Ozonation of Cowpea Grains: Alternative for the Control of Callosobruchus maculatus and Maintenance of Grain Quality
Abstract
1. Introduction
2. Materials and Methods
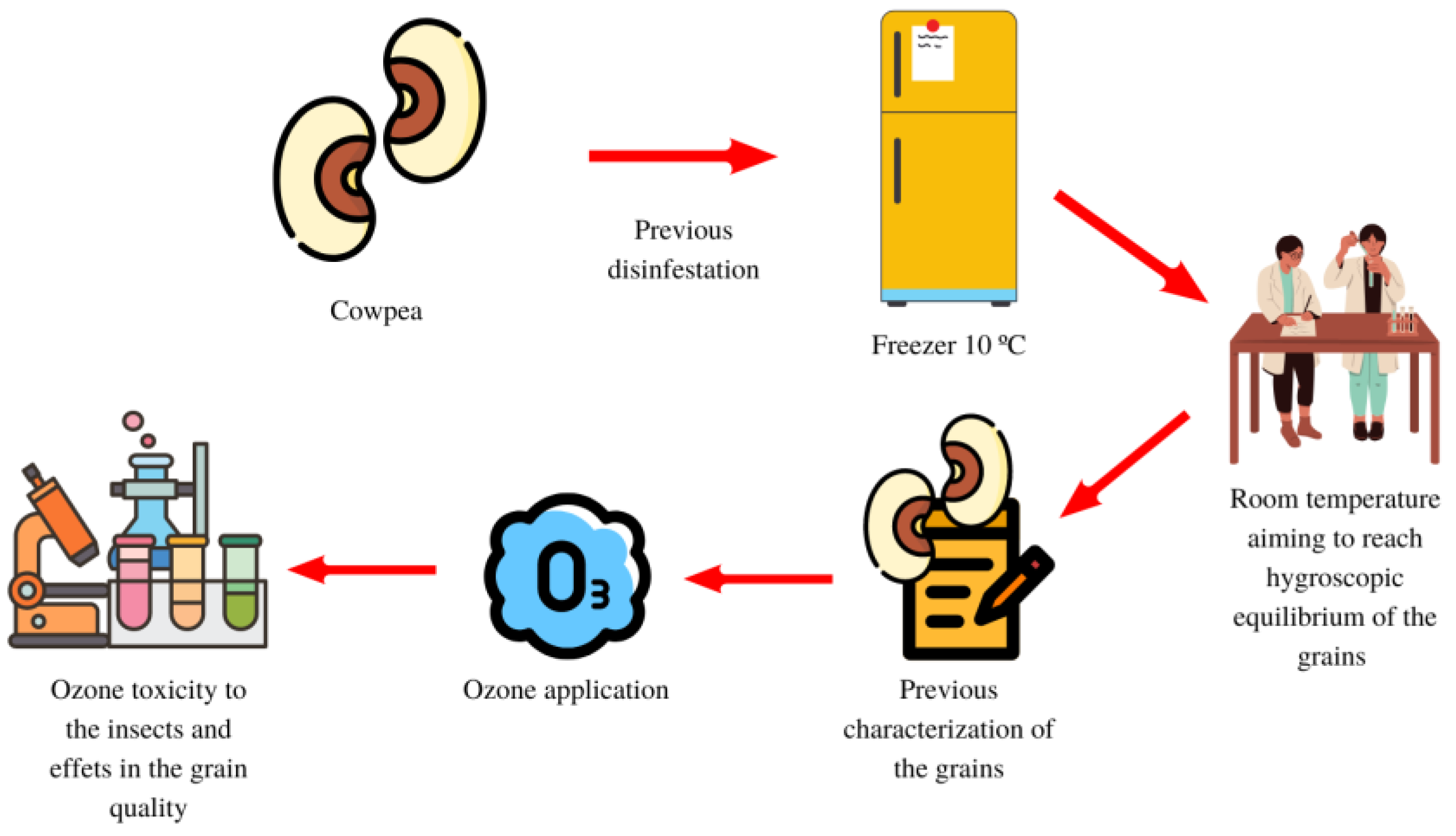
2.1. Samples and Experimental Design
2.2. Rearing of Callosobruchus maculatus
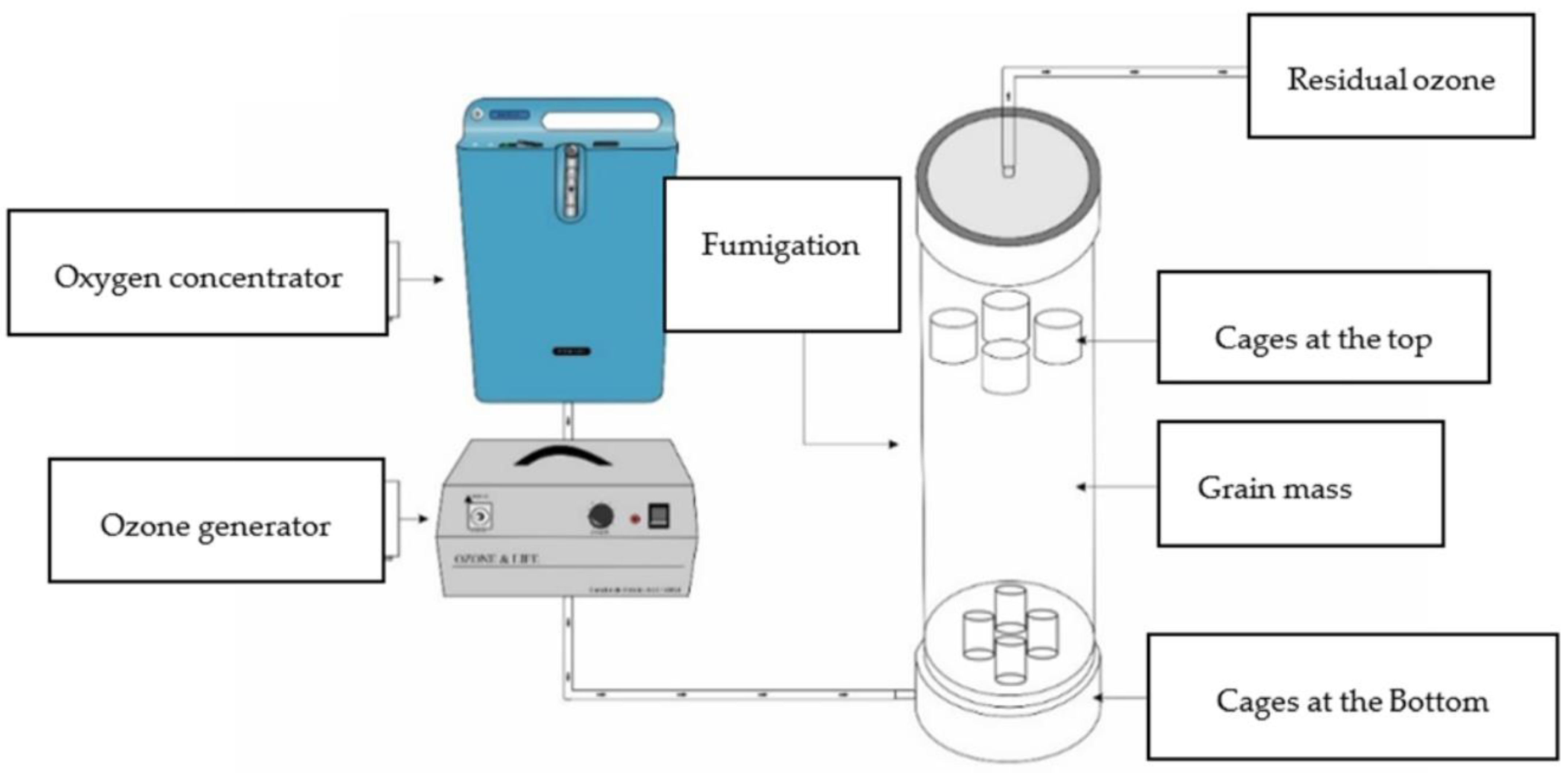
