Impact of Cypermethrin (Arpon G) on Soil Health and Zea mays Growth: A Microbiological and Enzymatic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Characterization
2.2. Experimental Design
2.3. Characteristics of the Insecticide Arpon G
2.4. DNA Isolation and Identification of Bacteria and Fungi Using the NGS Method
2.5. Enzymatic Analyses of Soils
2.6. Data Analysis and Statistical Processing
3. Results
3.1. The Effect of Cypermethrin on Z. mays Biomass and Greenness Index
3.2. The Reaction of Bacteria and Fungi to Cypermethrin and Z. mays
3.2.1. Distribution Characteristics of the Soil Bacterial Community
3.2.2. Distribution Characteristics of Soil Fungal Community
3.3. Response of Soil Enzymes to Cypermethrin and Z. mays
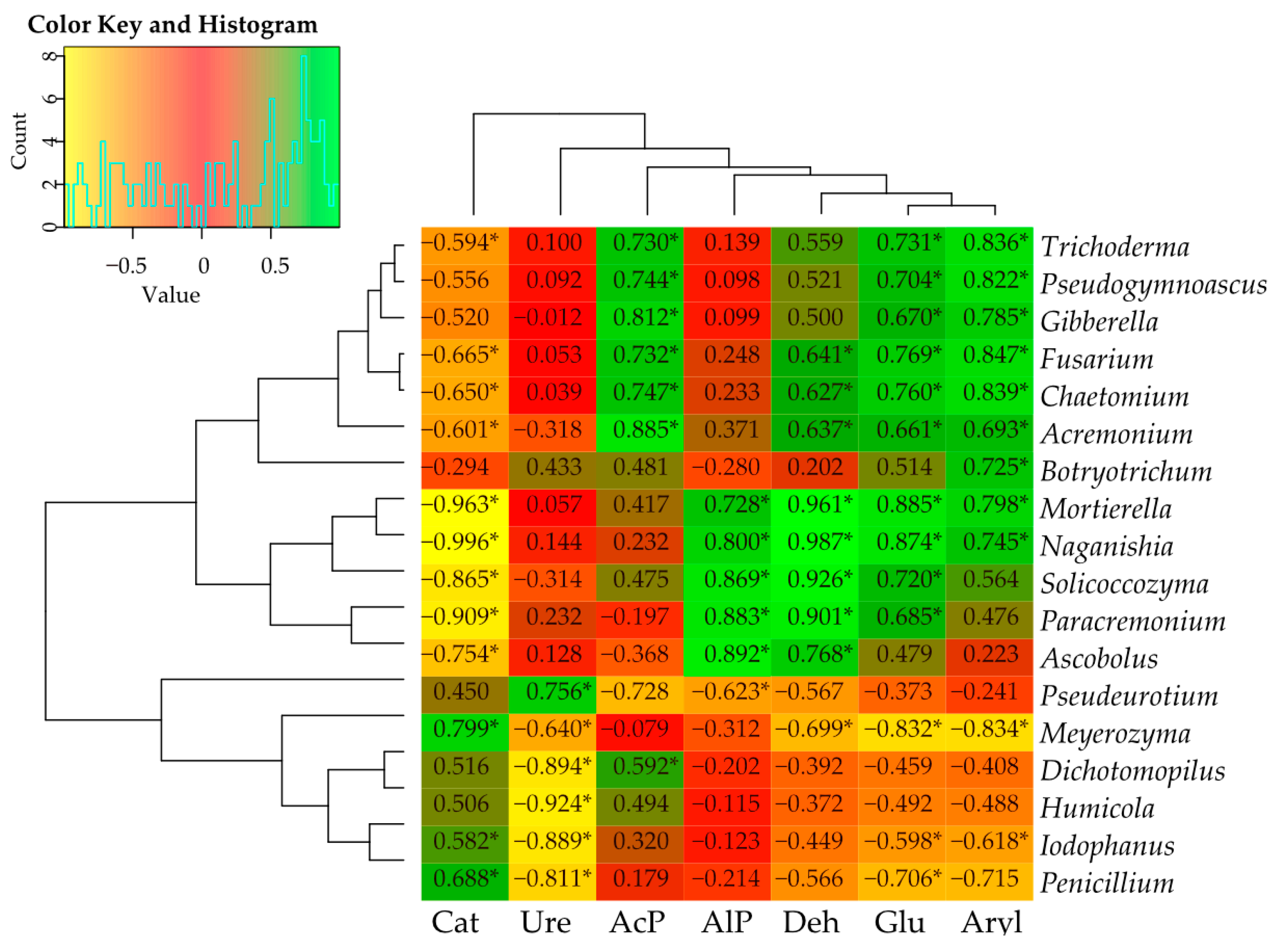
3.4. Interdependence between Bacteria and Fungi and Soil Enzyme Activity at the Genus Level
3.4.1. Correlation between Bacteria and Fungi
3.4.2. Correlation between Bacteria and Soil Enzymes
3.4.3. Correlation between Fungi and Soil Enzymes
4. Discussion
4.1. The Effect of Arpon-G on the Growth and Development of Z. mays
4.2. Response of Bacteria and Fungi to Cypermethrin
4.3. Response of Soil Enzymes to Cypermethrin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ashraf, S.A.; Siddiqui, A.J.; Elkhalifa, A.E.O.; Khan, M.I.; Patel, M.; Alreshidi, M.; Moin, A.; Singh, R.; Snoussi, M.; Adnan, M. Innovations in Nanoscience for the Sustainable Development of Food and Agriculture with Implications on Health and Environment. Sci. Total Environ. 2021, 768, 144990. [Google Scholar] [CrossRef] [PubMed]
