Abstract
As one of the essential cereal crops, wheat provides 20% of the calories and proteins consumed by humans. Due to population expansion, dietary shift and climate change, it is challenging for wheat breeders to develop new varieties for meeting wheat production requirements. Marker-assisted selection (MAS) has distinct advantages over conventional selection in plant breeding, such as being time-saving, cost-effective and goal-oriented. This review makes attempts to give a description of different molecular markers: sequence tagged site (STS), simple sequence repeat (SSR), genotyping by sequencing (GBS), single nucleotide polymorphism (SNP) arrays, exome capture, Kompetitive Allele Specific PCR (KASP), cleaved amplified polymorphic sequence (CAPS), semi-thermal asymmetric reverse PCR (STARP) and genotyping by target sequencing (GBTS). We also summarize some quantitative trait loci (QTL)/genes as well as their linked markers, which are potentially useful in MAS. This paper provides updated information on some markers linked to critical traits and their potential applications in wheat breeding programs.
1. Introduction
As an imperative food crop, bread wheat (Triticum aestivum L.) feeds ~40% of the global population [1]. With the growing population, annual progress in yield improvement will hardly fulfill wheat production requirements by 2050 [2]. In addition, many challenges threaten the increase in wheat yield; therefore, modern cultivars need to improve biotic resistance to various diseases, including powdery mildew, rusts and Fusarium head blight (FHB); enhance abiotic tolerance to adapt to climate change, such as heat, cold and drought; and finally have optimum end-use quality to meet dietary shifts [3]. Facing a growing population and complex climate, wheat breeders should increase yield potential to secure food supply for the future. Therefore, improving productivity and stability is a quite challenging task for wheat breeders.
Conventional breeding has played a critical role in wheat improvement in the last few decades. Despite not knowing the molecular mechanisms of inheritance, some key genes, such as reduced plant height Rht-B1b [4], vernalization Vrn1 [5], photoperiod response (Ppd-1) [6] and grain size (such as TaGW2 [7] and TaSus1 [8]) were positively selected. However, it was quite limited to manipulate more alleles of these genes for further improvement [2]. Furthermore, selection depending on phenotype is entirely inefficient for complex traits, which are less heritable and easily influenced by environment, and also require an extended period (about 10 years) to develop varieties [9]. Selection of cultivars for interest genes based on molecular markers is referred to as marker-assisted selection (MAS). As a requisite breeding tool, MAS could make it faster and easier to select target traits in breeding for breeders. Transferring rust and dough quality loci to an elite but susceptible line ‘Stylet’ using MAS in combination with doubled haploid (DH) technology, breeders in southern Australia developed a commercial cultivar within five years vs. the 12 years needed through conventional breeding [10]. MAS contributes to overcoming the shortcomings of traditional breeding based on phenotype and greatly improves crop breeding efficiency for pyramiding multi-allelic traits to produce high-yielding cultivars. It has many strengths over conventional breeding by: (1) allowing selection for a trait dependent on a single plant; (2) pyramiding a few individual QTL/genes at a time; (3) permitting selection of recessive genes efficiently without doing phenotypic evaluation at each generation; (4) selecting phenotypes where field conditions are restricted (e.g., evaluating root traits is costly and time-consuming, and disease screening needs to be infected by the relevant isolate(s) and evaluated at specific stages); and (5) decreasing the number of replications and increasing selection intensity [11,12].
The notable advances in wheat genetics and genomics have boosted the development and innovation of molecular markers since the first generation hybridization-based restriction fragment length polymorphism (RFLP) in the early 1990s [2]. Because of its low frequency, high cost and time-consumption, RFLP has been replaced by other markers and is rarely used nowadays. The second generation PCR-based markers, such as random amplified polymorphic DNA (RAPD) and simple sequence repeats (SSR), have been developed for QTL/gene discovery. Because of a lack of reproducibility and absence of information on their physical locations on the chromosome, RAPDs are not used any more [13], while SSRs have been extensively exploited in wheat genetics studies due to their abundance, high polymorphism and genome specificity. In the wake of DNA sequencing technologies, the third generation SNP markers have been given priority to map QTL/genes now, which increasingly augment the progress of molecular breeding. SNP markers have some distinct advantages, including high quantity, high density, high genetic stability and easy automatic detection; therefore, they are extensively applied to QTL and association analyses nowadays [14].
In this paper, we (1) briefly described different types of molecular markers, especially those genomic based markers, (2) summarized some QTL/genes and their linked markers that might be used in wheat breeding, (3) reviewed some progress on MAS in wheat and (4) discussed the prospective use of MAS in wheat breeding.
2. Molecular Markers
Molecular markers are pivotal components in wheat breeding. Markers, as a sign or flag, are associated with a QTL/gene. Since they are applied for gene mapping and MAS with varying degrees of efficiency, various marker technologies have been developed [2,12,14]. A brief timeline of marker types, a few key genes and technologies are depicted based on Rasheed et al. [1], which play pivotal roles in wheat improvement (Figure 1). RAPD, RFLP and AFLP (amplified fragment length polymorphism) have largely been replaced by other efficient markers, so we did not describe them in this review. Other gel-based markers (e.g., STS and SSR) are still in use to a certain degree, despite their low throughput and density.
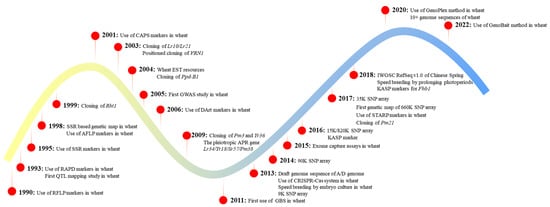
Figure 1.
Timeline of application of molecular markers in wheat breeding and research.
2.1. Sequence-Tagged-Site (STS) Marker
STSs are short and unique sequences that identify the specific loci. A large quantity of sequencing data and functional genes can be used for the development of STS markers. For example, peroxidase (POD) activity is correlated with flour color, processing and product quality. Through sequence blast against the wheat expressed sequence tag (EST) database, six STS markers were developed to characterize POD genes (TaPod-A2, TaPod-A3, TaPod-B1 and TaPod-D1) [15]. Two complementary dominant functional markers were further designed to distinguish two haplotypes (TaPod-D1a and TaPod-D1b) with different POD activities [15].
In recent years, many STS markers from ESTs and sequencing datasets were developed in wild relatives, such as Dasypyrum villosum [16] and Agropyron cristatum [17]. A total of 507 STS markers of Thinopyrum ponticum were developed to discriminate chromosome 1Js genetic stocks in wheat backgrounds by specific-locus amplified fragment sequencing (SLAF-seq) [18].
2.2. Simple Sequence Repeats (SSRs) Marker
SSRs are DNA stretches containing a variable number of short and simple sequence repeats. They are ubiquitous in wheat genomes, generally codominant and highly polymorphic, which allows for the direct detection of heterozygosity and makes them the marker of choice for a diversity of purposes, such as gene mapping and tagging [19]. Despite the frequent application of SSRs, there is limited potential in practical crop breeding. SSRs are also finite and distributed unevenly in a certain genome. It is challenging to properly identify precise information in terms of multiple alleles per locus and integrate or compare SSR data from different platforms or populations.
By developing new SSR markers using comparative genomics analysis, powdery mildew resistance gene Pm52 was placed in a 0.21 cM genetic bin on chromosome arm 2BL in wheat [20]. The two Pm52-linked markers, Xicsl234 and Xicscl817, could expedite the mining of Pm52 in the Liangxing99-derived materials. A total of 192 SSR markers were selected to evaluate 123 wheat stocks for lipoxygenase (LOX) activity, and association analysis found 22 marker loci with a significant correlation with LOX [21]. A novel QTL QFhb.cau-7DL for FHB resistance was identified from the wheat line AQ24788-83 (AQ) by genotyping recombinant inbred lines (RILs) using SSR, DArT and SNP markers [22]. This QTL was closely linked to the SSR marker gwm428, which could be used in the selection for QFhb.cau-7DL when it has polymorphisms in a given population.
2.3. Single Nucleotide Polymorphism (SNP) Markers
SNPs are the most common type of genetic variation, and are ideal markers for genetic discovery and molecular breeding. Their density varies from one per 370 bp to one per 540 bp in wheat [23]. SNPs are generally regarded as biallelic markers and classified based on nucleotide substitutions, genomic location or phenotype [14].
Next-generation sequencing (NGS) technology thoroughly promotes the development of SNP related platforms for genotyping in an ultra-high-throughput style, substantially improving QTL mapping and gene mining studies and genotyping large populations in a time-saving and cost-saving way, because these platforms can rapidly scan genomes at high-density with a range of multiplex levels and find robust allele calling with high call rates.
- GBS-SNP
Advances in NGS have greatly reduced the costs of DNA sequencing, so that genotyping by sequencing (GBS) is feasible for discovering and genotyping SNPs in wheat [24]. GBS is a high-throughput, cost-effective and rapid molecular tool for rating wheat complex genomes whose complexity is reduced through multiple enzyme digestions. GBS-SNPs provide better genome coverage and can be applied to construct high-density linkage maps, compared with conventional marker systems (e.g., SSRs) [25]. In addition, GBS does not depend on prior genome information for constructing genetic linkage maps. There are at least 15 restriction enzymes available and multiple genotyping strategies for GBS in crop plants [25,26]. However, there are some shortcomings. GBS is easily affected by the errors resulting from the low reads coverage and the poor capacity to identify true homozygotes [27]. Furthermore, GBS performance is extremely affected by the quality of the reference genome. The large genome size (16Gb) of hexaploid wheat and three homoeologous genomes also increases the occurrence of genotyping errors [2].
In wheat, GBS has been widely exploited for genomic selection for various traits. Using GBS technology, Hessian fly resistance genes h4 [28] and H7 [29] were mapped to chromosomes 1A and 6A, which explained 60.4–70.5% and 60.7–78.3% of the phenotypic variation, respectively. The GBS sequence data (102,147 SNP markers) of 439 elite spring wheat breeding lines were used for genome-wide association study (GWAS), which identified a QTL for FHB resistance on chromosome arm 1AL, explaining 5.3% of the total phenotypic variation [30]. The GWAS using 14,063 polymorphic GBS-SNP markers found eight QTL related with spot blotch disease resistance [31].
