SSR and SNP Marker-Based Investigation of Indian Rice Landraces in Relation to Their Genetic Diversity, Population Structure, and Geographical Isolation
Abstract
1. Introduction
2. Materials and Methods
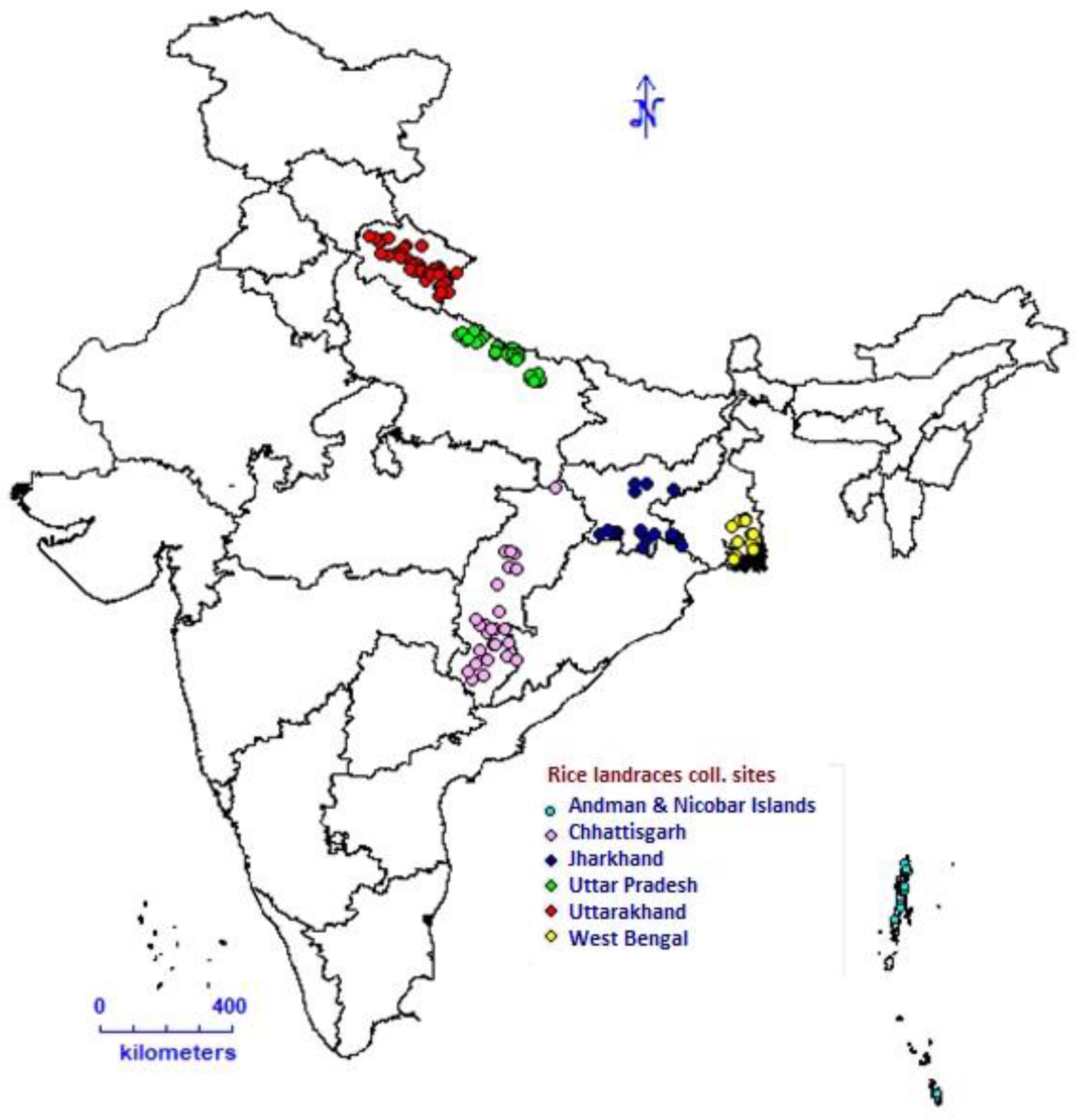
2.1. Plant Materials
2.2. DNA Extraction
2.3. Genotyping of Rice Landraces Using SSR Markers
2.4. Genotyping of Rice Landraces Using SNP Markers
2.5. Genetic Diversity Indices and Population Differentiation Using SSR Markers
2.6. SNP Filtering, Genetic Diversity Indices, and Population Differentiation Using SNP Markers
2.7. Discriminant Analysis of Principal Components (DAPC)
2.8. Analysis of Molecular Variance of 298 Rice Landraces Using SSR and SNP Markers
2.9. Study of the Index of Differentiation (Fst) and Mantel Test
3. Results
3.1. Study of Genetic Diversity Parameters of 298 Rice Landraces
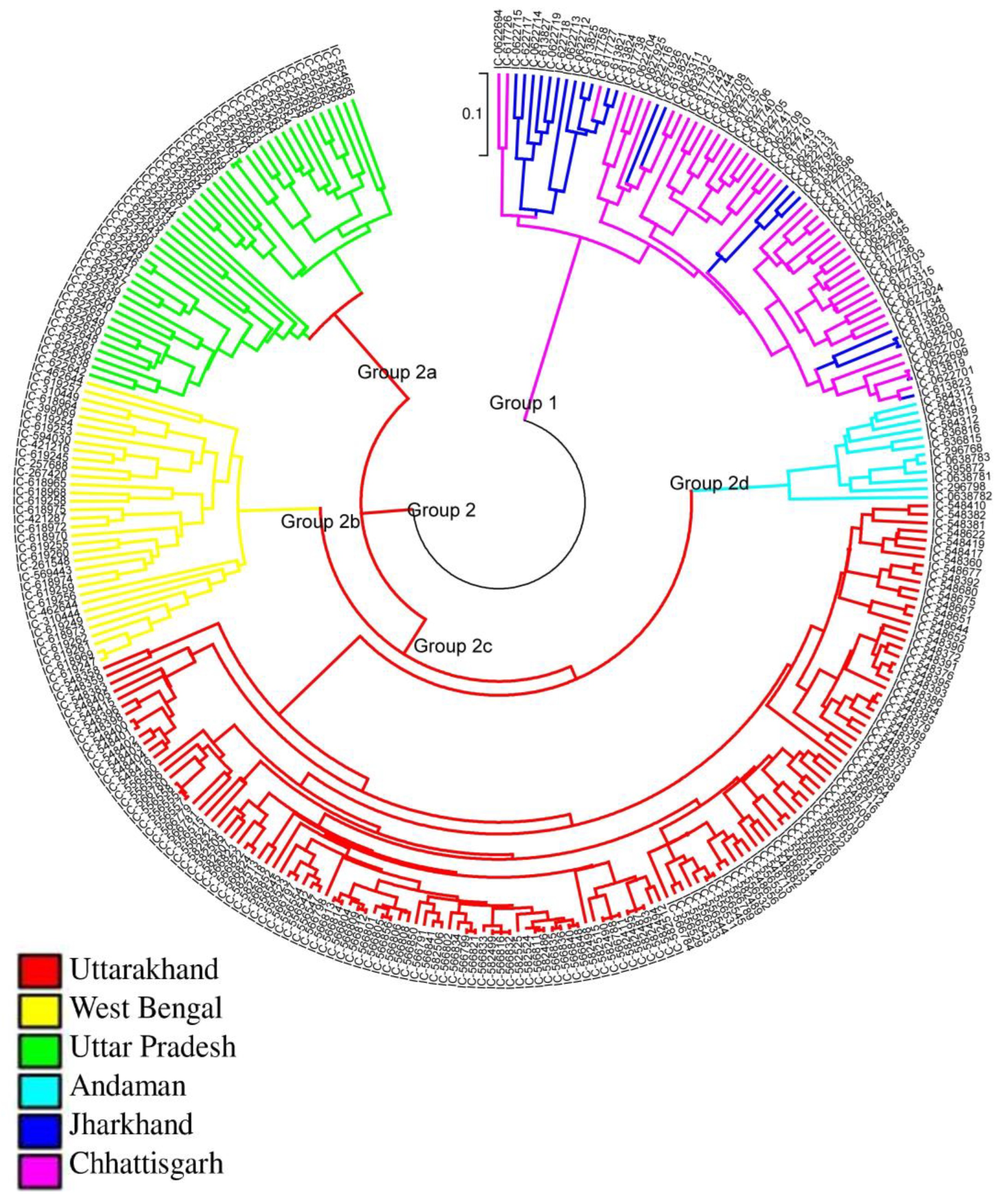
3.2. Genetic Relatedness Study of Rice Landraces Using SSR Markers
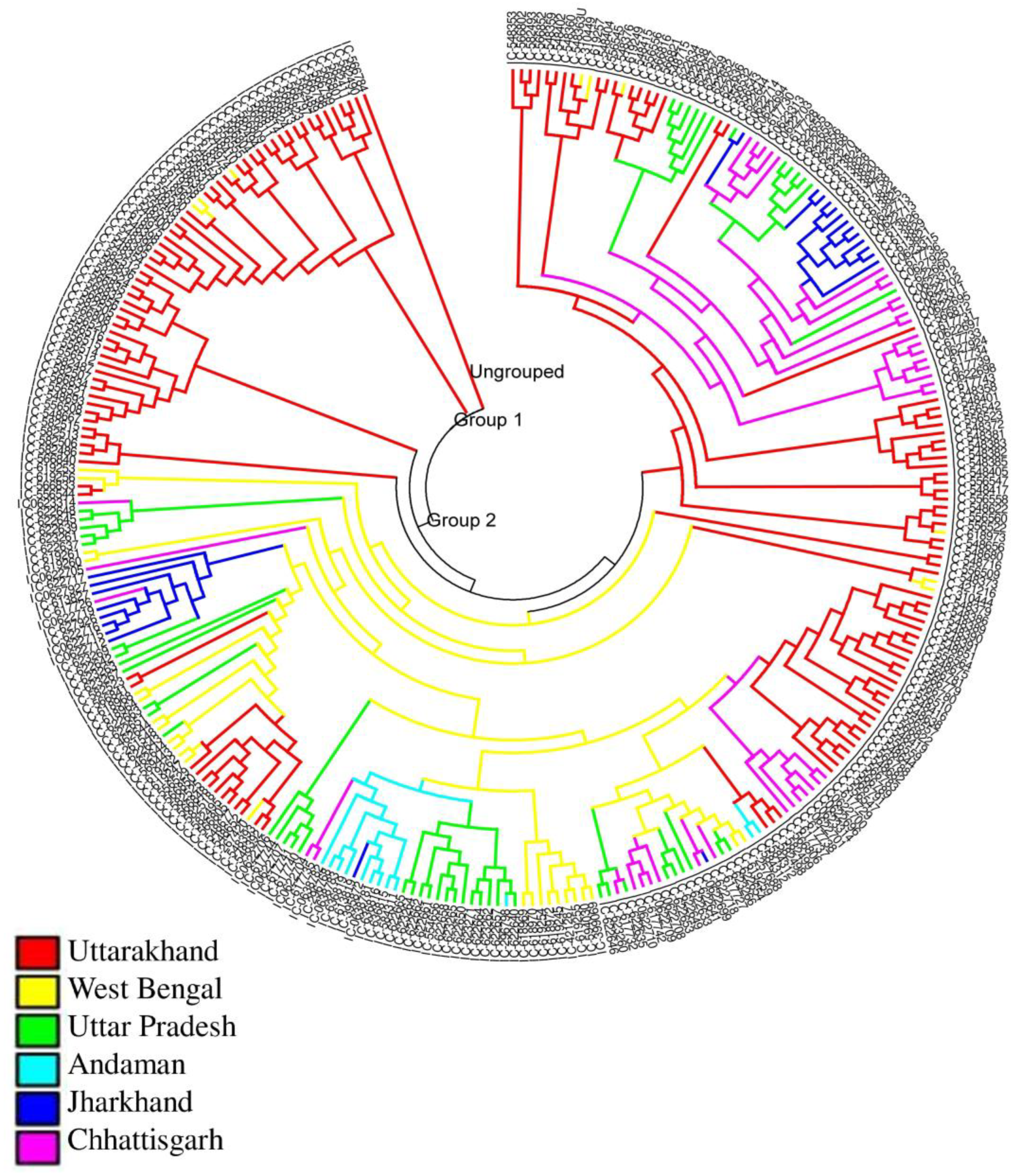
3.3. Study of Genetic Relatedness of Rice Landraces Using SNP Markers
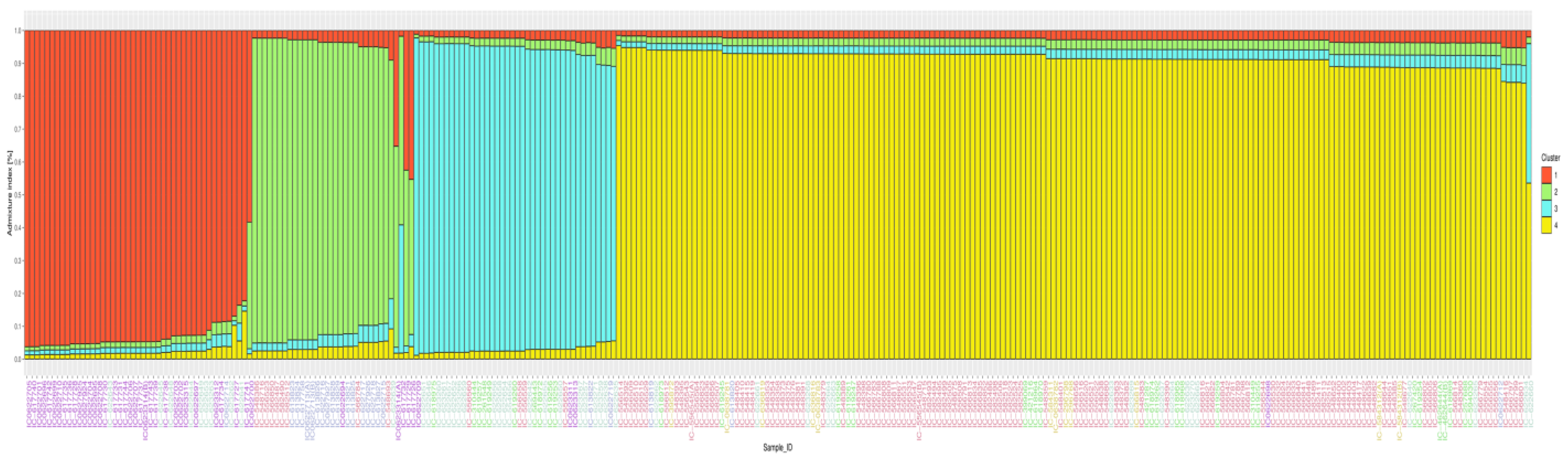
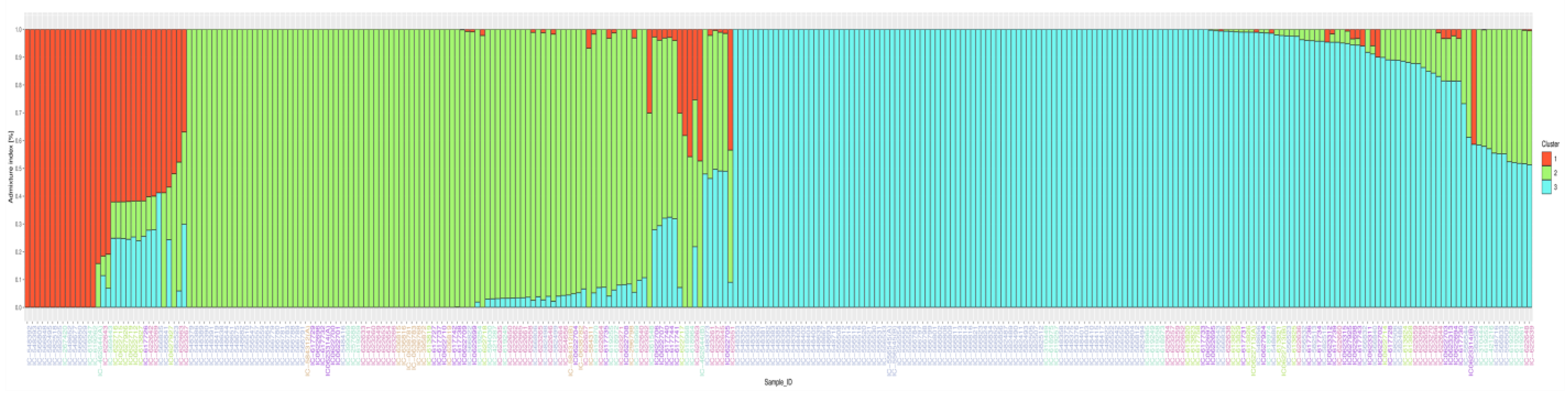
3.4. Population Structure Differentiation Using SSR Markers
3.5. Discriminant Analysis of Principal Components (DAPC) Using SSR Markers
3.6. Population Structure Differentiation Using SNP Markers
3.7. Discriminant Analysis of Principal Components (DAPC) Using SNP Markers
3.8. Analysis of Molecular Variance (AMOVA) from fastSTRUCTURE Populations Using SSR and SNP Markers
3.9. Region-Wise Analysis of Molecular Variance (AMOVA) of 298 Rice Landraces Using SSR and SNP Markers
3.10. Mantel Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasumathy, S.K.; Alagu, M. SSR marker-based genetic diversity analysis and SNP haplotyping of genes associating abiotic and biotic stress tolerance, rice growth, and development and yield across 93 rice landraces. Mol. Biol. Rep. 2021, 48, 5943–5953. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, V.V.; Muthurajan, R.; Duraialaguraja, S.; Sivakami, R.; Aravindhan Pandian, B.; Ponniah, G.; Gunasekaran, K.; Swaminathan, M.; Suji, K.K.; Sabariappan, R. Analysis of Population Structure and Genetic Diversity in Rice Germplasm Using SSR Markers: An Initiative Towards Association Mapping of Agronomic Traits in Oryza Sativa. Rice 2015, 8, 30–35. [Google Scholar] [CrossRef]
- Hour, A.; Hsieh, W.; Chang, S.; Wu, Y.P.; Chin, H.S.; Lin, Y.R. Genetic Diversity of Landraces and Improved Varieties of Rice (Oryza sativa L.) in Taiwan. Rice 2020, 13, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Londo, J.P.; Chiang, Y.C.; Hung, K.H.; Chiang, T.Y.; Schaal, B.A. Phylogeography of Asian Wild Rice, Oryza rufipogon, Reveals Multiple Independent Domestications of Cultivated Rice, Oryza sativa. Proc. Natl. Acad. Sci. USA 2006, 103, 9578–9583. [Google Scholar] [CrossRef] [PubMed]
- Pusadee, T.; Jamjod, S.; Chiang, Y.C.; Rerkasem, B.; Schaal, B.A. Genetic structure and isolation by distance in a landrace of Thai rice. Proc. Natl. Acad. Sci. USA 2009, 106, 13880–13885. [Google Scholar] [CrossRef]
- Roy, S.; Marndi, B.C.; Mawkhlieng, B.; Banerjee, A.; Yadav, R.M.; Misra, A.K.; Bansal, K.C. Genetic diversity and structure in hill rice (Oryza sativa L.) landraces from the North-Eastern Himalayas of India. BMC Genet. 2016, 17, 107–122. [Google Scholar] [CrossRef]
- Peringottillam, M.; Kunhiraman Vasumathy, S.; Selvakumar, H.K.; Alagu, M. Genetic diversity, and population structure of rice (Oryza sativa L.) landraces from Kerala, India analyzed through genotyping-by-sequencing. Mol. Genet. Genom. 2022, 297, 169–182. [Google Scholar] [CrossRef]
- Fujino, K.; Shirasawa, K. Fine-scale genetic structure of the rice landrace population in Japan. Mol. Genet. Genom. 2022, 297, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, S.D.; Kulwal, P.L.; Patil, J.V.; Sarawate, C.D.; Gaikwad, S.D.; Jadhav, A.S. Genetic Diversity and Population Structure in Landraces and Improved Rice Varieties from India. Rice Sci. 2015, 22, 99–107. [Google Scholar] [CrossRef]