- Ileriturk, M.; Kandemir, O.; Kandemir, F.M. Evaluation of Protective Effects of Quercetin against Cypermethrin-Induced Lung Toxicity in Rats via Oxidative Stress, Inflammation, Apoptosis, Autophagy, and Endoplasmic Reticulum Stress Pathway. Environ. Toxicol. 2022, 37, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Brain, R.A.; Anderson, J.C. The Agro-Enabled Urban Revolution, Pesticides, Politics, and Popular Culture: A Case Study of Land Use, Birds, and Insecticides in the USA. Environ. Sci. Pollut. Res. 2019, 26, 21717–21735. [Google Scholar] [CrossRef] [PubMed]
- Gagic, V.; Holding, M.; Venables, W.N.; Hulthen, A.D.; Schellhorn, N.A. Better Outcomes for Pest Pressure, Insecticide Use, and Yield in Less Intensive Agricultural Landscapes. Proc. Natl. Acad. Sci. USA 2021, 118, e2018100118. [Google Scholar] [CrossRef]
- Hernández-Suárez, E.M.; Beitia, F. Sustainable Management Methods of Orchard Insect Pests. Insects 2021, 12, 80. [Google Scholar] [CrossRef]
- Bravo, A.; Soberón, M. Can Microbial-Based Insecticides Replace Chemical Pesticides in Agricultural Production? Microb. Biotechnol. 2023, 16, 2011–2014. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/RP/visualize (accessed on 25 October 2023).
- US EPA. Toxics Release Inventory (TRI) Program. Available online: https://www.epa.gov/toxics-release-inventory-tri-program (accessed on 4 April 2023).
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/home/en (accessed on 25 October 2023).
- Environmental Protection Agency (EPA). Cypermethrin—Proposed Interim Registration Review Decision. 2020. Docket Number EPA-HQ-OPP-2012-0167. Available online: https://www.regulations.gov/docket/EPA-HQ-OPP-2012-0167/Document (accessed on 29 September 2022).
