Abstract
To reduce the application of phosphorus fertilizer and improve phosphorus efficiency in peanut (Arachis hypogaea L.) production, six peanut varieties with different phosphorus activation efficiencies were selected, and the root morphology, physiological indexes, and types and content of organic acids secreted were measured via a hydroponic experiment for 20 days. We analyzed the difference in calcium phosphate activation between peanut seedlings cultivated under low-phosphorus (LP, 0.01 mmol/L KH2PO4) and normal phosphorus (NP, 0.6 mmol/L KH2PO4) conditions and explored the physiological mechanisms of different peanut varieties on the activation efficiency of insoluble inorganic phosphorus. The results showed that under LP conditions, the root length, root surface area, root volume, root tip number, and root activity of the efficient P activation varieties were 18.31%, 17.50%, 15.23%, 20.00%, and 50.90% higher than those of the inefficient P activation varieties respectively. The reduction range of the nutrient solution pH of the high-efficiency varieties was 74.48% higher than that of the low-efficiency varieties under LP conditions. The total amount of organic acid secreted by the efficient P activation varieties increased by 236.07% on average under LP conditions compared with that under NP conditions. In comparison, the average increase in inefficient P activation varieties was only 16.36%. Under low P stress, the peanut varieties with high-efficiency P activation could increase the activation of insoluble inorganic P in the environment mainly by changing the root architecture and increasing the root-shoot ratio, root activity, and root proton and organic acid secretion.
1. Introduction
Phosphorus is an indispensable nutrient in crop growth, participating in various physiological and biochemical processes in plants in various forms [1,2]. As one of the three major oil crops in China, peanut (Arachis hypogaea L.) is renowned for its high nutritional value and abundant oil and protein content. It plays an important role in our agricultural economy and daily lives. Peanuts are a phosphorus-loving crop [3], and phosphorus plays a crucial role in the growth and physiological processes of peanuts [4]. There are many P fixation mechanisms in soil, including chemical precipitation, surface reaction, adsorption, and biological absorption. Therefore, various fast-acting phosphorus compounds are easily converted into slow-acting or ineffective compounds in soil [5], making it difficult for plant roots to directly absorb and utilize phosphorus in soil [6,7,8]. Soil P deficiency is not a lack of total P, but a lack of available P available to crops. The lack of available P in soil has become one of the most significant factors restricting crop production [9].
In most soils, inorganic P accounts for about 50–90% of the total amount of soil P. Inorganic P in soil can be subdivided into calcium phosphate (Ca-P), aluminum phosphate (Al-P), and iron phosphate (Fe-P), all of which are not easy for plants to use [10]. However, crops can activate insoluble phosphorus in the soil that is difficult to directly absorb. The rate of phosphorus activation reflects the ability of crops to activate insoluble phosphorus. Increasing the P activation rate is the key to improving the P availability of crops [11]. Improving the activation efficiency of inactive phosphorus in the environment can mitigate the problems caused by the excessive application of phosphorus fertilizer, such as water eutrophication [12].
The findings of previous studies have unequivocally demonstrated that a low-phosphorus environment contributes to the growth of roots, increases the APase activity secreted by roots, and promotes the efflux of flavonoids and organic acids exerted by roots, thereby promoting the recycling of insoluble phosphorus and organic phosphorus in soil [13]. Another study showed that maize lines from Embrapa can significantly alter root morphology under low-phosphorus conditions to enhance plant adaptability to low-phosphorus soils, while maize lines from DTMA reduce root length and surface area under phosphorus-deficient conditions [14], but the mechanism for this difference in peanuts has been scarcely studied. So, in this study, we examined the differences in biomass, the quantity of organic acids and protons secreted by roots exudate, and root morphology of peanut varieties with different P activation efficiencies under low P stress using the hydroponic method to explore the morphological and physiological mechanisms underlying the differences in the P activation efficiency of different peanut varieties. The objective was to identify advantageous physiological indicators of peanut varieties with high P activation efficiency. This research is of great significance for breeding new peanut varieties with enhanced phosphorus efficiency and optimizing the phosphorus fertilizer application technology applied to peanuts.
2. Materials and Methods
2.1. Location of Experiment
This research was conducted in September 2023 at the State Key Laboratory of Crop Biology, Shandong Agricultural University. (36°11′45″ N, 117°6′45″ E).
2.2. Material
In order to study the difference in the inorganic phosphorus activation ability of peanut varieties with different phosphorus activation efficiencies, six peanut varieties, including three high-efficiency phosphorus activation varieties (Fenghua 6, Luhua 12, and Shanhua 8) and three inefficient phosphorus activation varieties (Fenghua 2, 04H671, and Zhonghua 12), were used as plant materials. The P activation rates of the different peanut varieties were determined using an assay previously established in our laboratory [15]. Our group stored all the germplasms.