- SNP arrays
NGS technologies provide a surplus of sequencing data, which further enhance the development of chip-based marker platforms for high-throughput genotyping. Compared with NGS and PCR-based markers, SNP arrays are flexible in the light of sample and data point number customization, which helps its high-density scanning and high and powerful call rates. In wheat, various SNP solid chips are now available, including 15K [32], 35K [33], 55K [34], 90K [35], 660k [36] and 820K [37] in different platforms. Among them, each SNP array has its own advantages and disadvantages. For example, Wheat 820K SNP array were mainly customized by markers from wheat and its relatives [38] and designed for various stocks, such as landraces, synthetic hexaploids and wheat relatives, so that it is improper for breeders to specifically assess hexaploid germplasms. However, most markers of Wheat 660K SNP array were based on hexaploid and tetraploid wheats, emmer wheat and Aegilops tauschii, so they have a wide range of possible applications on wheat evaluation due to the genome-specificity, high-density and high-efficiency [39].
GBS-SNPs and chip-based SNPs are usually used together to discover loci of interest traits. Using 90K array and GBS SNPs, eight QTL for stripe rust resistance were identified on chromosomes 1A, 2A, 2B, 4A, 4B, 6B and 7D, and two of them were novel [40]. Flanking markers closely linked to green bug resistance gene Gb7 and Hessian fly resistance gene H32 were located on chromosome arms 7DL and 3DL, respectively [41].
2.4. Exome Capture
Given the advances in NGS techniques and reduced sequencing costs, it is now feasible to perform whole genome sequencing (WGS) to identify the genetic variants in a mapping population [42]. Nevertheless, WGS remains expensive in wheat due to its large and complex genome, making it necessary to have adequate coverage. To overcome this limitation, exome capture technology is an alternative solution to cover most gene coding regions [43]. On the other hand, the exome variants are the coding sequence information, which can facilitate the finding of the causative genes. On the contrary, using traditional techniques, forward mapping is a lengthy, multi-step process based on linked markers identification on the target trait in a mapping population and the candidate genes sequencing in a broad genetic region. Using this technology, some useful genes were identified, such as the leaf rust resistance genes (Lr1, Lr10, Lr21 and Lr34) and novel genes (on chromosomes 1A and 3D) [44], the stripe rust resistance locus QYr.ucw-1BL [45] and reduced plant height gene Rht-B1 [46]. Using bulked segregant exome capture sequencing (BSE-Seq) by combining the exome capture and sequencing of bulked segregant pools, a wheat yellow leaf mutant gene ygl1 was quickly mapped [47].
2.5. SNP-Converted Markers
SNPs have shown many benefits, including high quantity, high density, high genetic stability and easy automatic detection. Thus, NGS technologies (GBS and exome capture) and SNP arrays have been rapidly replacing traditional markers and are now widely applied in genetic studies and molecular breeding. The linked SNP markers are usually converted to fluorescence-based or gel-based markers, including KASP, CAPS and STARP described below, before they are further verified in wheat populations and finely mapped using haplotype analysis and MAS.
- KASP
Based on both PCR and fluorescence detection, Kompetitive Allele Specific PCR (KASP) detection technology meets both low- and high-throughput genotyping requirements. For KASP assays, the two forward primers have the 5′ tags carrying the standard FAM (5’-GAAGGTGACCAAGTTCATGCT-3’) or HEX (5’-GAAGGTCGGAGTCAACGGATT-3’) fluorescence when amplified in KASP master mix. A common genome-specific reverse primer is also needed, and the total amplicon length usually ranges from 50 to 125 bp. The PCR results are read with a microplate reader and visualized using an SNP allele calling software, such as KlusterCaller (LGC Genomics, Middlesex, UK). The genotypes in each cluster are analyzed to reveal their association with phenotypic data. Currently, the vast majority of studies choose KASP technologies to convert linked SNP markers [48].
- CAPS
Cleaved amplified polymorphic sequence (CAPS) technology combines PCR amplification and restriction enzyme treatment. A CAPS marker usually amplifies the same sized bands for the two alleles. If polymorphism exists in the restriction site near the SNP loci of one allele, the restriction enzyme digestion cleaves one amplified sequence and make different fragments in length, which can be easily analyzed by gel electrophoresis. However, in some cases, the SNP between alleles does not generate any polymorphic restriction site. This kind of SNP could be utilized for the development of ‘derived CAPS’ (dCAPS, [49]).
- STARP
Semi-thermal asymmetric reverse PCR (STARP) is another innovative way to perform flexible SNP genotyping depending on a similar competitive PCR reaction without requiring the third-party KASP master mix [50]. In this method, genotyping assay is conducted under unique PCR conditions using two universal priming element-adjustable primers (PEA-primers) [also called asymmetrically modified allele-specific primers (AMAS-primers)] and their common reverse primer. The two PEA-primers each have one base substituted at the third or fourth base of their 3′ regions to greatly increase the amplification specificity of the two alleles. In addition, a 4-10 bp insertion tailed at the 5′ end of one AMAS primer comes into the final PCR products between two genotypes, allowing the discrimination of allelic variants in traditional gel electrophoresis [51]. Wu et al. [51] developed 56 gene-specific STARP markers for 46 genes for wheat quality, tolerance to biotic and abiotic stresses, grain yield and adaptation-related traits, which provided a powerful and reliable marker toolkit for wheat breeding programs.
2.6. Genotyping by Target Sequencing (GBTS)
Molecular detection technologies have been profoundly innovated, from gel-based and fluorescence-based to solid chip-based and now liquid chip-based, as well as possibly automated genotyping platforms in the future. Solid-based SNP arrays from different platforms have been playing a vital role in gene discovery for genotyping large populations in wheat, but some shortcomings of their applications in crop improvement exists, such as low customization efficiency, little flexibility, expensive equipment and high cost [52]. On the contrary, GBTS can compensate for their limits [53,54]. GBTS is mainly divided into multiplex PCR-based target sequencing (GenoPlexs) and probe-in-solution-based target sequencing (GenoBaits). GBTS can accurately capture sequences at random positions and lengths, except for the areas with highly repeated sequences. In addition, it is upgradable so that newly mapped important loci can be joined into an existing marker panel without resynthesis. Using these two techniques, a set of marker panels can be developed to meet almost all the requirements of marker applications in the fields. In wheat, a few studies have begun to apply GBTS in genetic studies [55,56]. GBTS will potentially be used in wheat improvement on a large scale in the future.
3. Loci and Markers of Potential Applications in MAS
Many QTL/genes of interest and their tightly linked markers or functional markers (FMs) from linkage analysis, association analysis, gene cloning and sequencing have been reported in wheat [11,57,58]. More than 200 QTL underpinning yield-related traits have been documented in different wheat genotypes in last decade [59]. Around 150 FMs for important genes have been reported to select desirable characteristics in wheat [2]; 97 FMs that identify 93 alleles at 30 loci in wheat have been documented, including 56, 27 and 14 FMs for processing quality, agronomic and disease resistance traits, respectively [60]. These markers are of potential value in MAS for improving wheat agronomic traits, such as biotic stresses, yield-related traits and quality characters. Numerous loci for agronomic traits have been reported in recent years, and it is impossible to summarize all of them, so only some important loci of a few traits are detailed in this review (Table 1).
3.1. Loci for Resistance to Biotic Stresses
Wheat is exposed to many biotic threats, such as fungi, viruses and insects, which heavily affect its yield and the quality. On average, yield loss in wheat production is about 21.5% due to pathogens and pests [61]. Plant pathogens are ever-evolving at a high pace through mutations or recombinations. FHB, powdery mildew and rusts are the three main menaces; therefore, it is of vital significance to find novel genes against them.
FHB is a serious disease in vast wheat-growing areas of the globe where rainfall frequently occurs during flowering time and leads to heavy reductions in wheat yield worldwide. A total of 50 QTL, controlling different types of FHB resistance, have been reported with unique chromosome locations [62,63]. Among them, only seven QTL have been officially named, from Fhb1 on 3B [64] to Fhb7 on 7D [65], which have been described in detail [63]. Fhb1 from cultivar Sumai3 is the most frequent selection of FHB resistance in many breeding programs [66]. Two functional markers (TaHRC-GSM and TaHRC-KASP) for Fhb1 were developed based on the critical sequence deletion of the putative histidine-rich calcium-binding protein (TaHRC) and validated to be diagnostic in different types of populations [67]. Recently, a novel major QTL for FHB resistance (QFhb-2DL) was identified on chromosome 2D (Type II) in a Chinese wheat cultivar Ji5265, which can explain ~30% of the phenotypic variation for FHB resistance [68]. Two linked KASP markers (KASP10238 and KASP12056) were proved to be diagnostic in 2065 wheat accessions.
To date, more than 69 powdery mildew resistance loci (Pm1-Pm69) have been catalogued in wheat [69]. Among these named genes, some new alleles were found and more information is available for better utilization. For example, a previously uncategorized Pm60 allele was discovered from T. urartu and two molecular markers (M-Pm60-S1 and M-Pm60-S2) were developed to distinguish the functional Pm60a allele from the non-functional Pm60a′ allele, which were helpful for precisely identifying the Pm60 allele [70]. Furthermore, many new loci were identified, including MlIW39 [71], PmKN0816 [72] and Qpm-3BL [73]. A new adult plant resistance (APR) gene, QPm.caas-3BS, to powdery mildew pathogen was delimited from the RILs derived from the cross Zhou8425B/Chinese Spring. One linked SNP STARP marker was developed and validated on 103 wheat cultivars, showing that QPm.caas-3BS could reduce maximum disease severity by 5.3% [35].

Table 1.
Loci and linked or functional markers for some key traits.
Table 1.