- Bellisai, G.; Bernasconi, G.; Binaglia, M.; Brancato, A.; Cabrera, L.C.; Castellan, I.; Castoldi, A.F.; Chiusolo, A.; Crivellente, F.; Del Aguila, M.; et al. Review of the Existing Maximum Residue Levels for Cypermethrin According to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2023, 21, e07800. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The Production and Uses of Beauveria Bassiana as a Microbial Insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Scheepers, L.D.; Freercks, R.; Merwe, E. van der Acute Cypermethrin and Other Pyrethroid Poisoning—An Organophosphate-like Poisoning: A Case Report and Review. Toxicol. Rep. 2023, 11, 107–110. [Google Scholar] [CrossRef]
- Gad, H.A.; Ramadan, G.R.; El-Bakry, A.M.; El-Sabrout, A.M.; Abdelgaleil, S.A. Monoterpenes: Promising natural products for public health insect control- A review. Int. J. Trop. Insect Sci. 2022, 42, 1059–1075. [Google Scholar] [CrossRef]
- Saied, E.; Fouda, A.; Alemam, A.M.; Sultan, M.H.; Barghoth, M.G.; Radwan, A.A.; Desouky, S.G.; Azab, I.H.E.; Nahhas, N.E.; Hassan, S.E.-D. Evaluate the Toxicity of Pyrethroid Insecticide Cypermethrin before and after Biodegradation by Lysinibacillus Cresolivuorans Strain HIS7. Plants 2021, 10, 1903. [Google Scholar] [CrossRef] [PubMed]
- Akande, M.G.; Abraham, S.U.; Ogunnubi, J.C.; Akande, M.G.; Abraham, S.U.; Ogunnubi, J.C. Benefits and Risks of Pesticide Usage in Pets. In Pesticides—Updates on Toxicity, Efficacy and Risk Assessment; IntechOpen: La Plata, Argentina, 2022; ISBN 978-1-80356-039-7. [Google Scholar]
- Ihedioha, T.; Asuzu, I.; Nwanta, J. Retrospective Evaluation of the Patterns of Clinical Use of Anti-Parasitic Agents on Animals Presented for Veterinary Care at an Animal Hospital in Nigeria. Thai J. Vet. Med. 2021, 51, 75–83. [Google Scholar] [CrossRef]
- Carter, S.W. A Review of the Use of Synthetic Pyrethroids in Public Health and Vector Pest Control. Pestic. Sci. 1989, 27, 361–374. [Google Scholar] [CrossRef]
- Iľko, I.; Peterková, V.; Heregová, M.; Strelková, L.; Preinerová, K.; Derka, T.; Boršová, K.; Čabanová, V. The Study on Biocidal Resistance of Mosquitoes of Genus Culex and Aedes to Commonly Used Biocides Cypermethrin and Deltamethrin in Central Europe. Biologia 2023, 78, 2727–2736. [Google Scholar] [CrossRef]
- Moon, K.; Kim, W.J.; Lee, J.; Kim, D.; Choi, K.S.; Lee, S.H.; Kim, Y.H. Transcriptomic Comparison of Cypermethrin-Susceptible and -Tolerant Asian Longhorned Ticks (Haemaphysalis Longicornis Neumann). Entomol. Res. 2021, 51, 374–386. [Google Scholar] [CrossRef]
- Zortéa, T.; Baretta, D.; Maccari, A.P.; Segat, J.C.; Boiago, E.S.; Sousa, J.P.; Da Silva, A.S. Influence of Cypermethrin on Avoidance Behavior, Survival and Reproduction of Folsomia Candida in Soil. Chemosphere 2015, 122, 94–98. [Google Scholar] [CrossRef]
- Chen, S.; Geng, P.; Xiao, Y.; Hu, M. Bioremediation of β-Cypermethrin and 3-Phenoxybenzaldehyde Contaminated Soils Using Streptomyces Aureus HP-S-01. Appl. Microbiol. Biotechnol. 2012, 94, 505–515. [Google Scholar] [CrossRef]
- Cycoń, M.; Piotrowska-Seget, Z. Pyrethroid-Degrading Microorganisms and Their Potential for the Bioremediation of Contaminated Soils: A Review. Front. Microbiol. 2016, 7, 1463. [Google Scholar] [CrossRef]
- Bhatt, P.; Rene, E.R.; Huang, Y.; Wu, X.; Zhou, Z.; Li, J.; Kumar, A.J.; Sharma, A.; Chen, S. Indigenous Bacterial Consortium-Mediated Cypermethrin Degradation in the Presence of Organic Amendments and Zea Mays Plants. Environ. Res. 2022, 212, 113137. [Google Scholar] [CrossRef]
- Carrière, Y.; Brown, Z.; Aglasan, S.; Dutilleul, P.; Carroll, M.; Head, G.; Tabashnik, B.E.; Jørgensen, P.S.; Carroll, S.P. Crop Rotation Mitigates Impacts of Corn Rootworm Resistance to Transgenic Bt Corn. Proc. Natl. Acad. Sci. USA 2020, 117, 18385–18392. [Google Scholar] [CrossRef]