2.3. Experimental Design and Implementation
Initially, full and uniform-size peanut seeds were selected and then submerged in 0.1% hydrogen peroxide solution for 6 h. Subsequently, the peanut seeds were rinsed with deionized water four times and transferred to a germinating tray lined with absorbent cotton. Then, the tray was covered with plastic wrap after adding approximately 1 L of deionized water. After germination at 22–25 °C for 3 d in the dark, the peanut seeds were cultured with light applied until the cotyledon had fully unfolded. After the cotyledons had unfolded, the peanut seedlings were transferred to a 12-well hydroponic box containing 1.4 L of deionized water to balance for 3 d. Then, the peanut seedlings were cultured in Hoagland nutrient solution with low phosphorus (LP, 0.01 mmol/L KH2PO4) and normal phosphorus (NP, 0.6 mmol/L KH2PO4) for 16 d, respectively. During this 16 d period, the nutrient solution was changed every 4 d, the temperature was maintained at 24 ± 1 °C, and the light intensity was 20.9 Klux during the 16 h light cycle, followed by an 8-h darkness cycle.
After a 16-day treatment with LP and NP concentrations, the plants were transferred to a nutrient solution with a concentration of 2.86 mmol/L of calcium phosphate and a volume of 1.4 L. The pH of the nutrient solution was monitored daily. The calcium phosphate residual concentration in the nutrient solution, root morphology, phosphorus activation rate, root exudates, and other related parameters were determined after 4 d of treatment.
2.4. Determination Items and Methods
2.4.1. Peanut Root Morphological Index
The peanut plants were carefully removed from the hydroponic box, rinsed with deionized water, and then divided into ground and root parts. The root length, surface area, volume, average diameter, and number of root tips were analyzed using a WANSHEN root scanner.
2.4.2. Peanut Phosphorus Activation Rate
The P activation rate per plant (PAR, mg d−1 plant−1) [16] signifies the quantity of P activated per peanut plant in unit time. The specific approach is as follows: Firstly, the insoluble calcium phosphate adsorbed on the plant roots was washed off with deionized water. Secondly, the flushing solution was mixed with the nutrient solution, 10 mL of dilute hydrochloric acid was added to completely dissolve the insoluble calcium phosphate, the volume was fixed to 1.4 L, the mixture was stirred evenly using a glass rod, and then a 10 mL centrifugal tube was used for sampling. Lastly, the content of calcium phosphate in the nutrient solution was determined using the molybdenum antimony anti-colorimetric method.
Here, Q is the amount of insoluble phosphorus incorporated into the stock solution, C is the P concentration of the nutrient solution after activation, V is the volume of the nutrient solution after activation, h is the activation time, and N is the number of plants in each box.
Subsequently, the P activation rate per unit of root dry weight (PAR_RDW), the P activity rate per unit root length (PAR_RL), the P activation rate per unit root surface area (PAR_RSA), and the P activation rate per unit root volume (PAR_RV) were calculated further.
2.4.3. Root–Shoot Ratio
The above-ground and root portions of the plant were dried at 105 °C for half an hour and then at 80 °C for 48 h (until reaching a constant weight) separately, and the dry weight was determined to calculate the root–shoot ratio.
2.4.4. Root Activity
After 4 d of treatment with insoluble calcium phosphate, the aboveground and underground parts of the peanut seedlings were separated using scissors, and three complete roots were taken from each repeated treatment. Furthermore, 0.5 g of fresh peanut root tip samples with basically the same thickness and length were used to measure root activity via the staining method involving 2,3,5-triphenyl tetrazolium chloride (TTC) [17].
2.4.5. Root Secretion Capacity of H+
While being treated for 16 to 20 d, the nutrient solution pH values of each treatment were determined daily using a pH meter. Each treatment had three replicates, and each replicate was measured three times.
2.4.6. Collection and Determination of Organic Acids Secreted by Roots
The seedlings were carefully removed from the culture box, avoid inflicting root injuries. After washing the roots with deionized water, the roots were completely immersed in a triangle bottle containing 60 mL of distilled water (1 plant/bottle). Microbial inhibitors (thymol) were quickly added to the distilled water to suppress the decomposition of root secretions by microorganisms while keeping the root in darkness and exposing the aerial parts to light. After 2 h of reaction, the peanut seedlings were removed, and the collection solution was collected into a 15 mL centrifuge tube. The collection solution was blow-dried with nitrogen, and 1 mL of mobile phase was added; then, organic acid concentrations were determined using ultra-high performance liquid chromatography after filtration using a 0.22 μm filter membrane. In the ultra-high-performance liquid chromatography process, the flow rate was set to 1.0 mL min−1, the column temperature was set to room temperature, the sample size employed was 10 μL, and full-wavelength scanning was performed using a photo-diode array and octadecylsilyl. To ensure effective sample separation, detection was performed at 205 nm.
2.5. Statistical Analysis
Microsoft Excel 2016 and Origin 2022 were used for data analysis and chart generation. SPSS 26.0 statistical software was employed for descriptive statistical analysis, variance analysis, multiple comparisons (using the LSD method), and correlation analysis of the experimental data.
