. Obtaining Pineapple Aqueous Extracts
2.2. Collecting and Processing of Papaya Latex
2.3. Enzymatic Activity
2.4. Nematicidal Activity
2.5. Insecticidal Activity
2.5.1. Immersion Test with Larvae and Pupae
2.5.2. Preparation of an Artificial Diet for Tenebrio molitor Larvae
2.5.3. Food Deterrence Index and Nutritional Indices in Larvae
2.6. Statistical Analysis
3. Results and Discussion
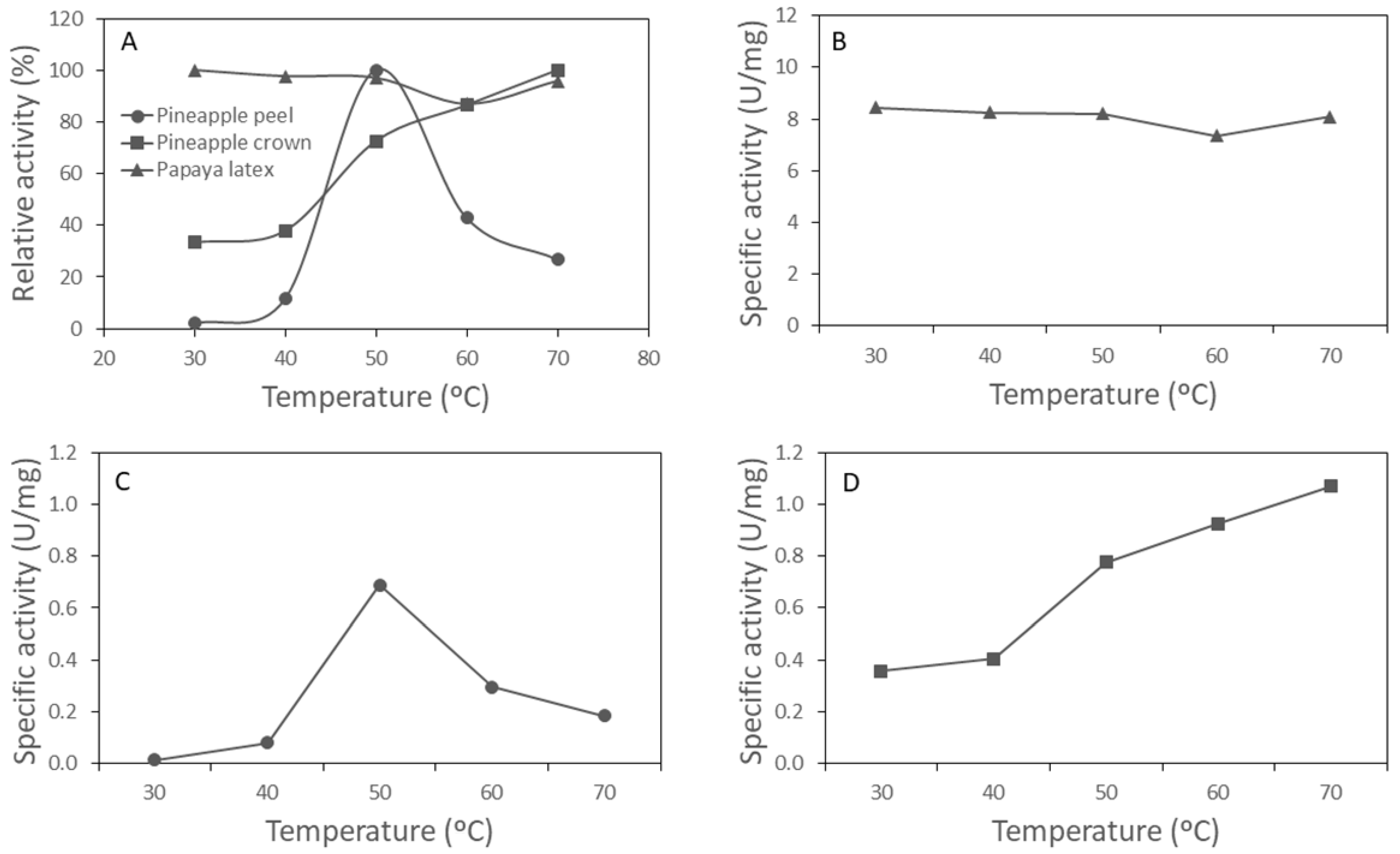
3.1. Protease Activity
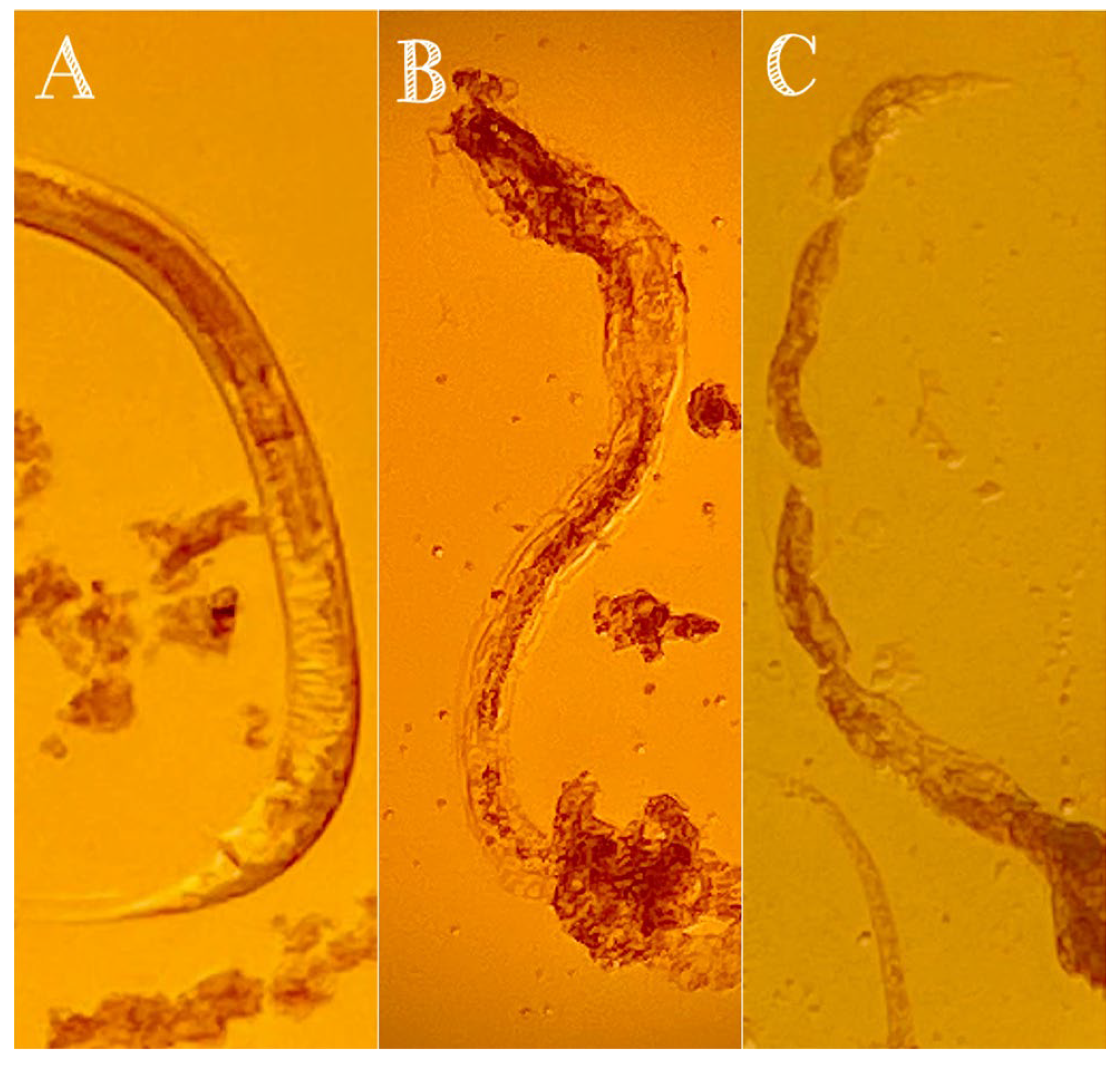
3.2. Nematicidal Activity
3.3. Insecticidal Activity
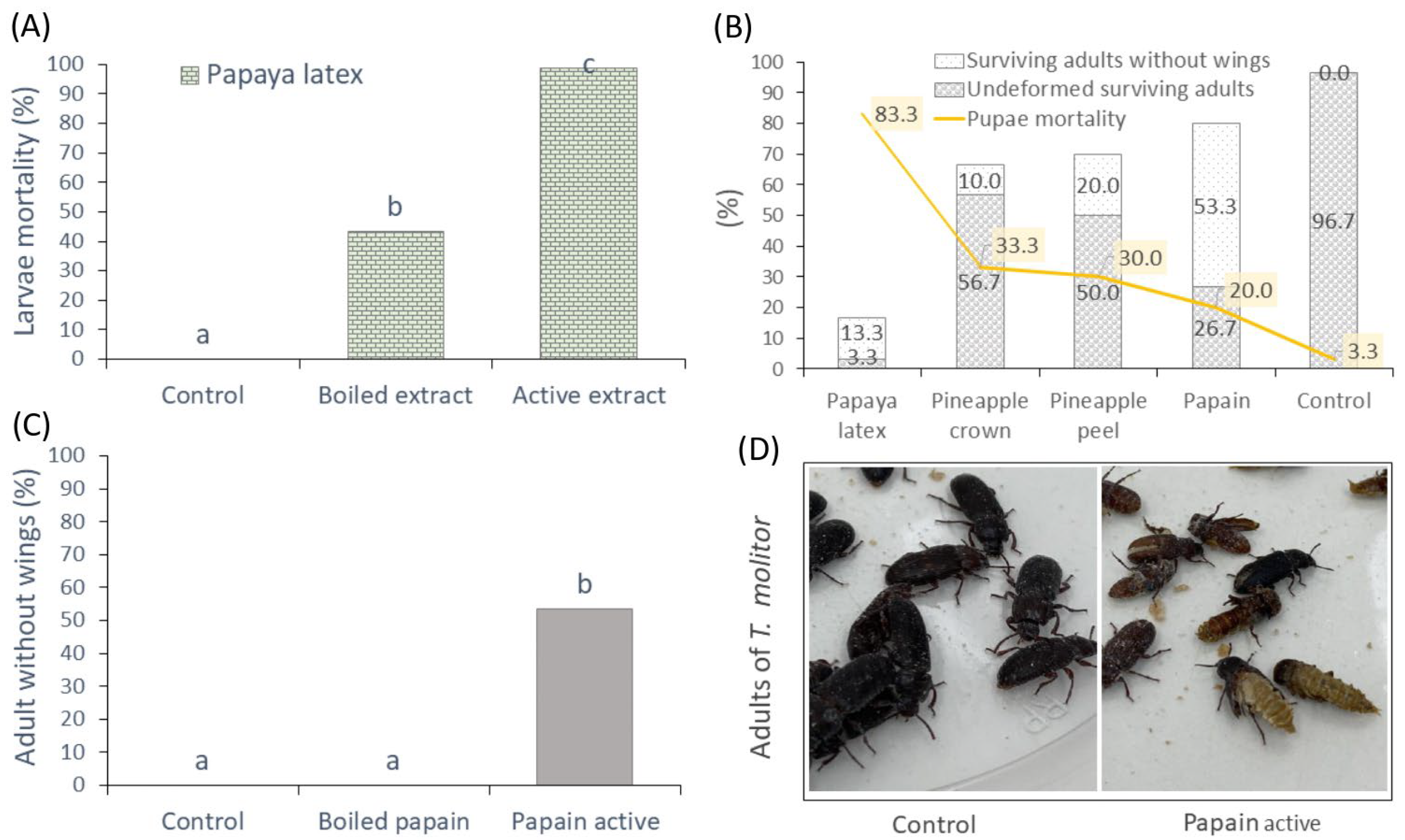
3.3.1. Mortality of Larvae and Pupae of Tenebrio molitor
3.3.2. Food Deterrence Index (ID) and Nutritional Parameters in Larvae of T. molitor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finegold, C.; Ried, J.; Denby, K.; Gurr, S. Global burden of crop loss. Gates Open Res. 2019, 3. [Google Scholar] [CrossRef]
- Kashyap, B.; Kumar, R. Sensing methodologies in agriculture for monitoring biotic stress in plants due to pathogens and pests. Inventions 2021, 6, 29. [Google Scholar] [CrossRef]
- Askary, T.H. Limitations, research needs and future prospects in the biological control of phytonematodes. In Biocontrol Agents of Phytonematodes; Askary, T.H., Martinelli, P.R.P., Eds.; CAB International: Wallingford, UK, 2015; pp. 446–454. [Google Scholar]
- Oliveira, C.M.; Auad, A.M.; Mendes, S.M.; Frizzas, M.R. Crop losses and the economic impact of insect pests on Brazilian agriculture. Crop Prot. 2014, 56, 50–54. [Google Scholar] [CrossRef]
- Smith, D.; Ryan, M.J.; Luke, B.; Djeddour, D.; Seier, M.K.; Varia, S.; Pollard, K.M.; Pratt, C.F.; Kurose, D.; Shaw, R.H. CABI UK and Nagoya Protocol Triggered Benefit Sharing; CABI: Wallingford, UK, 2021. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L.; Syed, J.H.; Cheng, Z.; Li, J.; Zhang, G.; Jones, K.C. Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: A comprehensive review of India. Sci. Total Environ. 2015, 511, 123–137. [Google Scholar] [CrossRef]