- Adu, G.B.; Awuku, F.J.; Amegbor, I.K.; Haruna, A.; Manigben, K.A.; Aboyadana, P.A. Genetic characterization and population structure of maize populations using SSR markers. Ann. Agric. Sci. 2019, 64, 47–54. [Google Scholar] [CrossRef]
- Farhangian-Kashani, S.; Azadi, A.; Khaghani, S.; Changizi, M.; Gomarian, M. Association analysis and evaluation of genetic diversity in wheat genotypes using SSR markers. Biol. Future 2021, 72, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Dangi, G.S.; Mendum, M.L.; Prins, B.H.; Walker, M.A.; Meredith, C.P.; Simon, C.J. Simple sequence repeat analysis of a clonally propagated species: A tool for managing a grape germplasm collection. Genome 2001, 44, 432–438. [Google Scholar] [CrossRef]
- Coombs, J.J.; Frank, L.M.; Douches, D.S. An applied fingerprinting system for cultivated potato using simple sequence repeats. Am. J. Potato Res. 2004, 81, 243–250. [Google Scholar] [CrossRef]
- Louarn, S.; Torp, A.M.; Holme, I.B.; Andersen, S.B.; Jensen, B.D. Database-derived microsatellite markers (SSRs) for cultivar differentiation in Brassica oleracea. Genet. Resour. Crop Evol. 2007, 54, 1717–1725. [Google Scholar] [CrossRef]
- Rahman, M.S.; Molla, M.R.; Alam, M.S.; Rahman, L. DNA fingerprinting of rice (Oryza sativa L.) cultivars using microsatellite markers. Aust. J. Crop Sci. 2009, 3, 122–128. [Google Scholar]
- Singh, N.; Jayaswal, P.K.; Panda, K.; Mandal, P.; Kumar, V.; Singh, B. Single-copy gene-based 50 K SNP chip for genetic studies and molecular breeding in rice. Sci. Rep. 2015, 5, 11600. [Google Scholar] [CrossRef]
- Chen, H.; Xie, W.; He, H.; Yu, H.; Chen, W.; Li, J.; Yu, R.; Yao, Y.; Zhang, W.; He, Y.; et al. A High-Density SNP Genotyping Array for Rice Biology and Molecular Breeding. Mol. Plant. 2014, 7, 541–553. [Google Scholar] [CrossRef]