3. Results
3.1. Phosphorus Activation Rate of Different Peanut Varieties
There were significant differences in PAR, PAR_RDW, PAR_RL, PAR_RSA, and PAR_RV among the six peanut varieties under NP conditions (Table 1). The P activation rate per plant for the three high-P activation efficiency varieties were notably elevated compared to those of the three low-P activation efficiency varieties. Remarkably, Fenghua 6 had the highest P activation rate per plant, being 162.18% higher than that of 04H671, which had the lowest P activation rate per plant. Similarly, the P activation rate per unit root dry weight of the six peanut varieties also exhibited considerable variation, with the rate for the high-activation varieties being significantly lower than those of the low-activation varieties. The P activation rate per unit root length of the high-activation varieties were distinctly higher than those of the low-activation varieties, with the average rate for the three high-activation varieties being 162.90% higher than that of the low-activation varieties. Furthermore, there were significant differences in the P activation rate per unit root surface area among the six peanut varieties. Luhua 12, with the highest P activation rate per unit root surface area, had a value that was 313.00% higher than that of 04H671, which had the lowest. Lastly, the P activation rate per unit root volume of the high-activation varieties was significantly higher than those of the low-activation varieties. The average P activation rate per unit root volume of the three efficient P activation varieties was 239.60% higher than that of the inefficient P activation varieties.

Table 1.
The phosphorus activation rate of six peanut varieties under NP conditions.
There were significant differences in PAR, PAR_RDW, PAR_RL, PAR_RSA, and PAR_RV among the six peanut varieties under LP conditions (Table 2). The P activation rate per plant of the efficient P activation varieties were significantly higher than those of the inefficient P activation varieties. Shanhua 8 had the highest P activation rate, being 207.61% higher than that of Fenghua 2, which had the lowest P activation rate per plant. Furthermore, the varieties with high activation showed a significantly lower phosphorus activation rate per unit root dry weight compared to those with low activation. The three varieties with high P activation efficiency showed significant advantages in P activation rate per root surface area and per root length. The average P activation rate per root surface area of the efficient P activation varieties was 137.61% higher than that of the inefficient P activation varieties. In terms of P activation rate per unit root volume, the efficient P activation varieties were significantly better than the inefficient P activation varieties, with an average increase of 141.03%.

Table 2.
The phosphorus activation rate of six peanut varieties under LP conditions.
3.2. Root Morphology of Peanut Varieties with Different P Activation Efficiencies
The root length of the six peanut varieties had extremely significantly increased under LP conditions compared with those under NP conditions (Figure 1, Table 3). There were significant differences in root length among the different peanut varieties under the same phosphorus level (p < 0.001). Under LP conditions, the root length of the high-efficiency varieties were significantly longer than those of the low-efficiency varieties. The root length of the three high-efficiency varieties was 18.31% higher than that of the three low-efficiency varieties. There were extremely significant differences in root surface area among the treatments and varieties. Compared with that under NP conditions, the root surface area of the efficient variety Fenghua 6 under LP conditions increased by 19.54%, while that of the inefficient variety Fenghua 2 decreased by 2.06%. There were significant differences in root volume, average root diameter, and root tip number among the different peanut varieties under conditions with the same phosphorus level. However, there were no significant differences in root volume, mean root diameter, and number of root tips between LP and NP for the same varieties. Comparing the same peanut variety under LP and NP conditions (Table 3), the root length and root surface area of the six peanut varieties under LP conditions were significantly higher than those under NP conditions, but their root volume, mean diameter, and root tips were not significantly higher than those under NP conditions.
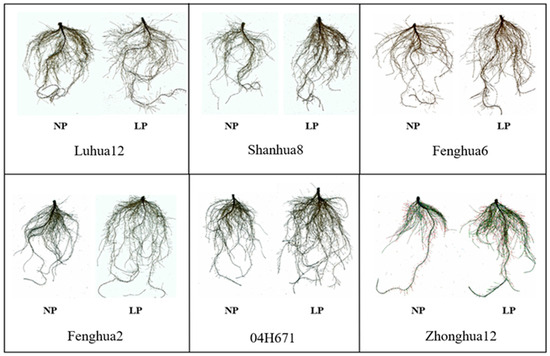
Figure 1.
Root morphology of six peanut varieties under NP and LP conditions.

Table 3.
Root morphological indexes of six peanut varieties under NP and LP conditions.
3.3. Root–Shoot Ratio of Peanut Varieties with Different P Activation Efficiencies
The root–shoot ratio is the ratio of the dry weight of a plant’s root to the dry weight of its aboveground part. Figure 2 shows that the root–shoot ratio of the three varieties with a high efficiency of phosphorus activation under LP conditions were significantly higher than those under NP conditions, with an average increase of 29.54%, among which Fenghua 6 had the highest increase of 32.09%. The increases in the root–shoot ratio of Luhua 12 and Shanhua 8 were 27.83% and 28.71%, respectively. The root–shoot ratio of the three varieties with low P activation efficiency did not significantly improve under LP conditions.