- Askary, T.H.; Rana, A.; Mehraj, S.; Khanum, T.A.; Jan, U.; Rafiq, S.; War, W.A.; Akhil, N. Prospects of biopesticides in pest management. In Pest Management: Methods Applications and Challenges; Askary, T.H., Ed.; Nova Science Publishers: New York, NY, USA, 2022; pp. 149–172. [Google Scholar]
- Henry, M.; Beguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, C.; Barot, S.; Capowiez, Y.; Hedde, M.; Vandenbulcke, F. Pesticides and earthworms. A review. Agron. Sustain. Dev. 2014, 34, 199–228. [Google Scholar] [CrossRef]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, X.; Li, J.A. 1961–2010 record of fertilizer use, pesticide application and cereal yields: A review. Agron Sustain Dev. 2015, 35, 83–93. [Google Scholar] [CrossRef]
- Mfarrej, M.F.B.; Rara, F.M. Competitive, sustainable natural pesticides. Acta Ecol. Sin. 2019, 39, 145–151. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G.M. Challenge of utilization vegetal extracts as natural plant protection products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Ogunnupebi, T.A.; Oluyori, A.P.; Dada, A.O.; Oladeji, O.S.; Inyinbor, A.A.; Egharevba, G.O. Promising natural products in crop protection and food preservation: Basis, advances, and future prospects. Int. J. Agron. 2020, 2020, 8840046. [Google Scholar] [CrossRef]
- Sebastián, D.I.; Guevara, M.G.; Rocío, T.F.; Virginia, T.C. An overview of plant Proteolytic enzymes. In Biotechnological Applications of Plant Proteolytic Enzymes; Springer: Cham, Switzerland, 2018; pp. 1–19. [Google Scholar] [CrossRef]
- Fernández-Lucas, J.; Castañeda, D.; Hormigo, D. New trends for a classical enzyme: Papain, a biotechnological success story in the food industry. Trends Food Sci. Technol. 2017, 68, 91–101. [Google Scholar] [CrossRef]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a potential bioactive compound: A comprehensive overview from a pharmacological perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef]
- Hagstrum, D. Atlas of Stored-Product Insects and Mites; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth performance and nutrient composition of mealworms (Tenebrio molitor) fed on fresh plant materials-supplemented diets. Foods 2020, 9, 151. [Google Scholar] [CrossRef]