Loci and linked or functional markers for some key traits.
| Traits | QTL/Gene | Marker Name | Marker Type | Source | Reference |
|---|---|---|---|---|---|
| Resistance to biotic stresses | |||||
| FHB | Fhb1 | TaHRC-GSM, TaHRC-KASP | Gene specific, KASP | Sumai3 | [66,67] |
| QFhb-2DL | KASP10238, KASP12056 | KASP | Ji5265 | [68] | |
| Powdery mildew | Pm60 | M-Pm60-S1, M-Pm60-S2 | / | Triticum urartu | [70] |
| QPm.caas-3BS | Str-IWB41105 | STARP | Zhou8452B | [35] | |
| Leaf rust | Lr22a | Kwh636, Kwh637 and Kwh638 | KASP | RL4495 | [74] |
| QLr-2BS | BS00092275_51 Kukri_c36783_91 | KASP | Zhoumai22 | [75] | |
| Stripe rust | QYr.AYH-5BL | KASP_AX-109337325, KASP_AX-110400764 | KASP | Anyuehong | [76] |
| Stem rust | Sr13 | KASPSr13, rwgsnp37 | KASP/STARP | tetraploid wheat | [77] |
| Resistance to abiotic stresses | |||||
| Drought | TaWRKY51 | AS, B-Hpa11 | Indel/CAPS | / | [78] |
| Cold | Fr-A2 | S1862541, S1298957, S1051014 | KASP | / | [79] |
| qCT5A.3 | k5A4692, k5A7728 | KASP | / | [80] | |
| Lodging | TaCOMT-3B | TaCOMT-3BM | Indel | / | [81] |
| Loci for yield-related traits | |||||
| TKW | TaTAP46 | / | KASP | / | [82] |
| TaSDIR1 | / | dCAPS | / | [83] | |
| QGw4B.4 | TaGW-4B | CAPS | Shannong 01-35 | [84] | |
| SL/SC | QSc/Sl.cib-5A, QSc/Sl.cib-6A | KASP_AX_110462709, KASP_AX-109308935 | KASP | Chunmai42 | [85] |
| FT | FT-D1 | SFT-D1 | STARP | Nongda4332 | [86] |
| SNPS | TaCol-B5 | TaCOL-B5 | CAPS | CItr 17600 | [87] |
| Loci for grain quality | |||||
| GPC | GPC | Kgpc-2B, Kgpc-2D, Kgpc-4A | KASP | / | [88] |
| Glu-D1 null | Glu-D1 | gwm642 | SSR | Nap Hal | [89] |
| Black point resistance | QBp.caas-3BL | / | KASP | Zhong892 | [90] |
| PHST | Qphs.ahau-1A | IA1142 | CAPS | / | [91] |
| Qphs.ahau-3B | WS5431 | dCAPS | / | [91] | |
| Qphs.ahau-6B | EX06323 | CAPS | / | [91] | |
Note: FHB, Fusarium head blight; TKW, thousand kernel weight; SL, spike length; SC, spike compactness; HD, heading date; PH, plant height; FT, flowering tine; SNPS, spikelet nodes per spike; GPC, grain protein content; PHST, pre-harvest sprouting tolerance.
Up to now, 82 leaf rust (Lr) resistance genes, 84 stripe rust (Yr) resistance genes and 63 stem rust (Sr) resistance genes have been permanently cataloged in wheat [69]. In addition to them, most genes can be chosen for improving wheat rust resistance, such as Lr22a [74], Lr80 [92], YrAS2388 [93] and Sr13 [77]. Some genes have not been extensively used in wheat cultivars, such as the broadly effective resistance gene Lr22a [74]. Three linked KASP markers (Kwh636, Kwh637 and Kwh638) were developed to reliably detect the presence or absence of Lr22a, which can facilitate Lr22a selection in MAS [74]. Some genes confer APR, such as QLr.cau-2BL [94] from wheat landrace Hongmazha, while QLr-2BS is a valuable all-stage resistance gene [76]. Their linked SSR or KASP markers were developed and verified in genetic populations and were potentially useful for introducing them into commercial wheat cultivars. A new APR to stripe rust loci, QYr.AYH-5BL from Chinese wheat landrace Anyuehong (AYH), was stably detected in all environments and could explain 13.6–21.4% of the phenotypic variation [76]. Its linked KASP markers have potential value for MAS to improve stripe rust resistance in breeding programs. One KASP and several STARP markers were developed to identify Sr13 haplotypes [77]. Both KASPSr13 and rwgsnp37 were robust markers for Sr13 and could be used by geneticists and breeders. STARP markers rwgsnp38, rwgsnp39 and rwgsnp40 could be ideally used to discriminate four haplotypes.
3.2. Loci for Resistance to Abiotic Stresses
In addition to biotic threatens, wheat growth and development are also badly impacted by various abiotic stresses. About 50% crop losses are, on average, caused by abiotic factors, including drought (9%), heat (20%), cold (7%) and other forms of stresses [48].
Improving water absorption capacity is a good means to improve drought tolerance in crops. Roots are in charge of the uptake of water from soil; strong root architecture is beneficial for crops to absorb water stored in soil and avoid drought stress [95]. There are numerous QTL/genes related to root traits as well as their reported linked markers [36,95,96,97]. TaWRKY51 is a positive regulator contributing to the root system and grain yield (GY). Hap-2A-I is a favorable haplotype for large spike, and Hap-2B-II and allele-G are elite haplotypes/alleles for long root. Their functional markers have been developed for the utilization in the MAS [78]. In addition to root-related genes, other markers for drought tolerance genes have been reported. Sixteen functional KASP markers for 16 alleles related to drought tolerance were used to assess 153 Pakistani wheat cultivars released from 1953 to 2016 [98]. Favored haplotypes of five genes were unconsciously pyramided and selected during selection breeding, while six genes had lower frequencies in favorable haplotypes among those stocks.
Winter hardiness is also a crucial breeding goal, since it is vital for wheat to adapt to harsh winter conditions. Fr-A2 is a major QTL for frost resistance on chromosome 5A, and its polymorphisms contribute to the variation in winter hardiness [79]. Two KASP markers (S1862541 and S1298957) could differentiate two haplotypes of Fr-A2. Among 11 cold tolerance QTL found by genotyping a panel of 768 wheat germplasms using GBS, two significantly associated SNPs of qCT5A.3 were converted into KASP markers and were validated successfully in a F2 population [80].
Lodging is also an important concern for reducing wheat yield and grain quality. Caffeic acid 3-O-methyltransferase (COMT) is a key enzyme involved in lignin biosynthesis contributing to lodging resistance and has two allelic variants [81]. A codominant Indel marker was developed to validate the association between allelic patterns and stem lignin content, showing that TaCOMT-3Ba was the excellent haplotype.
Additionally, wheat yield is seriously influenced by extremely hot weather, terminal heat stress in particular. It affects approximately 40% of the wheat-cultivating regions of the world. Sihag et al. [99] identified two microRNA (miRNA)-based SSR markers (miR159c and miR165b), which showed specific alleles and discriminated terminal heat-tolerant genotypes from the susceptible genotypes.
3.3. Loci for Yield-Related Traits
Agronomic traits such as plant height (PH), heading date (HD), spike length (SL) and thousand kernel weight (TKW) are critical factors affecting wheat yield. Many genes were cloned and their haplotypes were analyzed, such as vernalization genes Vrn1 [5], Vrn2/ZCCT1 [100] and Vrn3 [101]; and PH-related genes Rht-B1b, Rht-D1b [4], Rht8 and Rht24 [102]. These genes have been widely utilized in wheat breeding.
As a polygenic trait, TKW is the most stably inherited determinant of yield potential, exhibiting higher heritability values than overall yield and yield components. Many genes controlling grain weight as well as their haplotypes were analyzed, such as TaAGP [103], TaSus1 [8], TaSDIR1 [84], TaDA1 [104] and KAT-2B [105]. Fourteen distinct haplotypes of six TaCKX genes were explored by single-molecule real-time (SMRT) sequencing and seven functional markers were then developed (four Indel and three CAPS markers). Among them, six specific haplotypes were significantly correlated with high TKW and short plant height (PH) [106]. TaTAP46-5A (Type 2A-phosphatase-associated protein) has been proven to influence kernel size and TKW in transgenic wheat, which was rarely selected (less than 1%) in conventional breeding according to phenotype and experience [82]. A KASP functional marker could be used to select it to improve breeding efficiency. TaSDIR1-4A, a Salt- and Drought-Induced RING Finger 1 (SDIR1) member, was found to affect TKW [83]. A dCAPS marker can be used to differentiate good haplotypes from two genotypes.
In addition, a series of QTL for grain weight were finely mapped, including QTKW.caas-4BS [107], QTKW.caas-5DL [108], QTgw.caas-5B [109] and QYld.aww-1B.2 [110]. Duan et al. [84] dissected a major and stable QTL, QGW4B.4-17, for TKW with an increase of 2.19–3.06g and high phenotypic variation explained (PVE) of 22.5–36.3%. The corresponding CAPS marker was developed and verified in 205 wheat cultivars and showed a highly significant correlation with TKW.
Many QTL/genes have pleiotropic effects on multiple traits. Two QTL clusters (QSc/Sl.cib-5A and QSc/Sl.cib-6A) have pleiotropic effects on plant height, TKW and grain length; their related KASP markers might be potentially applicable in wheat breeding [85]. The FT-D1 gene controls spikelet number and heading date, and a robust STARP marker was reported to simplify and streamline MAS for this gene in wheat breeding [86]. A pleiotropic gene TaCol-B5 can not only increase the number of spikelet nodes per spike, but can also produce more tillers and spikes, thereby improving the yield of transgenic wheat under field conditions. It was found in only 33 of 1657 stocks from a global collection of wheat germplasms. A diagnostic marker identifying the SNP involving the Ser269/Gly269 substitution was developed to plausibly accelerate this rare allele in a variety of genetic backgrounds [88].
3.4. Loci for Grain Quality
Wheat grain protein content (GPC) is a major end-use quality. Wheat quality can be improved by the manipulation of the main storage protein genes, many of which have been efficiently utilized for wheat quality improvement, such as GluD1 (5 + 10) [111] and GluB1 (17 + 18) [112]. A robust and reliable KASP marker toolkit was reported for HMW-GS at Glu-A1, Glu-B1 and Glu-D1 loci; polyphenol oxidase (PPO) activity genes (Ppo-A1 and Ppo-D1) [113], which can distantly facilitate the selection and stacking of excellent genes in wheat breeding programs. Jiang et al. [88] successfully developed three KASP markers (Kgpc-2B, Kgpc-2D and Kgpc-4A) associated with GPC, which were applied to screen 15 lines with high GPC from 164 F6 breeding lines, indicating their high selective efficiency.
Breeding wheat with a weak and extensible gluten feature is another aspect aiming to improve biscuit making quality. Nap Hal is an Indian wheat landrace with weak gluten because of Glu-D1 double null [89]. The codominant marker (gwm642) tightly linked with Glu-D1 double null could be applied to transferring it into high yielding backgrounds.