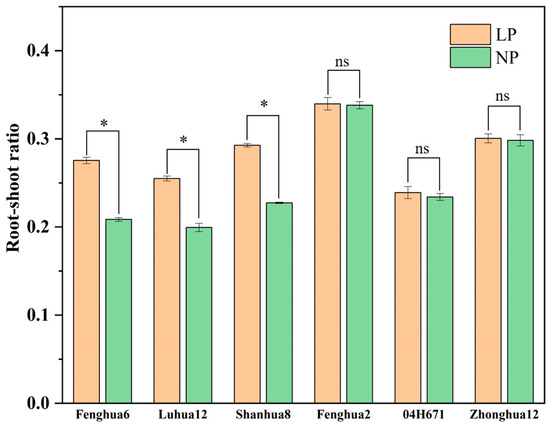
Figure 2.
Root–shoot ratio of six peanut varieties under NP and LP conditions. Note: “*” represents significant differences between the two treatments, while “ns” represents insignificant differences between the two treatments.
3.4. Root Activity of Peanut Varieties with Different P Activation Efficiencies
As shown in Figure 3, the root activity of the other five peanut varieties increased under LP conditions, except for that of Fenghua 2. Under NP conditions, Shanhua 8, Fenghua 2, and 04H671 had significantly higher root activity than the other varieties. Under LP conditions, the root activity of Luhua 12 and Shanhua 8 was significantly higher than that of the other varieties. The root activity of the high phosphorus activation varieties Fenghua 6, Luhua 12, and Shanhua 8 increased by 49.69%, 66.37%, and 40.91%, respectively, under LP conditions as compared with NP conditions. Compared with NP conditions, the root activity of 04H671 and Zhonghua 12 with low P activation efficiency increased by 1.59% and 14.22% under LP conditions, respectively, while that of Fenghua 2 with low P activation efficiency decreased by 13.85%. The increase in the root activity of the efficient P activation varieties was significantly higher than that of the inefficient P activation varieties.
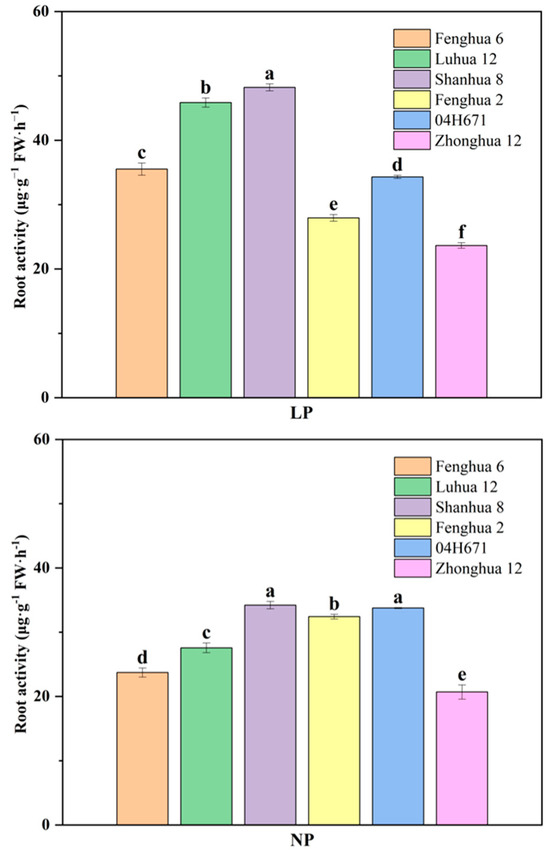
Figure 3.
Root activity of six peanut varieties under NP and LP conditions. Note: The different lowercase letter indicates that the difference has reached a significant level (p < 0.05).
3.5. H+ Secretion Ability Peanut Varieties with Different P Activation Efficiency
H+ secretion by peanut roots could keep the nutrient solution in a weakly acidic state, which was beneficial to the activation of insoluble calcium phosphate and its subsequent transformation into available phosphorus that could be directly utilized. The pH of the nutrient solution decreased under NP and LP conditions for all six peanut varieties, but the magnitudes of the decrease were different (Figure 4 and Figure 5). The varieties with efficient phosphorus activation (Fenghua 6, Luhua 12, and Shanhua 8) showed an average decrease in nutrient solution pH of 4.56% under NP conditions, and an average decrease in nutrient solution pH of 8.71% under LP conditions. The varieties with low-efficiency phosphorus activation (Fenghua 2, 04H671, and Zhonghua 12) showed an average decrease in nutrient solution pH of 4.12% under NP conditions and an average decrease in nutrient solution pH of 4.99% under LP conditions. Among them, the efficient variety Shanhua 8 exhibited a 384.30% higher decrease in the pH of nutrient solution under LP conditions than that under NP conditions, and the inefficient variety 04H671 exhibited a 0.81% higher decrease in the pH of the nutrient solution under LP conditions than that under NP conditions. These findings indicate that highly activated varieties exhibit superior H+ secretion ability under LP conditions, thereby facilitating the activation process of insoluble calcium phosphate.