- Chavhan, R.L.; Sable, S.; Narwade, A.V.; Hinge, V.R.; Kalbande, B.B.; Mukherjee, A.K.; Chakrabarty, P.K.; Kadam, U.S. Multiplex molecular marker-assisted analysis of significant pathogens of cotton (Gossypium sp.). Biocatal. Agric. Biotechnol. 2023, 47, 102557. [Google Scholar] [CrossRef]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghuge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U.S. Assessment of genetic diversity and volatile content of commercially grown banana (Musa spp.) cultivars. Sci. Rep. 2022, 12, 7979. [Google Scholar] [CrossRef]
- Murray, M.G.; Thomson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acid Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Singh, H.; Deshmukh, R.K.; Singh, A.; Singh, A.K.; Gaikwad, K.; Sharma, T.; Mohapatra, T.; Singh, N.K. Highly variable SSR markers suitable for Rice genotyping using Agarose gels. Mol. Breed. 2009, 25, 359–364. [Google Scholar] [CrossRef]
- Liu, K.; Muse, S.V. PowerMarker: An Integrated Analysis Environment for Genetic Marker Analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Tajima, F.; Tateno, Y. Accuracy of Estimated Phylogenetic Trees from Molecular Data. J. Mol. Evol. 1983, 19, 153–170. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 8, 242–245. [Google Scholar] [CrossRef]
- Raj, A.; Stephens, M.; Pritchard, J.K. fastSTRUCTURE: Variational inference of population structure in large SNP data sets. Genetics 2014, 197, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Liu, J.X. StructureSelector: A web-based software to select and visualize the optimal number of clusters using multiple methods. Mol. Ecol. Resour. 2018, 18, 176–177. [Google Scholar] [CrossRef]
- Puechmaille, S. The program STRUCTURE does not reliably recover the correct population structure when sampling is uneven: Sub-sampling and new estimators alleviate the problem. Mol. Ecol. 2016, 16, 608–627. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. Peer J. 2014, 2, e281. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2010, 24, 1403–1405. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Sengupta, S.; Parida, S.K.; Roy, B.; Ghosh, M.; Prasad, M.; Ghose, T.K. Genetic Diversity and Population Structure of rice Landraces from Eastern and North Eastern States of India. BMC Genet. 2013, 14, 71–85. [Google Scholar] [CrossRef]