2.3. Generation of Ozone Gas and Application System
2.4. Fumigation Chamber
2.5. Ozone Toxicity for C. maculatus
2.6. Quality Evaluation of Cowpea Grains
2.7. Characterization of Cowpea before Treatment with Ozone and Oxygen
2.8. Water Content and Germination
2.8.1. Water Content
2.8.2. Germination
2.9. Electrical Conductivity
2.10. Experimental Design and Statistical Analysis
3. Results
3.1. Ozone Gas Toxicity
3.2. Quality Evaluation of Cowpea Grains
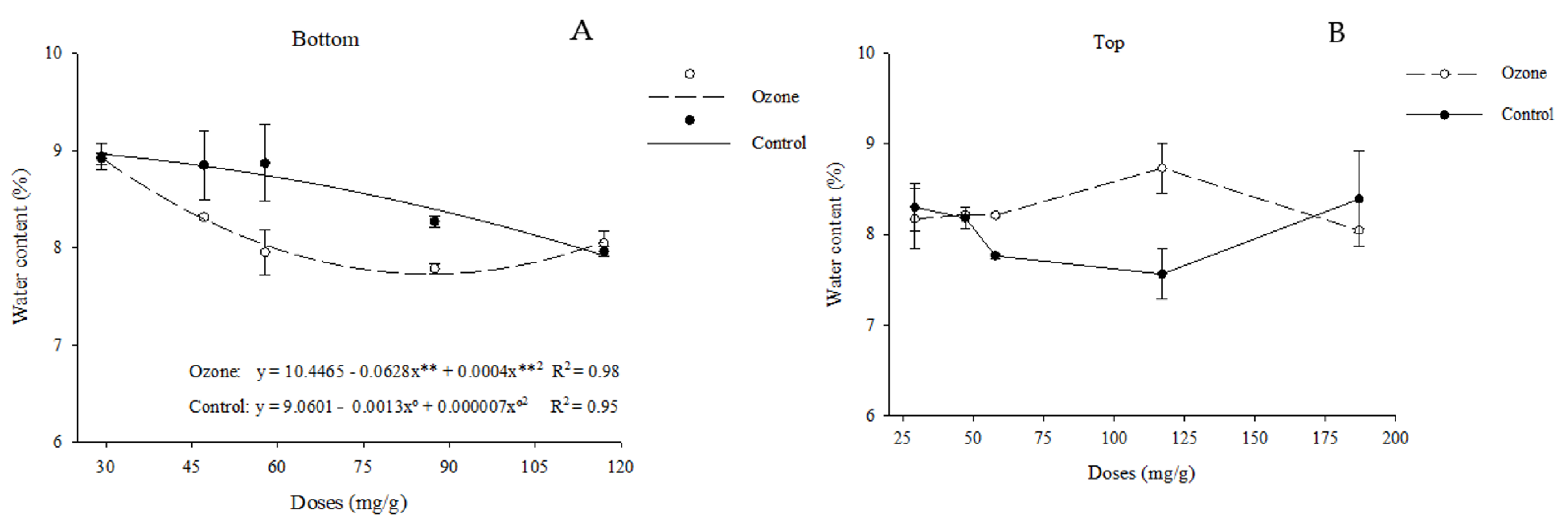
3.2.1. Water Content
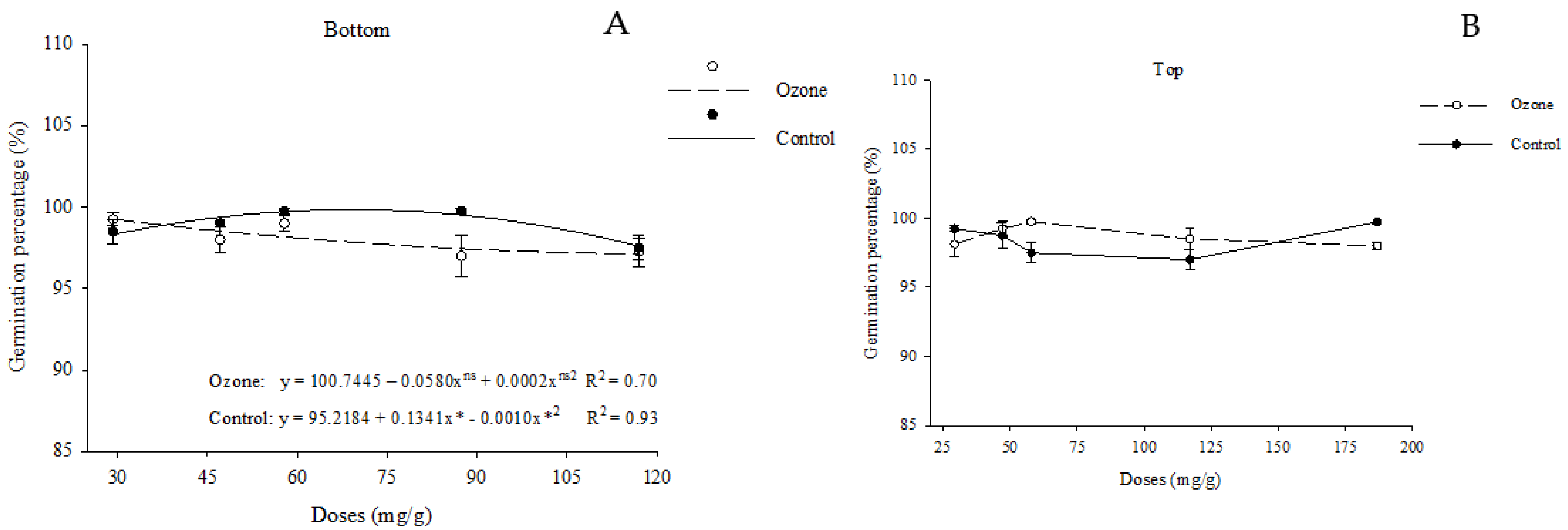
3.2.2. Germination
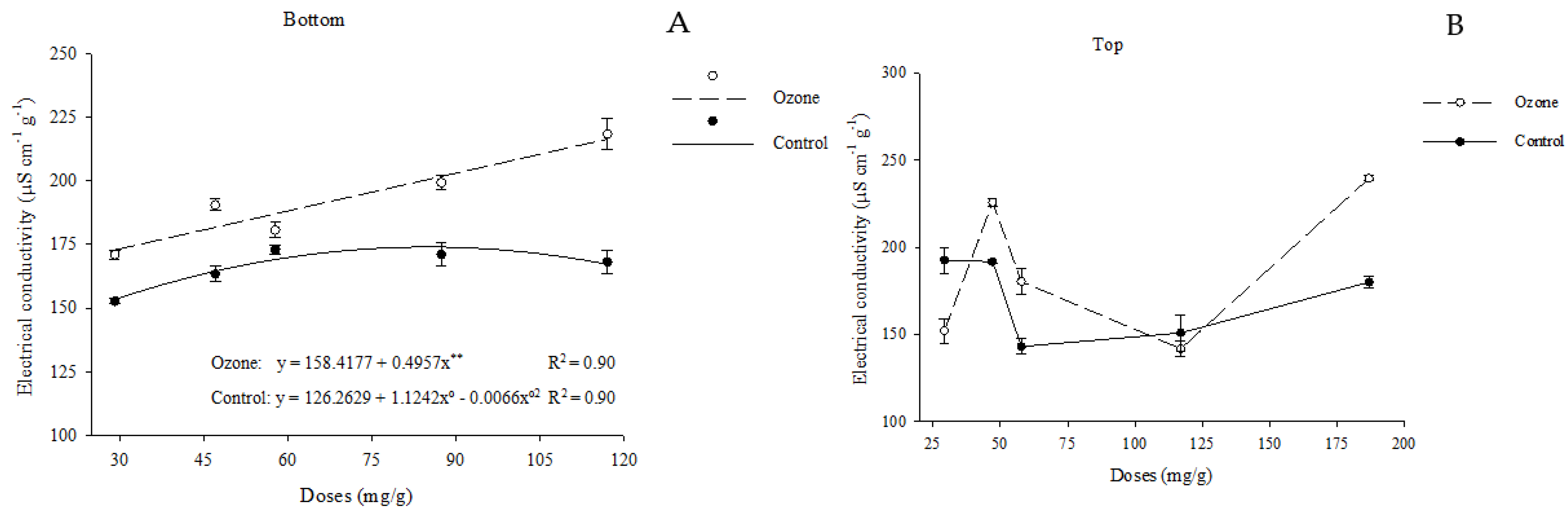
3.2.3. Electrical Conductivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, G.C.; Magalhães, R.C.; Sobreira, A.C.; Schmitz, R.; Silva, L.C. Rendimento de grãos secos e componentes de produção de genótipos de feijão-caupi em cultivo irrigado e de sequeiro. Rev. Agro Mbiente Line 2016, 4, 342–350. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. Crops. Cow Peas, Dry. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 6 June 2021).