- Silva, L.B.; de Souza, R.G.; da Silva, S.R.; Feitosa, A.D.C.; Lopes, E.C.; Lima, S.B.P.; Pavan, B.E. Development of Tenebrio molitor (Coleoptera: Tenebrionidae) on poultry litter-based diets: Effect on chemical composition of larvae. J. Insect Sci. 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Domingues, L.F.; Giglioti, R.; Feitosa, K.A.; Fantatto, R.R.; Rabelo, M.D.; de Sena Oliveira, M.C.; Bechara, G.H.; de Souza Chagas, A.C. In vitro activity of pineapple extracts (Ananas comosus, Bromeliaceae) on Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Exp. Parasitol. 2013, 134, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Domingues, L.F.; Giglioti, R.; Feitosa, K.A.; Fantatto, R.R.; Rabelo, M.D.; de Sena Oliveira, M.C.; Bechara, G.H.; de Oliveira, G.P.; Junior, W.B.; de Souza Chagas, A.C. In vitro and in vivo evaluation of the activity of pineapple (Ananas comosus) on Haemonchus contortus in Santa Inês sheep. Vet. Parasitol. 2013, 197, 263–270. [Google Scholar] [CrossRef]
- Kovendan, K.; Murugan, K.; Naresh Kumar, A.; Vincent, S.; Hwang, J.S. Bioefficacy of larvicdial and pupicidal properties of Carica papaya (Caricaceae) leaf extract and bacterial insecticide, spinosad, against chikungunya vector, Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2012, 110, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Peachey, L.E.; Pinchbeck, G.L.; Matthews, J.B.; Burden, F.A.; Behnke, J.M.; Hodgkinson, J.E. Papaya latex supernatant has a potent effect on the free-living stages of equid cyathostomins in vitro. Vet. Parasitol. 2016, 228, 23–29. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Seetharaman, P.; Krishnan, M.; Gnanasekar, S.; Sivaperumal, S. Carica papaya (Papaya) latex: A new paradigm to combat against dengue and filariasis vectors Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). 