- Hassan, D.A.; Hama-Ali, E.O. Evaluation of gene flow and genetic diversity in rice accessions across Kurdistan region-iraq using SSR markers. Mol. Biol. Rep. 2022, 49, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Sarri, V.; Baldoni, L.; Porceddu, A.; Cultrera, N.G.M.; Contento, A.; Frediani, M.; Belaj, A.; Trujillo, I.; Cionini, P. Microsatellite markers are powerful tools for discriminating among olive cultivars and assigning them to geographically defined populations. Genome 2006, 49, 1606–1615. [Google Scholar] [CrossRef]
- Yang, X.; Tan, B.; Liu, H.; Zhu, W.; Xu, L.; Wang, Y.; Fan, X.; Sha, L.; Zhang, H.; Zeng, J.; et al. Genetic diversity and population structure of Asian and Europian common wheat accessions based on genotyping–by–sequencing. Front. Genet. 2020, 11, 580782–580796. [Google Scholar] [CrossRef]
- Kumar, D.; Chhokar, V.; Sheoran, S.; Singh, R.; Sharma, P.; Jaiswal, S.; Jaisri, J.; Angadi, U.B. Characterization of genetic diversity and population structure in wheat using array-based SNP markers. Mol. Biol. Rep. 2020, 47, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Tomar, V.; Dhillon, G.S.; Singh, D.; Singh, R.P.; Poland, J.; Joshi, A.K.; Tiwari, B.S.; Kumar, U. Elucidating SNP-based genetic diversity and population structure of advanced breeding lines of bread wheat (Triticum aestivum L.). Peer J. 2021, 22, e11593. [Google Scholar] [CrossRef]
- Yu, Z.; Fredua-Agyeman, R.; Hwang, S.F.; Strelkov, S.E. Molecular genetic diversity and population structure analyses of rutabaga accessions from Nordic countries as revealed by single nucleotide polymorphism markers. BMC Genom. 2021, 22, 442–451. [Google Scholar] [CrossRef]