- Lopes, L.M.; Sousa, A.H.; Santos, V.B.; Silva, G.N.; Abreu, A.O. Development rates of Callosobruchus maculatus (Coleoptera: Chrysomelidae) in landrace cowpea varieties occurring in southwestern Amazonia. J. Stored Prod. Res. 2018, 76, 111–115. [Google Scholar] [CrossRef]
- Matos, L.F.; da Cruz Lima, E.; de Andrade Dutra, K.; Navarro, D.M.D.A.F.; Alves, J.L.R.; Silva, G.N. Chemical composition and insecticidal effect of essential oils from Illicium verum and Eugenia caryophyllus on Callosobruchus maculatus in cowpea. Ind. Crops Prod. 2020, 45, 112088. [Google Scholar] [CrossRef]
- Brito, R.C.; Fontes, L.S.; Silva, P.H.S.; Santana, C.S.; Barbosa, D.R.S. Essential oils from Betula lenta, Cinnamomum cassia, Citrus aurantium var. Amara and Acorus calamus as biopesticides against cowpea weevil. Int. J. Trop. Insect Sci. 2021, 42, 261–268. [Google Scholar] [CrossRef]
- Barbosa, D.R.S.; Oliveira, J.V.; Silva, P.H.S.; Santana, M.F.; Breda, M.O.; França, S.M.; De Miranda, V.L. Lethal and sublethal effects of chemical constituents from essential oils on Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae: Bruchinae) in cowpea stored grains. J. Plant Dis. Prot. 2021, 128, 1575–1586. [Google Scholar] [CrossRef]
- Cheng, W.; Lei, J.; Ahn, J.E.; Wnag, Y.; Lei, C.; Zhu-Salzman, K. CO2 enhances effects of hypoxia on mortality, development, and gene expression. In cowpea bruchid, Callosobruchus maculatus. J. Stored Prod. Res. 2013, 59, 1160–1168. [Google Scholar] [CrossRef]
- Vales, M.I.; Rao, G.V.R.; Sudini, H.; Patil, S.B.; Murdock, L.L. Effective and economic storage of pigeonpea seed in triple layer plactic bags. J. Stored Prod. Res. 2014, 58, 29–38. [Google Scholar] [CrossRef]
- Pimentel, M.A.G.; Faroni, L.R.D.; Guedes, R.N.C.; Sousa, A.H.; Tótola, M.R. Phospine resistance in Brazilian populations of Sitophilus zeamais Motschusky (Coleoptera: Curculionidae). J. Stored Prod. Res 2009, 45, 71–74. [Google Scholar] [CrossRef]
- Alves, M.S. Composição Química e Atividade Biológica dos óleos Essenciais de Cymbopogon citratus (DC) Stapf. E Cymbopogon nardus (L) Rendle, Sobre o Ciclo Reprodutivo, Enzimas de Resistência e a Composição lipídica do Callosobruchus maculatus (Fabricius, 1775) (Coleoptera: Bruchidae.), Inseto-Praga do Feijão Vigna unguiculata (L) Walp. Master’s Thesis, Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Lorini, I. Controle Integrado de Pragas de Grãos Armazenados, Embrapa Trigo-Documentos (INFOTECA-E); Embrapa: Brasilia, Brazil, 1998; p. 34. [Google Scholar]
- Ahmed, N.; Darshanee, H.L.C.; Khan, I.A.; Zhang, Z.F.; Liu, T.X. Host selection behavior of the green peach aphid, Myzus persicae, in response to volatile organic compounds and nitrogen contents of cabbage cultivars. Front. Plant Sci. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Nickhil, C.; Mohapatra, D.; Kar, A.; Giri, S.K.; Verma, U.S.; Sharma, Y.; Singh, K.K. Delineating the effect of gaseous ozone on disinfestation efficacy, protein quality, dehulling efficiency, cooking time and surface morphology of chickpea grains during storage. J. Stored Prod. Res. 2021, 93, 101823. [Google Scholar]