Wheat black point is unfavorable to grain appearance, processing and product quality. A locus, QBp.caas-3BL, for black point resistance was finely mapped in an interval of 1.7 Mb by 90K SNP array and the converted KASP marker from this study can be used for the selection of QBp.caas-3BL [90].
Pre-harvest sprouting is another aspect to ultimately affect the end product quality. Three novel QTL for pre-harvest sprouting tolerance (PHST) were detected based on the phenotypes of 192 wheat varieties (lines) and the corresponding genotypes [91]. SNP markers from 90K SNP array were tightly linked with these major QTL (Qphs.ahau-1A, Qphs.ahau-3B and Qphs.ahau-6B) and further converted to two CAPS and one dCAPS markers. The CAPS marker EX06323 for Qphs.ahau-6B co-segregated with a novel major QTL underlying PHST in a RIL population raised from the cross Jing 411/Wanxianbaimaizi. In addition, the allele EX06323-G revealed a highly significant correlation with all PHST traits in 374 wheat varieties.
Grain micronutrient content is also an important trait for the nutritional quality improvement for achieving biofortification. QTL and related markers for improving Fe or Zn contents in wheat were identified [114]. Among the many QTL/genes for Fe, Zn and Se contents in another review, two key QTL deserve attention, QGZn.cimmyt-7B_1P2 and QGFe.cimmyt-4A_P2, which explained the largest PVE of 32.7% for Zn and 21.14% for Fe, respectively [115].
4. Cases of MAS in wheat breeding
Using the linked markers related to key agronomic traits, wheat breeders could select their interested QTL/genes and rapidly pyramid them to a background by MAS. Currently, most stacking genes belong to disease resistance and a few quality loci, while the application of other yield-related genes has rarely been reported, such as TKW, probably due to uncertain hereditary effects. Some examples of MAS are shown in Table 2.
Using MAS, Fhb1 from H-SA (Hartog/Sumai 3, an Fhb1-Sr2 recombinant) has successfully introgressed into Quaiu, Munal, Super 152 and other leading cultivars by breeders at CIMMYT MAS. Breeders at the Chinese Academy of Agricultural Sciences (CAAS) have also introduced Fhb1 into other elite lines, such as Jimai 22 and Zhoumai 16, through MAS [66]. Three powdery mildew-susceptible wheat cultivars (Ningchun4, Ningchun47 and Ningchun50) were hybridized with a powdery mildew resistant line CB037 (carrying Pm21 from Dasypyrum villosum) [116]. Nine elite lines with strong powdery mildew resistance were developed by molecular marker selection, after backcrossing five times and four generations of selfing. Using linked markers Xbarc32, Xwgp5175, Xwmc557 and Xcfa2040, Yr59 from wheat line PI660061 was successfully introgressed into four advanced wheat varieties (Chuanmai 42, Jimai 22, Xinmai 26 and Zhengmai 9023), and 16 introgression lines showed higher levels of stripe rust resistance and better agronomic traits compared with their parents [117].

Table 2.
Examples of using MAS stacking some key genes.
Table 2.
Examples of using MAS stacking some key genes.
| Genes for MAS | Receptor | Marker Type | Reference |
|---|---|---|---|
| Fhb1 | Quaiu, Munal, Super 152, Jimai 22, Zhoumai 16 | KASP [67] | [66] |
| Pm21 | Ningchun4, Ningchun47, Ningchun50 | EST-STS | [116] |
| Yr59 | Chuanmai 42, Jimai 22, Xinmai 26, Zhengmai 9023 | SSR | [117] |
| Yr70/Lr76, Lr37/Yr17/Sr38, Gpc-B1/Yr36, QPhs.ccsu-3A.1, QGw.ccsu-1A.3, Lr24/Sr24 and Glu-A1-1/Glu-A1-2 | PBW343 | SSR/CAPS | [118] |
| ML91260, Yr26, Dx5 + Dy10 | Xiaoyan22 | SSR | [119] |
| Bx7OE, Gpc-B1 | CWHWS | SSR/CAPS | [120] |
| Ax2*/Bx7OE/Dx5 | JM22 | SSR | [121] |
Five grain quality and eight rust resistance genes were transferred into an erstwhile Indian wheat PBW343, including Yr70/Lr76, Lr37/Yr17/Sr38, Gpc-B1/Yr36, QPhs.ccsu-3A.1, QGw.ccsu-1A.3, Lr24/Sr24 and Glu-A1-1/Glu-A1-2, by crossing two improved lines [118]. Among 11 selected lines, one line (CCSU-7) showed superior grain yield and grain protein content compared with PBW343. The powdery mildew resistance gene (ML91260), stripe rust resistance gene (Yr26) and high molecular-weight glutenin subunits Dx5 + Dy10 were stacked into the dwarf mutant of Xiaoyan22, an elite wheat cultivar [119]. The pyramided wheat lines had more lodging and disease resistance, longer dough stability time and higher yield than Xiaoyan22.
Gpc-B1 and Glu-B1 loci encoding Bx7OE submit were combined in the background of CWHWS cultivars by MAS, improving GPC by 0.8–1.1% [120]. Yao et al. [121] stacked three high-quality subunit genes, Ax2*/Bx7OE/Dx5, into JM22 based on multiplex PCR systems, which can be used for the MAS of wheats with strong gluten.
5. Considerations in the Application of MAS
Indirect breeding by MAS has obvious advantages over conventional breeding in some aspects [122,123]. For example, MAS allows breeders to pyramid multiple disease genes in one cultivar to increase the durability of resistance, while it is difficult to evaluate and choose the gene-tagged plants by traditional phenotypic screening because of the existence of an additional disease gene. Since assays can be conducted at any time, it greatly improves selection efficiency to identify individual plants carrying target genes at early generations.
Despite the feasibility of MAS applications, the verification of putative QTL should be conducted before considering their applications [11]. Some markers linked to certain traits might only be useful for one gene pool and can only be used in one background. MAS also requires polymorphisms in the parents, so breeders should determine the robustness and reliability of molecular markers on differentiating the genotypes of the parent material before the program. In most cases, low levels of marker polymorphism are usually a problem for the germplasm based on a narrow gene pool [124]. The linkage tightness between markers and target genes should be also considered, in case gene recombination events make markers ineffective. Neutral DNA genetic markers might not be close to the targeted genes and their predictive value is affected by the degree of linkage between target genes and markers, while functional markers are completely linked with target genes and accurately discriminate their alleles.
Owing to the complexity of the wheat genome, the efficiency of MAS is also affected by the genetic attributes of the target traits. QTL with large effects in early generations is balanced by a higher rate of fixation of unfavorable alleles of QTL with small effects in later generations [11]. At present, the majority of the loci used in MAS control qualitative traits, such as many disease resistance genes, are Rht genes and a few quality loci. Few successful reports focused on transferring or pyramiding TKW genes directly by MAS. Therefore, it deserves serious considerations from breeders on how to find resultful loci for all the useful traits other than those for biotic resistances only.
A good molecular marker should be accurate, highly reproducible, high-throughput, cost-effective and labor-saving. As for the construction of a high-density linkage map and genetic mapping, SNP markers from sequencing (such as GBS) and arrays (such as 660K chip) are better than the conventional marker (e.g., SSR), because SNP markers are high quantity, high density, have high genetic stability and are easy for automatic detection. When the linked markers are applied in MAS, florescence-based KASP is a better choice than other gel-based markers at present, because KASP markers are more high-throughput, cost-effective and labor-saving. However, the visualization of this marker relies on expensive equipment, either a real-time PCR machine or a plate reader.
Given the fund inputs, breeders have their own marker platforms of gel-based, fluorescence-based detection or chip-based identification. For example, the linked SNP of a certain trait can be converted to gel-based STARP or florescence-based KASP markers.
6. Future Prospects
Conventional breeding is completely phenotype-based, time-consuming and inefficient. The MAS of molecular breeding is considered a research field that has become an effective and beneficial methodology, aiding plant breeders in achieving their targets of interest.
The booming wheat genomics will accelerate marker development, QTL mapping and gene cloning, as well as breeding in wheat. Many Triticeae species have been sequenced and released, i.e., A subgenome T. urartu [125], D subgenome Ae. tauschii [126], Tibetan semi-wild wheat Z1817 [127], Chinese Spring (IWGSC RefSeq v1.0 and v2.1), 10+genome (https://www.10wheatgenomes.com (accessed on 30 November 2022)) [128] and cultivar Kenong9204 [129]. A total of 667 wheat varieties have been re-sequenced (http://wheat.cau.edu.cn/WheatUnion/b_4/ (accessed on 30 November 2022)) and other datasets, such as RNA-seq, are available. These sequences make it easier to develop perfect molecular markers linked with target traits and increase the efficiency of MAS. The innovation of NGS technology also provides multiple high-throughput tools for gene discovery and marker development, such as GBS, resequencing [130] and exome sequencing [131].
It is of critical significance to establish simple, accurate, cost-effective and whole-genome ultra-high-throughput genotyping platforms for marker-assisted breeding. Wheat breeding platforms will adopt automated genotyping technologies, either chips or other genotyping platforms, explore functional polymorphisms of key traits, provide unprecedented opportunities to facilitate the accurate prediction and cloning of genes and finally enhance the universal applications under wide genetic backgrounds in the future. NGS technologies (e.g., GBS and exome capture), solid arrays (e.g., 660K SNPs) and liquid arrays (e.g., GBTS) provide effective, accurate and cost-cutting choices to perform genomic selection and target gene identification. KASP platform is of substantial interest to wheat researchers globally and seems to be a better choice in single marker detection due to its cost-effectiveness and high-throughput. Developing segregating populations and ‘pure lines’ is a fundamental element in wheat breeding, and it typically takes up to ten years to obtain new, advanced cultivars in conventional breeding [9]. To break this bottleneck, speed breeding (SB) could be used to shorten the breeding cycle and accelerate crop research through rapid generation advancement. By adopting extended photoperiod (22 h light/2 h dark) in a controlled environment room, up to four–six generations per year can be achieved [132]. The utilization of embryo rescue even allows the production of up to eight generations per year [133]. By exposing seeds at the soil surface and an extended photoperiod of 22 h day/2 h night at 10 °C, a vernalization method can accelerate the vernalization process of winter wheat [134]. Despite shortened breeding cycle time, it is almost impossible to evaluate the agronomic traits and obtain the desirable genotypes due to the abnormal growing environment. Therefore, MAS is an indispensable tool to track the target genes.