3 Biotech 2018, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.H.; Soares, F.E.F.; Souza, D.C.; Lima, L.T.; Sufiate, B.L.; Ferreira, T.F.; Queiroz, J.H. Role of Synadenium grantii latex proteases in nematicidal activity on Meloidogyne incognita and Panagrellus redivivus. Braz. J. Biol. 2018, 79, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Bioch. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sufiate, B.L.; Soares, F.E.F.; Roberti, Á.S.; Queiroz, J.H. Nematicidal activity of proteases from Euphorbia milii. Biocatal. Agric. Biotechnol. 2017, 10, 239–241. [Google Scholar] [CrossRef]
- Soares, F.E.F.; Braga, F.R.; Araújo, J.V.; Genier, H.L.; Gouveia, A.S.; Queiroz, J.H. Nematicidal activity of three novel extracellular proteases of the nematophagous fungus Monacrosporium sinense. Parasitol. Res. 2013, 112, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Rankic, I.; Zelinka, R.; Ridoskova, A.; Gagic, M.; Pelcova, P.; Huska, D. Nano/microparticles in conjunction with microalgae extractas novel insecticides against Mealworm beetles, Tenebrio molitor. Sci. Rep. 2021, 11, 17125. [Google Scholar] [CrossRef]
- Lima, J.K.A.; Chicuta, C.P.D.L.; de Macedo Costa, M.; da Costa, M.L.A.; Grillo, L.A.M.; dos Santos, A.F.; Gomes, F.S. Biotoxicity of aqueous extract of Genipa americana L. bark on red flour beetle Tribolium castaneum (Herbst). Ind. Crops Prod. 2020, 156, 112874. [Google Scholar] [CrossRef]
- Napoleão, T.H.; do Rego Belmonte, B.; Pontual, E.V.; de Albuquerque, L.P.; Sá, R.A.; Paiva, L.M.; Coelho, L.C.B.B.; Paiva, P.M.G. Deleterious effects of Myracrodruon urundeuva leaf extract and lectin on the maize weevil, Sitophilus zeamais (Coleoptera, Curculionidae). J. Stored Prod. Res. 2013, 54, 26–33. [Google Scholar] [CrossRef]
- Brito, A.M.; Oliveira, V.; Icimoto, M.Y.; Nantes-Cardoso, I.L. Collagenase activity of bromelain immobilized at gold nanoparticle interfaces for therapeutic applications. Pharmaceutics 2021, 13, 1143. [Google Scholar] [CrossRef]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef]
- Azarkan, M.; El Moussaoui, A.; Van Wuytswinkel, D.; Dehon, G.; Looze, Y. Fractionation and purification of the enzymes stored in the latex of Carica papaya. J. Chromatogr. B 2003, 790, 229–238. [Google Scholar] [CrossRef]
- Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Laticifers, latex, and their role in plant defense. Trends Plant Sci. 2019, 24, 553–567. [Google Scholar] [CrossRef]
- Nakhate, Y.D.; Talekar, K.S.; Giri, S.V.; Vasekar, R.D.; Mankar, H.C.; Tiwari, P.R. Pharmacological and chemical composition of Carica papaya: On overview. World J. Pharm. Res. 2019, 8, 811–821. [Google Scholar]
- Page, A.P.; Stepek, G.; Winter, A.D.; Pertab, D. Enzymology of the nematode cuticle: A potential drug target? Int. J. Parasitol. Drugs Drug. Resist. 2014, 4, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Njom, V.S.; Winks, T.; Diallo, O.; Lowe, A.; Behnke, J.; Dickman, M.J.; Duce, I.; Johnstone, I.; Buttle, D.J. The effects of plant cysteine proteinases on the nematode cuticle. Parasites Vectors 2021, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Herbert-Doctor, L.A.; Saavedra-Aguilar, M.; Villarreal, M.L.; Cardoso-Taketa, A.; Vite-Vallejo, O. Insecticidal and Nematicidal Effects of Agave tequilana Juice against Bemisia tabaci and Panagrellus redivivus. Southwest. Entomol. 2016, 41, 27–40. [Google Scholar] [CrossRef]
- Soares, F.E.F.; Nakajima, V.M.; Sufiate, B.L.; Satiro, L.A.S.; Gomes, E.H.; Fróes, F.V.; Sena, F.P.; Braga, F.R.; Queiroz, J.H. Proteolytic and nematicidal potential of the compost colonized by Hypsizygus marmoreus. Exp. Parasitol. 2019, 197, 16–19. [Google Scholar] [CrossRef]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Development and ultrastructure of the rigid dorsal and flexible ventral cuticles of the elytron of the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2017, 91, 21–33. [Google Scholar] [CrossRef]
- Hackman, R.H. Cuticle: Biochemistry. In Biology of the Integument; Springer: Berlin/Heidelberg, Germany, 1984; pp. 583–610. [Google Scholar] [CrossRef]