- Sousa, A.H.; Faroni, L.R.A.; Silva, G.N.; Guedes, R.N.C. Ozone Toxicity and Walking Response of Populations of Sitophilus zeamais (Coleoptera: Curculionidae). J. Econ. Entomol. 2012, 105, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Rozado, A.F.; Faroni, L.R.A.; Uruchi, W.M.I.; Guedes, R.N.C.; Paes, J.L. Ozone Aplicação de ozônio contra Sitophilus zeamais e Tribolium castaneum em milho armazenamento. Agriambi 2008, 12, 282–285. [Google Scholar]
- Horvitz, S.; Cantalejo, M.J. Application of ozone for the postharvest tretment of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2014, 54, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Xinyi, E.; Li, B.; Subramanyam, B. Efficacy of ozone against adults and immature stages of phosphine susceptible and resistant strains of Rhyzopertha dominica. J. Stored Prod. Res. 2019, 83, 110–116. [Google Scholar]
- Gad, H.A.; Sileem, T.M.; Hassan, R.S.; Abdelgaleil, S.A.M. Toxicity of gaseous ozone to the different life stages of cowpea beetle, Callosobruchus maculatus (Coleoptera: Bruchidae), under laboratory conditions. Hell. Plant Prot. J. 2021, 14, 31–38. [Google Scholar] [CrossRef]
- Ghazawy, N.A.; Zinhoum, R.A.; Ali, M.M.; Afify, A.; Hussain, H.B. Efficacy of ozone on mortality, super oxide dismutases, nitric oxide enzymes and ultrastructural alterations on some stored product insect larvae in Egypt. Afr. Entomol. 2021, 29, 17–31. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Subhashini, S.; Banuu Priya, E.P.; Kothakota, A.; Ramesh, S.V.; Shahir, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
- Seyedabadi, E.; Aran, M.; Moghaddam, R.M. Application of Ozone against the larvae of Plodia interpunctella (Hübner) and its Impacts on the organoleptic properties of walnuts. J. Food Prot. 2021, 84, 147–151. [Google Scholar] [CrossRef]
- Silva, Y.N.M.; Silva, P.R.R.; de França, S.M.; Silva, Y.D.M.; Brito, R.D.C.; Barbosa, D.R.E.S.; Silva, G.N. Ozonation of Lima Bean (Phaseolus Lunatus L.): Control of Zabrotes Subfasciatus (Boheman, 1833) (Coleoptera: Chrysomelidae: Bruchinae) and Maintenance of Grain Quality. Ozone Sci. Eng. 2022, 44, 351–362. [Google Scholar] [CrossRef]
- Brasil. Regras Para Análise de Sementes; Ministério da Agricultura e Reforma Agrária: Brasília, Brazil, 2009; pp. 21–398. [Google Scholar]
- Abreu, A.O.; Faroni, L.R.D.A.; de Assis Silva, M.V.; de Sousa, A.H.; de Alencar, E.R.; Silva, G.N. Ozone as an alternative fumigant for controlling Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae) in cowpea beans. J. Stored Prod. Res. 2022, 97, 101969. [Google Scholar] [CrossRef]