In conclusion, wheat breeding will be targeted, highly effective and large-scale in gene discovery and selection, marker-assisted identification and plant growth. The platforms are more portable, cost-effective, automatic, high-throughput and intelligent. In terms of genomic selection, huge amounts of sequencing data or customized flexible and high-flux platforms (e.g., GenoBaits) accelerate gene discoveries. Many different kinds of genes can be introgressed or pyramided for generating climate-resilient wheat cultivars with high yield and specific nutritional quality by MAS. During this process, KASP array, or even more high-throughput technology will be chosen for marker identification. In the future, the technological innovations might markedly make sequencing at very low cost, so sequencing-based detection would be the ultimate choice [55].
Author Contributions
Writing—original draft preparation, L.S.; writing—review and editing, R.W., X.Y., A.Z. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hebei Natural Science Foundation (22322913D, C2022204202, 2021HBQZYCXY005) and the Talents Program of Hebei Agricultural University in China (YJ2021016, YJ2021017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, L.; Zheng, Y.; Wang, Y.; Wang, S.; Wang, T.; Wang, C.; Chen, Y.; Zhang, K.; Zhang, N.; Dong, Z.; et al. A HST1-like gene controls tiller angle through regulating endogenous auxin in common wheat. Plant Biotechnol. J. 2023, 21, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Xia, X. From markers to genome-based breeding in wheat. Theor. Appl. Genet. 2019, 132, 767–784. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, B.; Yao, Y.; Guo, Z.; Jia, H.; Kong, L.; Zhang, A.; Ma, W.; Ni, Z.; Xu, S.; et al. Wheat genomic study for genetic improvement of traits in China. Sci. China Life Sci. 2022, 65, 1718–1775. [Google Scholar] [CrossRef]
- Peng, J.; Richards, D.; Hartley, N.; Murphy, G.; Devos, K.; Flintham, J.; Beales, J.; Fish, L.J.; Worland, A.J.; Pelica, F.; et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 1999, 400, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Loukoianov, A.; Tranquilli, G.; Helguera, M.; Fahima, T.; Dubcovsky, J. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 2003, 100, 6263–6268. [Google Scholar] [CrossRef] [PubMed]
- Mohler, V.; Lukman, R.; Ortiz-Islas, S.; William, M.; Worland, A.J.; van Beem, J.; Wenzel, G. Genetic and physical mapping of photoperiod insensitive gene Ppd-B1 in common wheat. Euphytica 2004, 138, 33–40. [Google Scholar] [CrossRef]
- Simmonds, J.; Scott, P.; Brinton, J.; Mestre, T.C.; Bush, M.; Blanco, A.D.; Dubcovsky, J.; Uauy, C. A splice acceptor site mutation in TaGW2-A1 increases thousand grain weight in tetraploid and hexaploid wheat through wider and longer grains. Theor. Appl. Genet. 2016, 129, 1099–1112. [Google Scholar] [CrossRef]
- Khalid, M.; Afzal, F.; Gul, A.; Amir, R.; Subhani, A.; Ahmed, Z.; Mahmood, Z.; Xia, X.; Rasheed, A.; He, Z. Molecular characterization of 87 functional genes in wheat diversity panel and their association with phenotypes under well-watered and water-limited conditions. Front. Plant Sci. 2019, 10, 717. [Google Scholar] [CrossRef]
- Anderson, J.A. Marker-assisted selection for Fusarium head blight resistance in wheat. Int. J. Food Microbiol. 2007, 119, 51–53. [Google Scholar] [CrossRef]
- Kuchel, H.F.R.; Hollamby, G.; Reinheimer, J.L.; Jefferies, S.P. The challenges of integrating new technologies into a wheat breeding programme. In 11th International Wheat Genetics Symposium, Proceedings of 11th International Wheat Genet Symposium, Brisbane, Australia, 24–29 August 2008; Appels, R., Eastwood, R., Lagudah, E., Langridge, P., Mackay, M., McIntyre, L., Sharp, P., Eds.; Sydney University Press: Sydney, Australia, 2008; pp. 1–5. [Google Scholar]
- Liu, S.; Banik, M.; Yu, K.; Park, S.J.; Poysa, V.; Guan, Y. Marker-assisted selection (MAS) in major cereal and legume crop breeding: Current progress and future directions. Int. J. Plant Breed. 2007, 1, 74–88. [Google Scholar]
- Paux, E.; Sourdille, P.; Mackay, I.; Feuillet, C. Sequence-based marker development in wheat: Advances and applications to breeding. Biotechnol. Adv. 2012, 30, 1071–1088. [Google Scholar] [CrossRef] [PubMed]
- Devos, K.M.; Gale, M.D. The use of random amplified polymorphic DNA markers in wheat. Theor. Appl. Genet. 1992, 84, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, S.W.C.; Nilsen, K.; Kumar, S. Introduction to Marker-Assisted Selection in Wheat Breeding. In Accelerated Breeding of Cereal Crops; Springer Protocols Handbooks; Bilichak, A., Laurie, J.D., Eds.; Humana: New York, NY, USA, 2022. [Google Scholar]
- Geng, H.; Shi, J.; Fuerst, E.P.; Wei, J.; Morris, C.F. Physical mapping of Peroxidase genes and development of functional markers for TaPod-D1 on bread wheat chromosome 7D. Front. Plant Sci. 2019, 10, 523. [Google Scholar] [CrossRef]
- Zhang, R.; Yao, R.; Sun, D.; Sun, B.; Feng, Y.; Zhang, W.; Zhang, M. Development of V chromosome alterations and physical mapping of molecular markers specific to Dasypyrum villosum. Mol. Breed. 2017, 37, 67. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Lu, Y.; Liu, Q.; Yang, X.; Li, X.; Li, L. A resource of large-scale molecular markers for monitoring Agropyron cristatum chromatin introgression in wheat background based on transcriptome sequences. Sci. Rep. 2017, 7, 11942. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Q.; Zhang, J.; Wang, S.; Chen, C.; Wang, C.; Zhang, H.; Wang, Y.; Ji, W. Cytogenetic analysis and molecular marker development for a new wheat–Thinopyrum ponticum 1Js (1D) disomic substitution line with resistance to stripe rust and powdery mildew. Front. Plant Sci. 2020, 11, 1282. [Google Scholar] [CrossRef]
- Gupta, P.K.; Varshney, R.K. The development and use of microsatellite markers for genetic analysis and plant breeding with emphasis on bread wheat. Euphytica 2000, 113, 163–185. [Google Scholar] [CrossRef]
- Wu, P.; Hu, J.; Zou, J.; Qiu, D.; Qu, Y.; Li, Y.; Li, T.; Zhang, H.; Yang, L.; Liu, H.; et al. Fine mapping of the wheat powdery mildew resistance gene Pm52 using comparative genomics analysis and the Chinese Spring reference genomic sequence. Theor. Appl. Genet. 2019, 132, 1451–1461. [Google Scholar] [CrossRef]
- Zheng, W.Y.; Zhao, L.; Li, Y.M.; Li, J.; Zhu, Z.H.; Yao, D.N. Association analysis between Lipoxygenase activity and SSR markers in wheat grains. Cereal Res. Commun. 2021, 50, 297–303. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Z.; Du, Z.; Che, M.; Zhang, Y.; Quan, W.; Wang, Y.; Jiang, X.; Zhang, Z. Detection and validation of a novel major QTL for resistance to Fusarium head blight from Triticum aestivum in the terminal region of chromosome 7DL. Theor. Appl. Genet. 2019, 132, 241–255. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Choudhary, S.; Hakki, E.E.; Akkaya, M.S.; Thomas, G. From RFLP to DArT: Molecular tools for wheat (Triticum spp.) diversity analysis. Genet. Resour. Crop Ev. 2014, 61, 1001–1032. [Google Scholar] [CrossRef]
- Lehnert, H.; Berner, T.; Lang, D.; Beier, S.; Stein, N.; Himmelbach, A.; Kilian, B.; Keilwagen, J. Insights into breeding history, hotspot regions of selection, and untapped allelic diversity for bread wheat breeding. Plant J. 2022, 112, 897–918. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.R.; Qureshi, N.; Pourkheirandish, N. Genotyping by Sequencing Advancements in Barley. Front. Plant Sci. 2022, 13, 931423. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yuan, Y.; Wang, H.; Yu, D.; Liu, Y.; Zhang, A.; Gowda, M.; Nair, S.K.; Hao, Z.; Lu, Y.; et al. Applications of genotyping-by-sequencing (GBS) in maize genetics and breeding. Sci. Rep. 2020, 10, 16308. [Google Scholar] [CrossRef]
- Reyes, V.P.; Kitonv, J.K.; Nishiuchi, S.; Makihara, D.; Doi, K. Utilization of Genotyping-by-Sequencing (GBS) for Rice Pre-Breeding and Improvement: A Review. Life 2022, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Niu, F.; Xu, Y.; Liu, X.; Zhao, L.; Bernardo, A.; Li, Y.; Liu, G.; Chen, M.S.; Cao, L.; Hu, Z.; et al. The Hessian fly recessive resistance gene h4 mapped to chromosome 1A of the wheat cultivar ‘Java’ using genotyping-by-sequencing. Theor. Appl. Genet. 2020, 133, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, X.; Xu, Y.; Bernardo, A.; Chen, M.; Li, Y.; Niu, F.; Zhao, L.; Bai, G. Reassigning Hessian fly resistance genes H7 and H8 to chromosomes 6A and 2B of the wheat cultivar ‘Seneca’ using genotyping-by-sequencing. Crop Sci. 2020, 60, 1488–1498. [Google Scholar] [CrossRef]
- Liu, Y.; Salsman, E.; Fiedler, J.D.; Hegstad, J.B.; Green, A.; Mergoum, M.; Zhong, S.; Li, X. Genetic mapping and prediction analysis of FHB resistance in a hard red spring wheat breeding population. Front. Plant Sci. 2019, 10, 1007. [Google Scholar] [CrossRef]