- Chander, S.; Garcia-Oliveira, A.L.; Gedil, M.; Shah, T.; Otusanya, G.O.; Asiedu, R.; Chigeza, G. Genetic Diversity and Population Structure of Soybean Lines Adapted to Sub-Saharan Africa Using Single Nucleotide Polymorphism (SNP) Markers. Agronomy 2021, 11, 604–618. [Google Scholar] [CrossRef]
- Courtois, B.; Frouin, J.; Greco, R.; Bruschi, G.; Droc, G.; Hamelin, C.; Ruiz, M.; Clément, G.; Evrard, J.-C.; van Coppenole, S. Genetic diversity and population structure in a European collection of rice. Crop. Sci. 2012, 52, 1663–1675. [Google Scholar] [CrossRef]
- Van Inghelandt, D.; Melchinger, A.E.; Lebreton, C.; Stich, B. Population structure and genetic diversity in a commercial maize breeding program assessed with SSR and SNP markers. Theor. Appl. Genet. 2010, 120, 1289–1299. [Google Scholar] [CrossRef]
- Guichoux, E.; Lagache, L.; Wagner, S.; Chaumeil, P.; Léger, P.; Lepais, O.; Lepoittevin, C.; Malausa, T.; Revardel, E.; Salin, F.; et al. Current trends in microsatellite genotyping. Mol. Ecol. Resour. 2011, 11, 591–611. [Google Scholar] [CrossRef]
- García, C.; Guichoux, E.; Hampe, A. A comparative analysis between SNPs and SSRs to investigate genetic variation in a juniper species (Juniperus phoenicea ssp. turbinata). Tree Genet. Genomes 2017, 14, 87–95. [Google Scholar] [CrossRef]
- Tsykun, T.; Rellstab, C.; Dutech, C.; Sipos, G.; Prospero, S. Comparative assessment of SSR and SNP markers for inferring the population genetic structure of the common fungus Armillaria cepistipes. Heredity 2017, 119, 371–380. [Google Scholar] [CrossRef]
- Tanhuanpää, P.; Erkkilä, M.; Tenhola-Roininen, T.; Tanskanen, J.; Manninen, O. SNP diversity within and among Brassica rapa accessions reveals no geographic differentiation. Genome 2016, 59, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Choudhury, D.R.; Singh, A.K.; Kumar, S.; Srinivasan, K.; Tyagi, R.K.; Singh, N.K.; Singh, R. Comparison of SSR and SNP markers in the estimation of genetic diversity and population structure of Indian rice varieties. PLoS ONE 2013, 8, e84136. [Google Scholar] [CrossRef]