- Rakness, K.; Gordon, G.; Langlais, B.; Masschelein, W.; Matsumoto, N.; Richard, Y.; Robson, M.; Somyia, I. Guideline for measurement of ozone concentration in the process gas from an ozone generator. Ozone Sci. Eng. 1996, 18, 209–229. [Google Scholar] [CrossRef]
- Silva, G.N.; Cecom, P.R.; Faroni, L.R.A.; Sousa, A.H. Ozone to control Rhyzopertha dominica (Coleoptera: Bostrichidae) in stored wheat grains. J. Stored Prod. Postharvest Res. 2016, 7, 37–44. [Google Scholar]
- Van Leeuwen, J. Proposed OS&E requirement: Measuring ozone dosage. Ozone Sci. Eng. 2015, 37, 191–192. [Google Scholar]
- ASAE—American Society of Agricultural Engineers. Moisture Measurement—Unground Grain and Seeds; ASAE: St. Joseph, MO, USA, 2004. [Google Scholar]
- Vieira, R.D.; Carvalho, N.M. Testes de Vigor em Sementes; Funep/Unesp: Jaboticabal, Brazil, 1994; pp. 103–132. [Google Scholar]
- SAS Institute. User’s Guide, Version 8.02, TS Level 2MO.; SAS Institute Inc.: Cary, NC, USA, 2001. [Google Scholar]
- SPSS Inc. Sigma Plot User’s Guide Version 7.0 (Revised Edition); SPSS Inc.: Chicago, IL, USA, 2001. [Google Scholar]
- Işikber, A.A.; Öztekin, S. Comparison of susceptibility of two stored-product insects, Ephestia kuehniella Zeller and Tribolium confusum du Val to gaseous ozone. J. Stored Prod. Res. 2009, 45, 159–164. [Google Scholar] [CrossRef]
- Kells, S.A.; Mason, L.J.; Maier, D.E.; Woloshuck, C.P. Efficacy and fumigation characteristics of ozone in stored maize. J. Stored Prod. Res. 2001, 37, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Hardin, J.A.; Jones, C.L.; Bonjour, E.L.; Noyes, R.T.; Beeby, R.L.; Eltiste, D.A.; Decker, S. Ozone fumigation of stored grain; closed-loop recirculation and the rate of ozone consumption. J. Stored Prod. Res. 2010, 467, 149–154. [Google Scholar] [CrossRef]
- Kim, J.G.; Yousef, A.E.; Dave, S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Stored Prod. Res. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Mendez, F.; Maier, D.E.; Mason, L.J.; Woloshuck, C.P. Penetration of ozone into columns of stored grains and effects on chemical composition and processing performance. J. Stored Prod. Res. 2003, 39, 33–44. [Google Scholar] [CrossRef]
- Isikber, A.A.; Athanassiou, C.G. The use of ozone gas for the control of insects and micro-organisms in stored products. J. Stored Prod. Res. 2015, 64, 139–145. [Google Scholar] [CrossRef]
- Bechlin, T.R.; Granella, S.J.; Christ, D.; Coelho, S.R.M.; Viecelli, C.A. Evaluation of grain and oil quality of packaged and ozonized flaxseed. J. Stored Prod. Res. 2019, 83, 311–316. [Google Scholar] [CrossRef]
- Oliveira, J.M.; ALencar, E.R.; Blum, L.E.; Ferreira, W.F.S.; Botelho, S.C.C.; Racanicci, A.M.C.; Leandro, E.S.; Mendonça, M.A.; Moscon, E.S.; Bizerra, L.V.A.S.; et al. Ozonation of Brazil Nuts: Decomposition kinetics, control of Aspergillus flavus and the effect on color and on raw oil quality. Food Sci. Technol. 2020, 123, 106–109. [Google Scholar] [CrossRef]