- Tomar, V.; Singh, D.; Dhillon, G.S.; Singh, R.P.; Poland, J.; Joshi, A.K.; Singh, P.K.; Bhati, P.K.; Kumar, S.; Rahman, M.; et al. New QTLs for spot blotch disease resistance in wheat (Triticum aestivum L.) using genome-wide association mapping. Front. Genet. 2021, 11, 613217. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Sun, Y.; Li, D.; Gao, D.; Zhan, K.; Cheng, S. Identification of quantitative trait loci for Fusarium head blight (FHB) resistance in the cross between wheat landrace N553 and elite cultivar Yangmai 13. Mol. Breed. 2021, 41, 24. [Google Scholar] [CrossRef]
- Mu, J.; Huang, S.; Liu, S.; Zeng, Q.; Dai, M.; Wang, Q.; Wu, J.; Yu, S.; Kang, Z.; Han, D. Genetic architecture of wheat stripe rust resistance revealed by combining QTL mapping using SNP-based genetic maps and bulked segregant analysis. Theor. Appl. Genet. 2019, 132, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Li, Y.; Guo, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Liu, L. Genetic mapping by integration of 55K SNP array and KASP markers reveals candidate genes for important agronomic traits in hexaploid wheat. Front. Plant Sci. 2021, 12, 628478. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.; Ren, Y.; Gao, F.; Yin, G.; Liu, J.; Guo, L.; Zheng, J.; He, Z.; Xia, X. Mapping and validation of a new QTL for adult-plant resistance to powdery mildew in Chinese elite bread wheat line Zhou8425B. Theor. Appl. Genet. 2018, 131, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, W.; Zhang, N.; Chen, M.; Zheng, S.; Zhao, C.; Han, J.; Liu, J.; Zhang, X.; Song, L.; et al. Identification of QTL regions for seedling root traits and their effect on nitrogen use efficiency in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2018, 131, 2677–2698. [Google Scholar] [CrossRef] [PubMed]
- Winfield, M.O.; Allen, A.M.; Burridge, A.J.; Barker, G.L.A.; Benbow, H.R.; Wilkinson, P.A.; Coghill, J.; Waterfall, C.; Davassi, A.; Scopes, G.; et al. High-density SNP genotyping array for hexaploid wheat and its secondary and tertiary gene pool. Plant Biotechnol. J. 2016, 14, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Winfield, M.O.; Burridge, A.J.; Downie, R.C.; Benbow, H.R.; Barker, G.L.A.; Wilkinson, P.A.; Coghill, J.; Waterfall, C.; Davassi, A.; et al. Characterization of a wheat breeders’ array suitable for high-throughput SNP genotyping of global accessions of hexaploid bread wheat (Triticum aestivum). Plant Biotechnol. J. 2017, 15, 390–401. [Google Scholar] [CrossRef]
- Sun, C.; Dong, Z.; Zhao, L.; Ren, Y.; Zhang, N.; Chen, F. The Wheat 660K SNP array demonstrates great potential for marker-assisted selection in polyploid wheat. Plant Biotechnol. J. 2020, 18, 1354–1360. [Google Scholar] [CrossRef]
- Yang, Y.; Basnet, B.R.; Ibrahim, A.M.H.; Rudd, J.C.; Chen, X.; Bowden, R.L.; Xue, Q.; Wang, S.; Johnson, C.D.; Metz, R.; et al. Developing KASP markers on a major stripe rust resistance QTL in a popular wheat TAM 111 using 90K array and genotyping-by-sequencing SNPs. Crop Sci. 2019, 59, 165–175. [Google Scholar] [CrossRef]
- Tan, C.; Yu, H.; Yang, Y.; Xu, X.; Chen, M.; Rudd, J.; Xue, Q.; Ibrahim, A.M.; Garza, L.; Wang, S.; et al. Development and validation of KASP markers for the greenbug resistance gene Gb7 and the Hessian fly resistance gene H32 in wheat. Theor. Appl. Genet. 2017, 130, 1867–1884. [Google Scholar] [CrossRef]
- Schneeberger, K. Using next-generation sequencing to isolate mutant genes from forward genetic screens. Nat. Rev. Genet. 2014, 15, 662–676. [Google Scholar] [CrossRef]
- He, F.; Pasam, R.; Shi, F.; Kant, S.; Keeble-Gagnere, G.; Kay, P.; Forrest, K.; Fritz, A.; Hucl, P.; Wiebe, K.; et al. Exome sequencing highlights the role of wild-relative introgression in shaping the adaptive landscape of the wheat genome. Nat. Genet. 2019, 51, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, Y.; Beier, S.; Jiang, Y.; Thorwarth, P.H.; Longin, C.F.; Ganal, M.; Himmelbach, A.; Reif, J.C.; Schulthess, A.W. Exome association analysis sheds light onto leaf rust (Puccinia triticina) resistance genes currently used in wheat breeding (Triticum aestivum L.). Plant Biotechnol. J. 2020, 18, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Cobo, N.; Wanjugi, H.; Lagudah, E.; Dubcovsky, J. A high-resolution map of wheat QYr.ucw-1BL, an adult plant stripe rust resistance locus in the same chromosomal region as Yr29. Plant Genome. 2019, 12, 180055. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Howell, T.; Vasquez-Gross, H.; de Haro, L.A.; Dubcovsky, J.; Pearce, S. Mapping causal mutations by exome sequencing in a wheat TILLING population: A tall mutant case study. Mol. Genet. Genom. 2018, 293, 463–477. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, L.; Chen, Z.; Xia, C.; Gu, Y.; Wang, J.; Li, D.; Xie, Z.; Zhang, Q.; Zhang, X.; et al. Combining a new exome capture panel with an effective varBScore Algorithm accelerates BSA-based gene cloning in wheat. Front. Plant Sci. 2020, 11, 1249. [Google Scholar] [CrossRef]
- Kaur, B.; Mavi, G.S.; Gill, M.S.; Saini, D.K. Utilization of KASP technology for wheat improvement. Cereal Res. Commun. 2020, 48, 409–421. [Google Scholar] [CrossRef]
- Hrubá, M. dCAPS method: Advantages, troubles and solution. Plant Soil Environ. 2007, 53, 417–420. [Google Scholar] [CrossRef]
- Long, Y.M.; Chao, W.S.; Ma, G.J.; Xu, S.S.; Qi, L.L. An innovative SNP genotyping method adapting to multiple platforms and throughputs. Theor. Appl. Genet. 2017, 130, 597–607. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; He, Z.; Dreisigacker, S.; Wen, W.; Jin, H.; Zhai, S.; Li, F.; Gao, F.; Liu, J.; et al. Development and validation of high-throughput and low-cost STARP assays for genes underpinning economically important traits in wheat. Theor. Appl. Genet. 2020, 133, 2431–2450. [Google Scholar] [CrossRef]
- Rasheed, A.; Hao, Y.; Xia, X.; Khan, A.; Xu, Y.; Varshney, R.K.; He, Z. Crop breeding chips and genotyping platforms: Progress, challenges, and perspectives. Mol. Plant 2017, 10, 1047–1064. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, H.; Tao, J.; Ren, Y.; Xu, C.; Wu, K.; Zou, C.; Zhang, J.; Xu, Y. Development of multiple SNP marker panels affordable to breeders through genotyping by target sequencing (GBTS) in maize. Mol. Breed. 2019, 39, 37. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Q.; Zheng, H.; Xu, Y.; Sang, Z.; Guo, Z.; Peng, H.; Zhang, C.; Lan, H.; Wang, Y.; et al. Genotyping by target sequencing (GBTS) and its applications. Sci. Agric. Sin. 2020, 53, 15. [Google Scholar]
- Hou, J.; Liu, Y.; Hao, C.; Li, T.; Liu, H.; Zhang, X. Starch metabolism in wheat: Gene variation and association analysis reveal additive effects on kernel weight. Front Plant. Sci. 2020, 11, 562008. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Li, H.; Wang, J.; Zhao, J.; Zheng, X.; Wu, B.; Du, W.; Wang, J.; Zheng, J. Analysis of genetic regions related to field grain number per spike from Chinese wheat founder parent Linfen 5064. Front Plant. Sci. 2022, 12, 808136. [Google Scholar] [CrossRef] [PubMed]
- Arabzai, M.G.; Gul, H. Application techniques of molecular marker and achievement of marker assisted selection (MAS) in three major crops rice, wheat and maize. Int. J. Appl. Sci. Biotechnol. 2021, 8, 82–93. [Google Scholar] [CrossRef]
- Gupta, P.K.; Langridge, P.; Mir, R.R. Marker-assisted wheat breeding: Present status and future possibilities. Mol. Breed. 2010, 26, 145–161. [Google Scholar] [CrossRef]
- Cao, S.; Xu, D.; Hanif, M.; Xia, X.; He, Z. Genetic architecture underpinning yield component traits in wheat. Theor. Appl. Genet. 2020, 133, 1811–1823. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Appels, R.; Xia, X. Functional markers in wheat: Current status and future prospects. Theor. Appl. Genet. 2012, 125, 1–10. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Liu, S.; Hall, M.D.; Griffey, C.A.; McKendry, A.L. Meta-Analysis of QTL Associated with Fusarium Head Blight Resistance in Wheat. Crop Sci. 2009, 49, 1955–1968. [Google Scholar] [CrossRef]
- Bai, G.; Su, Z.; Cai, J. Wheat resistance to Fusarium head blight. Can. J. Plant Pathol. 2018, 40, 336–346. [Google Scholar] [CrossRef]
- Cuthbert, P.A.; Somers, D.J.; Thomas, J.; Cloutier, S.; Brulé-Babel, A. Fine mapping Fhb1, a major gene controlling fusarium head blight resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 112, 1465. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, X.; Hou, Y.; Cai, J.; Shen, X.; Zhou, T.; Xu, H.; Ohm, H.W.; Wang, H.; Li, A.; et al. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor. Appl. Genet. 2015, 128, 2301–2316. [Google Scholar] [CrossRef]
- Hao, Y.; Rasheed, A.; Zhu, Z.; Wulff, B.B.H.; He, Z. Harnessing wheat Fhb1 for Fusarium resistance. Trends Plant Sci. 2020, 25, 1–3. [Google Scholar] [CrossRef]