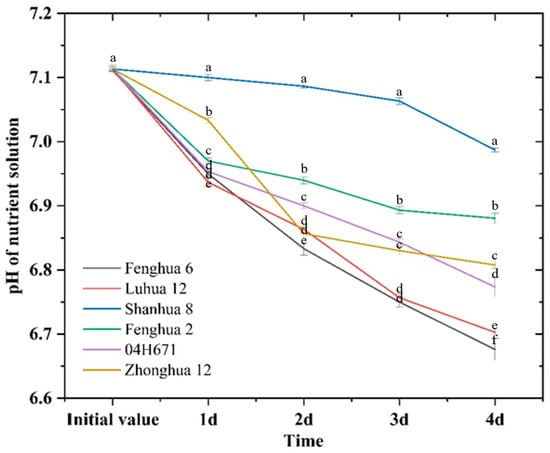
Figure 4.
pH variations of nutrient solution after the addition of calcium phosphate under NP conditions. Note: the different lowercase letters in each column indicates that the difference has reached a significant level (p < 0.05).
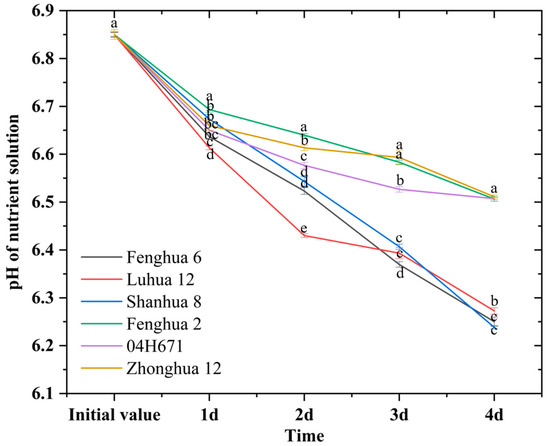
Figure 5.
pH variations of nutrient solution after the addition of calcium phosphate under LP conditions. Note: the different lowercase letters in each column indicates that the difference has reached a significant level (p < 0.05).
3.6. Organic Acids Secreted by Roots of Peanut Varieties with Different P Activation Efficiencies
Oxalic acid, tartaric acid, formic acid, and acetic acid were secreted by the six peanut varieties under both NP and LP conditions (Table 4). There were significant differences in the quantity of organic acids secreted by the roots of the different peanut varieties both under NP and LP conditions. The ratio of oxalic acid content to total organic acid secretion was the largest among the four detected organic acids. Under NP and LP conditions, the average oxalic acid secretion levels of the six peanut varieties accounted for 91.04% and 93.24% of the total amount of organic acid secretion, respectively. The levels of organic acids secreted by the three varieties with a high efficiency of phosphorus activation under LP conditions were 236.07% higher than those under NP conditions, while the levels of organic acids secreted by the three varieties with a low efficiency of phosphorus activation under LP conditions were 16.36% higher than those under NP conditions. Fenghua 6 was the peanut variety with the highest total amount of organic acid secretion in roots under both NP and LP conditions. Compared with that of roots under NP conditions, the total amount of organic acid secreted by the roots of the efficient variety Luhua 12 was strikingly increased by 649.82% under LP conditions, constituting the highest amount among all the varieties. The results showed that the P-activated efficient varieties could secrete more organic acids under LP conditions for the purpose of activating insoluble inorganic phosphorus in the low-phosphorus environment.

Table 4.
The types and concentrations of organic acids secreted by different peanut varieties under NP and LP conditions (mg/g).
3.7. Correlation Analysis of Root Morphology and Root Exudates in Peanut Varieties with Different P Activation Efficiencies
According to Figure 6, the P activation amount per plant was significantly positively correlated with the P activation rate per root length (0.61 *), root tip number (0.67 *), and root activity (0.65 *). It was very significantly positively correlated with root length (0.74 **), root surface area (0.76 **), the pH reduction value of the nutrient solution (0.82 **), and the organic acid amount secreted by roots (0.86 **) but very significant negatively correlated with P activation rate per root dry weight (−0.82 *). Additionally, the reduction in nutrient solution pH was very significant positively correlated with root length (0.77 **), root surface area (0.78 **), the amount of organic acids secreted by roots (0.82 **), and the number of root tips (0.62 **).
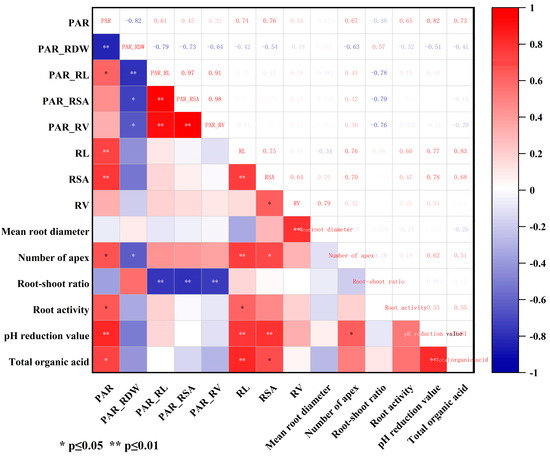
Figure 6.
Correlation analysis of root morphology and root exudates of 6 peanut varieties.