- Straumite, E.; Rucins, A.; Viesturs, D.; Kleperis, J.; Kristins, A. Evaluation of ozone influence on wheat grain quality during active drying. Agron. Res. 2021, 19, 1308–1317. [Google Scholar]
- Woodstock, L.M. Physiological and biochemical tests for seed vigor. Seed Sci. Technol. 1973, 1, 127–157. [Google Scholar]
- Rego, J.M. Qualidade Fisiológica e Sanitária de Sementes de Soja Ozonizadas Durante a Secagem. Master’s Thesis, Universidade Estadual do Oeste do Paraná, Cascavel, Brazil, 2017. [Google Scholar]
- Rosa, C.C.; Alencar, E.R.; Souza, N.O.S.; Bastos, C.S.; Suinaga, F.A.; Ferreira, W.F.S. Physiological Quality of Corn Seeds Treated with Gaseous Ozone. Ozone Sci. Eng. 2021, 44, 117–126. [Google Scholar] [CrossRef]
- Silva, M.V.A.; Faroni, L.R.A.; Sousa, A.H.; Prates, L.H.F.; Abreu, A.O. Kinetics of the ozone gas reaction in popcorn kernels. J. Stored Prod. Res. 2019, 83, 168–175. [Google Scholar] [CrossRef]
| Position | n | DF | Slope ± SE | LD50 (95% CI) | TR50 | LD95 (95% CI) | TR95 | χ2 | p |
|---|---|---|---|---|---|---|---|---|---|
| Bottom | 1000 | 3 | 2.29 ± 0.20 | 45.52 (41.10–49.64) | 1.44 | 236.95 (189.49–324.08) | 1.07 | 3.55 | 0.31 |
| Top | 1000 | 3 | 2.80 ± 0.16 | 65.97 (61.38–70.87) | - | 254.78 (217.68–309.83) | - | 0.76 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, G.Y.R.; Silva, G.N.; Silva, Y.N.M.; Silva, Y.d.M.; Marques, I.S.; da Silva, G.L.; Carvalho, M.S.; Faroni, L.R.D.; Rodrigues Lima, S.K.; Arcanjo, D.D.R.; et al. Ozonation of Cowpea Grains: Alternative for the Control of Callosobruchus maculatus and Maintenance of Grain Quality. Agriculture 2023, 13, 1052. https://doi.org/10.3390/agriculture13051052
Ramos GYR, Silva GN, Silva YNM, Silva YdM, Marques IS, da Silva GL, Carvalho MS, Faroni LRD, Rodrigues Lima SK, Arcanjo DDR, et al. Ozonation of Cowpea Grains: Alternative for the Control of Callosobruchus maculatus and Maintenance of Grain Quality. Agriculture. 2023; 13(5):1052. https://doi.org/10.3390/agriculture13051052
Chicago/Turabian StyleRamos, Gustavo Yves Rodrigues, Gutierres Nelson Silva, Ynayanna Nariza Medeiros Silva, Yago de Medeiros Silva, Izaias Santos Marques, Giovana Lopes da Silva, Marcela Silva Carvalho, Leda Rita D’antonino Faroni, Simone Kelly Rodrigues Lima, Daniel Dias Rufino Arcanjo, and et al. 2023. "Ozonation of Cowpea Grains: Alternative for the Control of Callosobruchus maculatus and Maintenance of Grain Quality" Agriculture 13, no. 5: 1052. https://doi.org/10.3390/agriculture13051052
APA StyleRamos, G. Y. R., Silva, G. N., Silva, Y. N. M., Silva, Y. d. M., Marques, I. S., da Silva, G. L., Carvalho, M. S., Faroni, L. R. D., Rodrigues Lima, S. K., Arcanjo, D. D. R., Lucarini, M., Durazzo, A., & Barbosa, D. R. e. S. (2023). Ozonation of Cowpea Grains: Alternative for the Control of Callosobruchus maculatus and Maintenance of Grain Quality. Agriculture, 13(5), 1052. https://doi.org/10.3390/agriculture13051052