- Su, Z.; Jin, S.; Zhang, D.; Bai, G. Development and validation of diagnostic markers for Fhb1 region, a major QTL for Fusarium head blight resistance in wheat. Theor. Appl. Genet. 2018, 131, 2371–2380. [Google Scholar] [CrossRef]
- Li, H.; Zhang, F.; Zhao, J.; Bai, G.; Amand, P.S.; Bernardo, A.; Ni, Z.; Sun, Q.; Su, Z. Identification of a novel major QTL from Chinese wheat cultivar Ji5265 for Fusarium head blight resistance in greenhouse. Theor. Appl. Genet. 2022, 135, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Xia, X.C.; Raupp, W.J.V. Catalogue of gene symbols for wheat. Annu. Wheat Newsl. 2022, 68, 68–81. [Google Scholar]
- Zhao, F.; Li, Y.; Yang, B.; Yuan, H.; Jin, C.; Zhou, L.; Pei, H.; Zhao, L.; Li, Y.; Zhou, Y.; et al. Powdery mildew disease resistance and marker-assisted screening at the Pm60 locus in wild diploid wheat Triticum urartu. Crop J. 2020, 8, 252–259. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, N.; Wang, H.; Shi, X.; Li, F.; Zhang, Q.; Wang, W.; Guo, W.; Hu, Z.; Li, H.; et al. Fine mapping of a powdery mildew resistance gene MlIW39 derived from wild emmer wheat (Triticum turgidum ssp. dicoccoides). Theor. Appl. Genet. 2021, 134, 2469–2479. [Google Scholar] [CrossRef]
- Wang, W.; He, H.; Gao, H.; Xu, H.; Song, W.; Zhang, X.; Zhang, L.; Song, J.; Liu, C.; Liu, K.; et al. Characterization of the powdery mildew resistance gene in wheat breeding line KN0816 and its evaluation in marker-assisted selection. Plant Dis. 2021, 105, 4042–4050. [Google Scholar] [CrossRef]
- Du, X.; Xu, W.; Peng, C.; Li, C.; Zhang, Y.; Hu, L. Identification and validation of a novel locus, Qpm-3BL, for adult plant resistance to powdery mildew in wheat using multilocus GWAS. BMC Plant Biol. 2021, 21, 357. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.S.; McCallum, B.D.; Hiebert, C.W. Development of single nucleotide polymorphism-based functional molecular markers from the Lr22a gene sequence in wheat (Triticum aestivum). Plant Breed. 2022, 141, 204–211. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, X.; Gebrewahid, T.W.; Zhou, Y.; Yang, E.; Xia, X.; He, Z.; Li, Z.; Liu, D. Genome-wide association mapping of leaf rust and stripe rust resistance in wheat accessions using the 90K SNP array. Theor. Appl. Genet. 2021, 134, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Yao, F.; Guan, F.; Cheng, Y.K.; Duan, L.; Zhao, X.; Li, H.; Pu, Z.; Li, W.; Jiang, Q.; et al. A stable QTL on chromosome 5BL combined with Yr18 conferring high-level adult-plant resistance to stripe rust in Chinese wheat landrace Anyuehong. Phytopathology 2021, 111, 1594–1601. [Google Scholar] [CrossRef]
- Gill, B.K.; Klindworth, D.L.; Rouse, M.N.; Zhang, J.; Zhang, Q.; Sharma, J.S.; Chu, C.; Long, Y.; Chao, S.; Olivera, P.D.; et al. Function and evolution of allelic variations of Sr13 conferring resistance to stem rust in tetraploid wheat (Triticum turgidum L.). Plant J. 2021, 106, 1674–1691. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, Y.F.; Li, C.A.; Chen, X.; Yang, L.L.; Zhang, J.; Wang, J.Y.; Li, L.; Reynolds, M.P.; Jing, R.L.; et al. Transcription factor TaWRKY51 is a positive regulator in root architecture and grain yield contributing traits. Front. Plant Sci. 2021, 12, 734614. [Google Scholar] [CrossRef]
- Pang, Y.; Wu, Y.; Liu, C.; Li, W.; Amand, P., St.; Bernardo, A.; Wang, D.; Dong, L.; Yuan, X.; Zhang, H.; et al. High-resolution genome-wide association study and genomic prediction for disease resistance and cold tolerance in wheat. Theor. Appl. Genet. 2021, 134, 2857–2873. [Google Scholar] [CrossRef]
- Würschum, T.; Longin, C.F.H.; Hahn, V.; Tucker, M.R.; Leiser, W.L. Copy number variations of CBF genes at the Fr-A2 locus are essential components of winter hardiness in wheat. Plant J. 2017, 89, 764–773. [Google Scholar] [CrossRef]
- Fu, L.P.; Xiao, Y.G.; Yan, J.; Liu, J.D.; Wen, W.E.; Zhang, Y.; Xia, X.C.; He, Z.H. Characterization of TaCOMT genes associated with stem lignin content in common wheat and development of a gene-specific marker. J. Integr. Agric. 2019, 18, 939–947. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.; Geng, Y.; Wang, Y.; Liu, Y.; Li, H.; Hao, C.; Wang, H.; Shang, X.; Zhang, X. Identification and development of a KASP functional marker of TaTAP46-5A associated with kernel weight in wheat (Triticum aestivum). Plant Breed. 2021, 140, 585–594. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Mao, X.; Zhang, J.; Liu, Y.; Xie, Q.; Yang, X.; Chang, X.; Li, C.; Zhang, X.; et al. RING finger ubiquitin E3 ligase gene TaSDIR1-4A contributes to determination of grain size in common wheat. J. Exp. Bot. 2020, 71, 5377–5388. [Google Scholar] [CrossRef]
- Duan, X.; Yu, H.; Ma, W.; Sun, J.; Zhao, Y.; Yang, R.; Ning, T.; Li, Q.; Liu, Q.; Guo, T.; et al. A major and stable QTL controlling wheat thousand grain weight: Identification, characterization, and CAPS marker development. Mol Breed. 2020, 40, 68. [Google Scholar] [CrossRef]
- Li, T.; Deng, G.; Su, Y.; Yang, Z.; Tang, Y.; Wang, J.; Qiu, X.; Pu, X.; Li, J.; Liu, Z.; et al. Identification and validation of two major QTLs for spike compactness and length in bread wheat (Triticum aestivum L.) showing pleiotropic effects on yield-related traits. Theor. Appl. Genet. 2021, 134, 3625–3641. [Google Scholar] [CrossRef]
- Chen, Z.; Ke, W.; He, F.; Chai, L.; Cheng, X.; Xu, H.; Wang, X.; Du, D.; Zhao, Y.; Chen, X.; et al. A single nucleotide deletion in the third exon of FT-D1 increases the spikelet number and delays heading date in wheat (Triticum aestivum L.). Plant Biotechnol. J. 2022, 20, 920–933. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, H.; Li, T.; Wu, J.; Nagarajan, R.; Lei, L.; Powers, C.; Kan, C.; Hua, W.; Liu, Z.; et al. TaCol-B5 modifies spike architecture and enhances grain yield in wheat. Science 2022, 376, 180–183. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, P.; Wu, L.; He, Y.; Li, C.; Ma, H.; Zhang, X. Linkage and association mapping and Kompetitive allele-specific PCR marker development for improving grain protein content in wheat. Theor. Appl. Genet. 2021, 134, 3563–3575. [Google Scholar] [CrossRef]
- Ram, S.; Devi, R.; Singh, R.B.; Narwal, S.; Singh, B.; Singh, G.P. Identification of codominant marker linked with Glu-D1 double null and its utilization in improving wheat for biscuit making quality. J. Cereal Sci. 2019, 90, 102853. [Google Scholar] [CrossRef]
- Liu, C.; Song, J.; Liu, S.; Liu, J.; Xu, D.; Tian, X.; Bian, Y.; Dong, Y.; Wang, F.; Wang, R.; et al. Molecular mapping and characterization of QBp.caas-3BL for black point resistance in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2021, 134, 3279–3286. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Wei, W.; Xie, H.; Liu, K.; Zhang, C.; Wu, Z.; Jiang, H.; Cao, J.; Zhao, L.; et al. Genome-wide association study of pre-harvest sprouting tolerance using a 90K SNP array in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2019, 132, 2947–2963. [Google Scholar] [CrossRef]
- Kumar, S.; Bhardwaj, S.C.; Gangwar, O.P.; Sharma, A.; Qureshi, N.; Kumaran, V.V.; Khan, H.; Prasad, P.; Miah, H.; Singh, G.P.; et al. Lr80: A new and widely effective source of leaf rust resistance of wheat for enhancing diversity of resistance among modern cultivars. Theor. Appl. Genet. 2021, 134, 849–858. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, X.; Wang, F.; He, Y.; Feng, L.; Jiang, B.; Hao, M.; Ning, S.; Yuan, Z.; Wu, J.; et al. Development and validation of gene-specific KASP markers for YrAS2388R conferring stripe rust resistance in wheat. Euphytica 2021, 217, 206. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Quan, W.; Zhang, X.; Feng, J.; Ren, J.; Jiang, X.; Zhang, Z. Mapping of a QTL with major effect on reducing leaf rust severity at the adult plant growth stage on chromosome 2BL in wheat landrace Hongmazha. Theor. Appl. Genet. 2021, 134, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Li, C.N.; Li, L.; Reynolds, M.P.; Wang, J.Y.; Chang, X.P.; Mao, X.G.; Jing, R.L. Recognizing the hidden half in wheat: Root system attributes associated with drought tolerance. J. Exp. Bot. 2021, 72, 5117–5133. [Google Scholar] [CrossRef]
- Ma, J.; Luo, W.; Zhang, H.; Zhou, X.H.; Qin, N.N.; Wei, Y.M.; Liu, Y.X.; Jiang, Q.T.; Chen, G.Y.; Zheng, Y.L.; et al. Identification of quantitative trait loci for seedling root traits from Tibetan semi-wild wheat (Triticum aestivum subsp tibetanum). Genome 2017, 60, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Fleury, D.; Jefferies, S.; Kuchel, H.; Langridge, P. Genetic and genomic tools to improve drought tolerance in wheat. J. Exp. Bot. 2010, 61, 3211–3222. [Google Scholar] [CrossRef]
- Rehman, S.U.; Sher, M.A.; Saddique, M.A.; Ali, Z.; Khan, M.A.; Mao, X.G.; Irshad, A.; Sajjad, M.; Ikram, R.M.; Naeem, M.; et al. Development and exploitation of KASP Assays for genes underpinning drought tolerance among wheat cultivars from Pakistan. Front. Genet. 2021, 12, 684702. [Google Scholar] [CrossRef] [PubMed]