- Gołębiowski, M.; Maliński, E.; Boguś, M.I.; Kumirska, J.; Stepnowski, P. The cuticular fatty acids of Calliphora vicina, Dendrolimus pini and Galleria mellonella larvae and their role in resistance to fungal infection. Insect Biochem. Mol. Biol. 2008, 38, 619–627. [Google Scholar] [CrossRef]
- Andersen, S.O. Characteristic properties of proteins from pre-ecdysial cuticle of larvae and pupae of the mealworm Tenebrio molitor. Insect Biochem. Mol. Biol. 2022, 32, 1077–1087. [Google Scholar] [CrossRef]
- Liu, Z.L.; Goh, S.H.; Ho, S.H. Screening of Chinese medicinal herbs for bioactivity against Sitophilus zeamais Motschulsky and Tribolium castaneum (Herbst). J. Stored Prod. Res. 2007, 43, 290–296. [Google Scholar] [CrossRef]
- Oliveira, A.P.S.; Agra-Neto, A.C.; Pontual, E.V.; de Albuquerque Lima, T.; Cruz, K.C.V.; de Melo, K.R.; de Oliveira, A.S.; Coelho, L.C.B.B.; Ferreira, M.R.A.; Soares, L.A.L.; et al. Evaluation of the insecticidal activity of Moringa oleifera seed extract and lectin (WSMoL) against Sitophilus zeamais. J. Stored Prod. Res. 2020, 87, 101615. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, H.L.B.; Alves, J.C.d.S.; Gladenucci, J.; Marucci, R.C.; Soares, F.E.d.F. Nematicidal and Insecticidal Activity of Proteases from Carica papaya and Ananas comosus. Agriculture 2023, 13, 1119. https://doi.org/10.3390/agriculture13061119
Castro HLB, Alves JCdS, Gladenucci J, Marucci RC, Soares FEdF. Nematicidal and Insecticidal Activity of Proteases from Carica papaya and Ananas comosus. Agriculture. 2023; 13(6):1119. https://doi.org/10.3390/agriculture13061119
Chicago/Turabian StyleCastro, Henry Leonel Bueso, Jhennifer Cristina de Souza Alves, Joanina Gladenucci, Rosangela Cristina Marucci, and Filippe Elias de Freitas Soares. 2023. "Nematicidal and Insecticidal Activity of Proteases from Carica papaya and Ananas comosus" Agriculture 13, no. 6: 1119. https://doi.org/10.3390/agriculture13061119
APA StyleCastro, H. L. B., Alves, J. C. d. S., Gladenucci, J., Marucci, R. C., & Soares, F. E. d. F. (2023). Nematicidal and Insecticidal Activity of Proteases from Carica papaya and Ananas comosus. Agriculture, 13(6), 1119. https://doi.org/10.3390/agriculture13061119