4. Discussion
4.1. Determination of the Amount of Activated Phosphorus
The difference between the two calcium phosphates over a four-day period lay in the amount of calcium phosphate activated in the peanut roots. This was calculated while operating on the assumption that the activated calcium phosphate was completely absorbed, but in the actual process, there is still activated but not absorbed available phosphorus left in the nutrient solution, which will lead to a relatively low activation amount of peanut measured, thus reducing the calculated activation rate of the peanut plants. Although this method still needs to be further optimized, it can accurately reflect the activation efficiency of peanut varieties with different phosphorus activation efficiencies and has little effect on the analysis of the physiological mechanism of their activation efficiency.
4.2. Root Morphology of Peanut Varieties under Different P Concentrations
Wu [18] found that plants have higher root plasticity under low P conditions so that they can better absorb phosphorus in the environment. Fan [19] pointed out that crops will expand their root system and increase their exploration of soil so as to obtain more phosphorus from phosphate-deficient soil. Meng [20] found that P deficiency significantly reduced the dry weight of leaves and stems of grapefruit (Citrus paradisi Mcfad.) seedlings, but the dry weight of roots remained unchanged, which ensured the ability of the roots to activate P. In this study, the root morphology of the six peanut varieties changed under the two different conditions tested, i.e., NP and LP, and the root lengths of the three high-efficiency phosphorus activation varieties under LP conditions were significantly higher than those of the three low-efficiency phosphorus activation varieties. The root lengths of the six peanut varieties under LP treatment were significantly longer than those under NP treatment, and this finding is consistent with the findings reported in a study by Ao [21]. Under LP conditions, there were significant differences in root surface area among the six peanut varieties. The root surface areas of the peanut varieties with efficient phosphorus activation were significantly higher than those of the peanut varieties with inefficient phosphorus activation. The increase in root surface area was conducive to increasing the contact area between peanut roots and the external environment and expanding the scope of rhizosphere soil. According to Nguyen [22], the higher root P concentration in wheat (Triticum aestivum L.) may be due to increased root length and decreased root diameter, which increase the surface area for P uptake. Maize (Zea mays L.) and soybean (Glycine max (L.) Merr.) had higher root biomass and root length and enhanced P spatial availability by expanding their root surface area. There was no significant difference in the root volume of the peanuts under different P concentrations, but there was a significant difference among the different peanut varieties, indicating that the root volume was mainly related to the tested variety. It is worth noting that Luhua 12 has the highest root volume and mean diameter under LP conditions among the six varieties. The high-efficiency phosphorus activation varieties exhibited superior root morphology compared to the low-efficiency phosphorus activation varieties. Specifically, the root length, root surface area, and root tip number of the high-efficiency phosphorus activation varieties were significantly higher than those of the low-efficiency phosphorus activation varieties. Greater root length and surface area aid peanut roots in coming into contact with more phosphorus in the environment, while a larger number of root tips facilitates phosphorus absorption by peanuts. Specifically, under LP conditions, both root length and root tips of Fenghua 6 are the highest among the six varieties, but Luhua 12 has the highest root surface area.
4.3. Organic Acid Secretion in Roots of Peanut Varieties under Different P Conditions
Previous studies have shown that crop roots are induced and secrete organic acids to reduce the pH of the rhizosphere when sensing low-P stress. Such an acidic environment is conducive to dissolving insoluble phosphate, converting it into soluble available P, and promoting plant P absorption [23,24,25,26]. The secretion of organic acids is part of a plant’s survival strategy for coping with nutrient deficiencies in the environment [27]. In this experiment, the total organic acid secretion of the high-efficiency phosphorus activation varieties was significantly higher than that of low-efficiency phosphorus activation varieties, indicating that high-efficiency phosphorus activation varieties have better adaptability under low-phosphorus conditions than the low-efficiency phosphorus activation varieties; this conclusion is consistent with the above research results. Leguminous plants such as pigeon pea (Cajanus cajan L. mills sp.) [28] and narrow-leafed lupin (Lupinus albus L.) [29] significantly increase their secretion of low-molecular-weight organic acids under low-phosphorus conditions. Studies have demonstrated that various organic acids exhibit differing capacities to activate insoluble phosphates, with citric acid demonstrating the highest efficacy, followed by oxalic acid, tartaric acid, malic acid, and acetic acid [30]. Mander [31] found that oxalic acid can chelate calcium bound to phosphate or compete with phosphate for adsorption sites through its hydroxyl and carboxyl groups. This mechanism results in the dissolution and increased availability of mineral phosphate [32]. In this study, four kinds of organic acids were detected, among which oxalic acid accounted for the largest proportion (83.48–96.85%), and this finding is consistent with the results of previous studies. The study by Zhao [33] revealed that plant roots secrete a larger quantity of organic acids under phosphorus deficiency conditions relative to those administered a phosphorus supply treatment, thereby enhancing the activation and absorption capacity of rice roots towards insoluble phosphorus. In this experiment, peanut roots secreted a greater amount of organic acid under the low-phosphorus (LP) treatment compared to that under the normal-phosphorus (NP) treatment. Specifically, the average total organic acid secretion of the high-efficiency varieties under low-phosphorus conditions was 122.37% higher than that of the low-efficiency varieties. The organic acid secretion ability of peanuts under LP conditions was higher than that under NP conditions, and the organic acid secretion ability of the varieties with a high efficiency of phosphorus activation was higher than that of the varieties with a low efficiency of phosphorus activation. It is worth noting that the secretion of oxalic, tartaric, formic, and acetic acid of Fenghua 6 under LP conditions was significantly higher than that of the other five varieties, and its secretion of total organic acids under LP and NP conditions was also significantly higher than that of the other varieties, which may be the reason for the high phosphorus activation efficiency of Fenghua 6, and this also the physiological mechanism of the difference in activation efficiency from the aspect of organic acid secretion in roots.