- Sihag, P.; Sagwal, V.; Kumar, A.; Balyan, P.; Mir, R.R.; Dhankher, O.P.; Kumar, U. Discovery of miRNAs and development of heat-responsive miRNA-SSR markers for characterization of wheat germplasm for terminal heat tolerance breeding. Front. Genet. 2021, 12, 699420. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Blechl, A.; Tranquill, G.; Ramakrishna, W.; SanMiguel, P.; Bennetzen, J.L.; Echenique, V.; Dubcovsky, J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef]
- Yan, L.; Fu, D.; Li, C.; Blechl, A.; Tranquilli, G.; Bonafede, M.; Sanchez, A.; Valarik, M.; Yasuda, S.; Dubcovsky, J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 2006, 103, 19581–19586. [Google Scholar] [CrossRef]
- Tian, X.; Xie, L.; Fu, L.; Xu, D.; Fu, C.; Wang, D.; Chen, X.; Xia, X.; Chen, Q.; He, Z.; et al. Molecular mapping of reduced plant height gene Rht24 in bread wheat. Front. Plant Sci. 2017, 8, 1379. [Google Scholar] [CrossRef]
- Hou, J.; Li, T.; Wang, Y.; Hao, C.; Liu, H.; Zhang, X. ADP-glucose pyrophosphorylase genes, associated with kernel weight, underwent selection during wheat domestication and breeding. Plant Biotechnol. J. 2017, 15, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, H.; Hao, C.; Wang, K.; Wang, Y.; Qin, L.; An, D.; Li, T.; Zhang, X. TaDA1, a conserved negative regulator of kernel size, has an additive effect with TaGW2 in common wheat (Triticum aestivum L.). Plant Biotechnol. J. 2020, 18, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, Y.; Wu, T.T.; Zhang, G.L.; Yin, H.; Chen, W.; Wang, S.; Chang, F.; Gou, J.Y. Cloning of wheat keto-acyl thiolase 2B reveals a role of jasmonic acid in grain weight determination. Nat. Commun. 2020, 11, 6266. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Yang, W.; Shan, Q.; Sun, L.; Wang, D.; Sajjad, M.; Li, X.; Sun, J.; Liu, D.; Zhan, K.; et al. TaCKX gene family, at large, is associated with thousand-grain weight and plant height in common wheat. Theor. Appl. Genet. 2020, 133, 3151–3163. [Google Scholar] [CrossRef]
- Li, F.J.; He, Z.H.; Liu, J.D.; Jin, H.; Cao, S.H.; Geng, H.W.; Yan, J.; Zhang, P.Z.; Wan, Y.X.; Xia, X.C. Genome-wide linkage mapping of yield-related traits in three Chinese bread wheat populations using high-density SNP markers. Theor. Appl. Genet. 2018, 131, 1903–1924. [Google Scholar] [CrossRef]
- Song, J.; Xu, D.; Dong, Y.; Li, F.; Bian, Y.; Li, L.; Luo, X.; Fei, S.; Li, L.; Zhao, C.; et al. Fine mapping and characterization of a major QTL for grain weight on wheat chromosome arm 5DL. Theor. Appl. Genet. 2022, 135, 3237–3246. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, L.; Liu, D.; Zeng, J.; Cao, S.; Xia, X.; Yan, J.; Song, X.; He, Z.; Zhang, Y. Fine mapping and validation of a major QTL for grain weight on chromosome 5B in bread wheat. Theor. Appl. Genet. 2021, 134, 3731–3741. [Google Scholar] [CrossRef] [PubMed]
- Tura, H.; Edwards, J.; Gahlaut, V.; Garcia, M.; Sznajder, B.; Baumann, U.; Shahinnia, F.; Reynolds, M.; Langridge, P.; Balyan, H.S.; et al. QTL analysis and fine mapping of a QTL for yield-related traits in wheat grown in dry and hot environments. Theor. Appl. Genet. 2020, 133, 239–257. [Google Scholar] [CrossRef]
- Altpeter, F.; Popelka, J.C.; Wieser, H. Stable expression of 1Dx5 and 1Dy10 high-molecular-weight glutenin subunit genes in transgenic rye drastically increases the polymeric glutelin fraction in rye flour. Plant Mol. Biol. 2004, 54, 783–792. [Google Scholar] [CrossRef]
- Payne, P.I. Genetics of wheat storage proteins and the effect of allelic variation on bread-making quality. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1987, 38, 141–153. [Google Scholar] [CrossRef]
- Rasheed, A.; Wen, W.; Gao, F.; Zhai, S.; Jin, H.; Liu, J.; Guo, Q.; Zhang, Y.; Dreisigacker, S.; Xia, X.; et al. Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Biofortification and bioavailability of Zn, Fe and Se in wheat: Present status and future prospects. Theor. Appl. Genet. 2021, 134, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, K.B.; Rani, S.; Kumar, M.; Gupta, V.; Babu, P.H.; Bainsla, N.K.; Yadav, R. Enhancing the nutritional quality of major food crops through conventional and genomics-assisted breeding. Front. Nutr. 2020, 7, 533453. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhang, S.; Li, S.; Wang, J.; Chen, H.; Wang, K.; Lin, Z.; Wei, Y.; Du, L.; Yan, Y. Improvement of three commercial spring wheat varieties for powdery mildew resistance by marker-assisted selection. Crop Prot. 2019, 125, 104889. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, T.; Zhou, X.; Chen, X.; Li, X.; Feng, J.; Yang, S.; Kang, Z. Combination of marker-assisted backcross selection of Yr59 and phenotypic selection to improve stripe rust resistance and agronomic performance in four elite wheat cultivars. Agronomy 2022, 12, 497. [Google Scholar] [CrossRef]
- Gautam, T.; Dhillon, G.S.; Saripalli, G.; Rakhi; Singh, V.P.; Prasad, P.; Kaur, S.; Chhuneja, P.; Sharma, P.K.; Balyan, H.S.; et al. Marker-assisted pyramiding of genes/QTL for grain quality and rust resistance in wheat (Triticum aestivum L.). Mol. Breed. 2020, 40, 49. [Google Scholar] [CrossRef]
- Zheng, W.; Li, S.; Liu, Z.; Zhou, Q.; Feng, Y.; Chai, S. Molecular marker assisted gene stacking for disease resistance and quality genes in the dwarf mutant of an elite common wheat cultivar Xiaoyan22. BMC Genet. 2020, 21, 45. [Google Scholar] [CrossRef]
- Brar, G.S.; Pozniak, C.J.; Briggs, C.; Hucl, P.J. Combined selection of Gpc-B1 and Glu-B1 locus encoding the Bx7OE subunit for improving end-use quality of hard white wheat. J. Cereal Sci. 2021, 100, 103260. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, C.; Bi, C.; Zhou, S.; Dong, F.; Liu, Y.; Yang, F.; Jiao, B.; Zhao, H.; Lyu, M.; et al. Establishment and application of multiplex PCR systems based on molecular markers for HMW-GSs in wheat. Agriculture 2022, 12, 556. [Google Scholar] [CrossRef]
- William, H.M.; Trethowan, R.; Crosby-Galvan, E.M. Wheat breeding assisted by markers: CIMMYT’s experience. Euphytica 2007, 157, 307–319. [Google Scholar] [CrossRef]
- Miedaner, T.; Korzun, V. Marker-assisted selection for disease resistance in wheat and barley breeding. Phytopathology 2012, 102, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Langridge, P.; Lagudah, E.S.; Holton, T.A.; Appels, R.; Sharp, P.L.; Chalmers, K.J. Trends in genetic and genome analyses in wheat: A review. Aust. J. Agric. Res. 2001, 52, 1043–1077. [Google Scholar] [CrossRef]
- Ling, H.Q.; Ma, B.; Shi, X.; Liu, H.; Dong, L.; Sun, H.; Cao, Y.; Gao, Q.; Zheng, S.; Li, Y.; et al. Genome sequence of the progenitor of wheat A subgenome Triticum urartu. Nature 2018, 557, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhao, S.; Kong, X.; Li, Y.; Zhao, G.; He, W.; Appels, R.; Pfeifer, M.; Tao, Y.; Zhang, X.; et al. Aegilops tauschii draft genome sequence reveals a gene repertoire for wheat adaptation. Nature 2013, 496, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Xin, M.; Wang, Z.; Yao, Y.; Hu, Z.; Song, W.; Yu, K.; Chen, Y.; Wang, X.; Guan, P.; et al. Origin and adaptation to high altitude of Tibetan semi-wild wheat. Nat. Commun. 2020, 11, 5085. [Google Scholar] [CrossRef]
- Walkowiak, S.; Gao, L.; Monat, C.; Haberer, G.; Kassa, M.T.; Brinton, J.; Ramirez-Gonzalez, R.H.; Kolodziej, M.C.; Delorean, E.; Thambugala, D.; et al. Multiple wheat genomes reveal global variation in modern breeding. Nature 2020, 588, 277–283. [Google Scholar] [CrossRef]
- Shi, X.; Cui, F.; Han, X.; He, Y.; Zhao, L.; Zhang, N.; Zhang, H.; Zhu, H.; Liu, Z.; Ma, B.; et al. Comparative genomic and transcriptomic analyses uncover the molecular basis of high nitrogen-use efficiency in the wheat cultivar Kenong 9204. Mol. Plant 2022, 15, 1440–1456. [Google Scholar] [CrossRef]
- Nave, M.; Taş, M.; Raupp, J.; Tiwari, V.K.; Ozkan, H.; Poland, J.; Hale, I.; Komatsuda, T.; Distelfeld, A. The independent domestication of Timopheev’s wheat: Insights from haplotype analysis of the Brittle rachis 1 (BTR1-A) gene. Genes 2021, 12, 338. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, H.; Zhang, J.; Guo, H.; Zhou, C.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; et al. Genome-wide and exome-capturing sequencing of a gamma-ray-induced mutant reveals biased variations in common wheat. Front. Plant Sci. 2022, 12, 793496. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, H.B.; Chen, G.D.; Yan, G.J.; Liu, C.J. A procedure allowing up to eight generations of wheat and nine generations of barley per annum. Euphytica 2013, 191, 311–316. [Google Scholar] [CrossRef]
- Cha, J.K.; O’Connor, K.; Alahmad, S.; Lee, J.H.; Dinglasan, E.; Park, H.; Lee, S.M.; Hirsz, D.; Kwon, S.W.; Kwon, Y.; et al. Speed vernalization to accelerate generation advance in winter cereal crops. Mol. Plant 2022, 15, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).