- Umakanth, B.; Vishalakshi, B.; Sathish Kumar, P.; Rama Devi, S.J.S.; Bhadana, V.P.; Senguttuvel, P.; Kumar, S.; Kumar, S.S.; Sharma, P.K.; Prasad, M.S.; et al. Diverse Rice Landraces of North-East India Enables the Identification of Novel Genetic Resources for Magnaporthe Resistance. Front. Plant Sci. 2017, 8, 1500. [Google Scholar] [CrossRef]
- Chen, R.; Shimono, A.; Aono, M.; Nakajima, N.; Ohsawa, R.; Yoshioka, Y. Genetic diversity and population structure of feral rapeseed (Brassica napus L.) in Japan. PLoS ONE 2020, 15, e0227990. [Google Scholar] [CrossRef] [PubMed]
- Tehseen, M.M.; Tonk, F.A.; Tosun, M.; Istipliler, D.; Amri, A.; Sansaloni, C.P.; Kurtulus, E.; Mubarik, M.S.; Nazari, K. Exploring the Genetic Diversity and Population Structure of Wheat Landrace Population Conserved at ICARDA Genebank. Front. Genet. 2022, 13, 900572. [Google Scholar] [CrossRef]
- Gadissa, F.; Tesfaye, K.; Dagne, K.; Geleta, M. Genetic diversity and population structure analyses of Plectranthus edulis (Vatke) Agnew collections from diverse agro-ecologies in Ethiopia using newly developed EST-SSRs marker system. BMC Genet. 2018, 19, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Melaku, G.; Labroo, M.; Liyu, H.; Shilai, Z.; Guangfu, H.; Jing, Z.; Tesfaye, K.; Haileselassie, T.; Hu, F. Genetic diversity and differentiation of the African wild rice (Oryza longistaminata chev. et roehr) in Ethiopia. Sci. Afr. 2019, 6, e00138. [Google Scholar] [CrossRef]
- Aesomnuk, W.; Ruengphayak, S.; Ruanjaichon, V.; Sreewongchai, T.; Malumpong, C.; Vanavichit, A.; Toojinda, T.; Wanchana, S.; Arikit, S. Estimation of the Genetic Diversity and Population Structure of Thailand’s Rice Landraces Using SNP Markers. Agronomy 2021, 11, 995. [Google Scholar] [CrossRef]



| SSR Marker | ||||||
| Andaman | West Bengal | Chhattisgarh | Jharkhand | UP | Uttarakhand | |
| Major Allele Frequency | 0.78 | 0.70 | 0.69 | 0.71 | 0.67 | 0.80 |
| Gene Diversity | 0.27 | 0.39 | 0.38 | 0.38 | 0.42 | 0.26 |
| Heterozygosity | 0.19 | 0.10 | 0.30 | 0.27 | 0.21 | 0.13 |