5. Conclusions
Peanut varieties with high-efficiency P activation could activate insoluble inorganic P by changing their root architecture, improving their root–shoot ratio and root activity, and increasing root H+ and organic acid secretion under low-phosphorus stress. The varieties with high P activation efficiency showed better adaptability than varieties with low P activation efficiency. The mechanisms by which different phosphorus activated efficient varieties respond to low-phosphorus stress are different. Specifically, under LP conditions, Fenghua 6 has a higher amount of organic acid secretion, Luhua12 has a good root morphology, and Shanhua 8 has a high root–shoot ratio and high root vitality. The traits identified in this study can serve as valuable screening criteria for evaluating the phosphorus activation efficiency of different peanut varieties, and the activated varieties with high phosphorus efficiency can be used as excellent materials for peanut breeding. However, the means by which these experimental results can be better applied under hydroponic conditions to production practice will be the focus of our future research.
Author Contributions
Conceptualization, Z.T. and K.Z.; formal analysis, S.Z.; funding acquisition, K.Z.; investigation, J.Z.; methodology, Z.T.; resources, F.L. and Y.W.; supervision, Y.W.; writing—original draft, Z.T.; writing—review and editing, K.Z. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Earmarked Fund for CARS-13 and the Agriculture Research System in Shandong Province of China, grant number SDAIT-04-03.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that are presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dissanayaka, D.M.S.B.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent insights into the metabolic adaptations of phosphorus-deprived plants. J. Exp. Bot. 2021, 72, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Oberson, A.; Culvenor, R.A.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; et al. Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant Soil 2011, 349, 89–120. [Google Scholar] [CrossRef]
- Naab, J.B.; Boote, K.J.; Jones, J.W.; Porter, C.H. Adapting and evaluating the CROPGRO-peanut model for response to phosphorus on a sandy-loam soil under semi-arid tropical conditions. Field Crops Res. 2015, 176, 71–86. [Google Scholar] [CrossRef]
- Kadirimangalam, S.R.; Jadhav, Y.; Nagamadhuri, K.V. Genetic approaches for assessment of phosphorus use efficiency in groundnut (Arachis hypogaea L.). Sci. Rep. 2022, 12, 21552. [Google Scholar] [CrossRef]
- George, T.S.; Hinsinger, P.; Turner, B.L. Phosphorus in soils and plants-facing phosphorus scarcity. Plant Soil 2016, 401, 1–6. [Google Scholar] [CrossRef]
- van de Wiel, C.C.M.; van der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2015, 207, 1–22. [Google Scholar] [CrossRef]
- Li, B.; Li, P.; Zeng, X.C.; Huang, Y.F.; Wang, G.Q.; Young, B.R. Assessing the sustainability of Phosphorus use in China: Flow patterns from 1980 to 2015. Sci. Total Environ. 2019, 704, 135305. [Google Scholar] [CrossRef]
- Ghodszad, L.; Reyhanitabar, A.; Reza, M.; Lajayer, B.A. Biochar affects the fate of phosphorus in soil and water: A critical review. Chemosphere 2021, 283, 131176. [Google Scholar] [CrossRef]
- Kellogg, L.E.; Bridgham, S.D. Phosphorus retention and movement across an ombrotrophic-minerotrophic peatland gradient. Biogeochemistry 2003, 63, 299–315. [Google Scholar] [CrossRef]
- Job, M.T.P.; Souza, J.L.; Oliveira, J.R.; Filho, J.F.L.; Oliveira, T.S. Changes in inorganic phosphorus fractions in weathered soils under long-term intensive cultivation and irrigation. Arch. Agron. Soil Sci. 2023, 69, 1177–1192. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Devau, N.; Hinsinger, P.; Cadre, E.L.; Colomb, B.; Gérard, F. Fertilization and pH effects on processes and mechanisms controlling dissolved inorganic phosphorus in soils. Geochim. Et Cosmochim. Acta 2011, 75, 2980–2996. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, C.; Zhao, X.; Yang, L.; Liu, C.; Jiang, L.; Liu, G.; Liu, P.; Luo, L. Multi-omics-based identification of purple acid phosphatases and metabolites involved in phosphorus recycling in stylo root exudates. Int. J. Biol. Macromol. 2023, 241, 124569. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.A.G.; de Sousa Tinoco, S.M.; de Souza, V.F.; Negri, B.F.; Gault, C.M.; Pastina, M.M.; Magalhaes, J.V.; Guimarães, L.J.M.; de Barros, E.G.; Buckler, E.S. Genome-Wide Association Study for Root Morphology and Phosphorus Acquisition Efficiency in Diverse Maize Panels. Int. J. Mol. Sci. 2023, 24, 6233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Luo, L.; Zhang, X.; Zhao, M.; Wang, X.; Zhang, J.; Wan, Q.; Li, X.; Wan, Y.; Zhang, K.; et al. Study on the Relationship of Root Morphology and Phosphorus Absorption Efficiency with Phosphorus Uptake Capacity in 235 Peanut (Arachis hypogaea L.) Germplasms. Front. Environ. Sci. 2022, 10, 855815. [Google Scholar] [CrossRef]
- Wu, Y. Identification and Evaluation of Phosphorus Efficiency of New Varieties from National Consortium for Peanut Seed Research. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2022. [Google Scholar] [CrossRef]
- Wang, S.; Wu, M.; Zhong, S.; Sun, J.; Mao, X.; Qiu, N.; Zhou, F. A Rapid and Quantitative Method for Determining Seed Viability Using 2,3,5-Triphenyl Tetrazolium Chloride (TTC): With the Example of Wheat Seed. Molecules 2023, 28, 6828. [Google Scholar] [CrossRef]
- Wu, A.; Fang, Y.; Liu, S.; Wang, H.; Xu, B.; Zhang, S.; Deng, X. Root morphology and rhizosheath acid phosphatase activity in legume and graminoid species respond differently to low phosphorus supply. Rhizosphere 2021, 19, 100391. [Google Scholar] [CrossRef]
- Fan, J.W.; Du, Y.L.; Turner, N.C.; Wang, B.R.; Fang, Y.; Xi, Y.; Guo, X.R.; Li, F.M. Changes in root morphology and physiology to limited phosphorus and moisture in a locally-selected cultivar and an introduced cultivar of Medicago sativa L. growing in alkaline soil. Plant Soil 2015, 392, 215–226. [Google Scholar] [CrossRef]
- Meng, X.; Chen, W.W.; Wang, Y.Y.; Huang, Z.R.; Ye, X.; Chen, L.S.; Yang, L.T. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef]
- Ao, J.; Fu, J.; Tian, J. Genetic variability for root morph-architecture traits and root growth dynamics as related to phosphorus efficiency in soybean. Funct. Plant Biol. 2010, 37, 304–312. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Stangoulis, J. Variation in root system architecture and morphology of two wheat genotypes is a predictor of their tolerance to phosphorus deficiency. Acta Physiol. Plant. 2019, 41, 109. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 1999, 211, 121–130. [Google Scholar] [CrossRef]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil 2019, 447, 135–156. [Google Scholar] [CrossRef]
- Justi, M.; Silva, C.A.; Rosa, S.D. Organic acids as complexing agents for iron and their effects on the nutrition and growth of maize and soybean. Arch. Agron. Soil Sci. 2022, 68, 1369–1384. [Google Scholar] [CrossRef]
- Raghothama, K.G.; Karthikeyan, A.S. Phosphate Acquisition. Plant Soil 2005, 274, 37–49. [Google Scholar] [CrossRef]
- Krishnappa, R.; Aftab Hussain, I.S. Phosphorus acquisition from deficient soil: Involvement of organic acids and acid phosphatase in pigeon pea (Cajanus cajan L. mills sp.). Indian J. Plant Physiol. 2014, 19, 197–204. [Google Scholar] [CrossRef]
- Chen, Y.; Dunbabin, V.M.; Diggle, A.J.; Siddique, K.H.M.; Rengel, Z. Phosphorus starvation boosts carboxylate secretion in P-deficient genotypes of Lupinus angustifolius with contrasting root structure. Crop Pasture Sci. 2013, 64, 588–599. [Google Scholar] [CrossRef]
- Dinkelaker, B.; Römheld, V.; Marschner, H. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant Cell Environ. 1989, 12, 285–292. [Google Scholar] [CrossRef]
- Mander, C.; Wakelin, S.; Young, S.; Condron, L.; O’Callaghan, M. Incidence and diversity of phosphate-solubilising bacteria are linked to phosphorus status in grassland soils. Soil Biol. Biochem. 2012, 44, 93–101. [Google Scholar] [CrossRef]
- Jalali, M.; Jalali, M. Effect of Low-Molecular-Weight Organic Acids on the Release of Phosphorus from Amended Calcareous Soils: Experimental and Modeling. J. Soil Sci. Plant Nutr. 2022, 22, 4179–4193. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, Y. Rhizosphere calcareous soil P-extraction at the expense of organic carbon from root-exuded organic acids induced by phosphorus deficiency in several plant species. Soil Sci. Plant Nutr. 2014, 60, 640–650. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).