| PIC | 0.22 | 0.34 | 0.31 | 0.32 | 0.38 | 0.21 |
| SNP Marker | ||||||
| Andaman | West Bengal | Chhattisgarh | Jharkhand | UP | Uttarakhand | |
| Major Allele frequency | 0.45 | 0.44 | 0.42 | 0.42 | 0.44 | 0.44 |
| Shannon Diversity | 0.48 | 0.49 | 0.48 | 0.47 | 0.49 | 0.49 |
| Heterozygosity | 0.81 | 0.67 | 0.74 | 0. 76 | 0.7 | 0.75 |
| PIC | 0.37 | 0.37 | 0.36 | 0.36 | 0.37 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, D.R.; Kumar, R.; Maurya, A.; Semwal, D.P.; Rathi, R.S.; Gautam, R.K.; Trivedi, A.K.; Bishnoi, S.K.; Ahlawat, S.P.; Singh, K.; et al. SSR and SNP Marker-Based Investigation of Indian Rice Landraces in Relation to Their Genetic Diversity, Population Structure, and Geographical Isolation. Agriculture 2023, 13, 823. https://doi.org/10.3390/agriculture13040823
Choudhury DR, Kumar R, Maurya A, Semwal DP, Rathi RS, Gautam RK, Trivedi AK, Bishnoi SK, Ahlawat SP, Singh K, et al. SSR and SNP Marker-Based Investigation of Indian Rice Landraces in Relation to Their Genetic Diversity, Population Structure, and Geographical Isolation. Agriculture. 2023; 13(4):823. https://doi.org/10.3390/agriculture13040823
Chicago/Turabian StyleChoudhury, Debjani Roy, Ramesh Kumar, Avantika Maurya, Dinesh P. Semwal, Ranbir S. Rathi, Raj K. Gautam, Ajaya K. Trivedi, Santosh K. Bishnoi, Sudhir P. Ahlawat, Kuldeep Singh, and et al. 2023. "SSR and SNP Marker-Based Investigation of Indian Rice Landraces in Relation to Their Genetic Diversity, Population Structure, and Geographical Isolation" Agriculture 13, no. 4: 823. https://doi.org/10.3390/agriculture13040823
APA StyleChoudhury, D. R., Kumar, R., Maurya, A., Semwal, D. P., Rathi, R. S., Gautam, R. K., Trivedi, A. K., Bishnoi, S. K., Ahlawat, S. P., Singh, K., Singh, N. K., & Singh, R. (2023). SSR and SNP Marker-Based Investigation of Indian Rice Landraces in Relation to Their Genetic Diversity, Population Structure, and Geographical Isolation. Agriculture, 13(4), 823. https://doi.org/10.3390/agriculture13040823