- Jiménez-Galindo, J.C.; Castillo-Rosales, A.; Castellanos-Pérez, G.; Orozco-González, F.; Ortega-Ortega, A.; Padilla-Chacón, D.; Butrón, A.; Revilla, P.; Malvar, R.A. Identification of Resistance to the Corn Weevil (Sitophilus Zeamais M.) in Mexican Maize Races (Zea Mays L.). Agronomy 2023, 13, 312. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The Usability of Sorbents in Restoring Enzymatic Activity in Soils Polluted with Petroleum-Derived Products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Sensitivity of Zea Mays and Soil Microorganisms to the Toxic Effect of Chromium (VI). Int. J. Mol. Sci. 2023, 24, 178. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J.; Borowik, A.; Kaczyński, P. Possibilities of Restoring Homeostasis of Soil Exposed to Terbuthylazine by Its Supplementation with HumiAgra Preparation. Appl. Soil Ecol. 2022, 178, 104582. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Borowik, A.; Kucharski, J. The Response of the Soil Microbiome to Contamination with Cadmium, Cobalt, and Nickel in Soil Sown with Brassica Napus. Minerals 2021, 11, 498. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. Microbial Diversity and Enzyme Activity as Indicators of Permethrin-Exposed Soil Health. Molecules 2023, 28, 4756. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M.S. Ecotoxicological Implications of Residual Pesticides to Beneficial Soil Bacteria: A Review. Pestic. Biochem. Physiol. 2022, 188, 105272. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Pingree, M.R.A.; Gao, S. Chapter 16—Assessing Soil Biological Health in Forest Soils. In Developments in Soil Science; Busse, M., Giardina, C.P., Morris, D.M., Page-Dumroese, D.S., Eds.; Global Change and Forest Soils; Elsevier: Amsterdam, The Netherlands, 2019; Volume 36, pp. 397–426. [Google Scholar]
- Tejada, M.; García, C.; Hernández, T.; Gómez, I. Response of Soil Microbial Activity and Biodiversity in Soils Polluted with Different Concentrations of Cypermethrin Insecticide. Arch. Environ. Contam. Toxicol. 2015, 69, 8–19. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. The Impact of Permethrin and Cypermethrin on Plants, Soil Enzyme Activity, and Microbial Communities. Int. J. Mol. Sci. 2023, 24, 2892. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Wyszkowska, J.; Borowik, A.; Kordala, N. Contamination of Soil with Diesel Oil, Application of Sewage Sludge and Content of Macroelements in Oats. Water. Air. Soil Pollut. 2020, 231, 546. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of Aerobic Microorganisms and Soil Enzyme Response to Soil Contamination with Ekodiesel Ultra Fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef] [PubMed]
- Bragança, I.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Assessment of Pyrethroid Pesticides in Topsoils in Northern Portugal. Water Air Soil Pollut. 2019, 230, 166. [Google Scholar] [CrossRef]
- Statista—The Statistics Portal for Market Data, Market Research, and Market Studies. Available online: https://www.statista.com/ (accessed on 4 April 2023).
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinformatics 2015, 16, 169. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Citing RStudio. Available online: https://support.posit.co/hc/en-us/articles/206212048-Citing-RStudio (accessed on 4 April 2023).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data; R Package Version 2.17.0. 2020. Available online: https://CRAN.R-Project.Org/Package=gplots (accessed on 8 December 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria. 2019. Available online: https://www.R-project.org (accessed on 8 December 2022).
- Doran, J.W.; Zeiss, M.R. Soil Health and Sustainability: Managing the Biotic Component of Soil Quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Pandey, J.K.; Dubey, G.; Gopal, R. Prolonged Use of Insecticide Dimethoate Inhibits Growth and Photosynthetic Activity of Wheat Seedlings: A Study by Laser-Induced Chlorophyll Fluorescence Spectroscopy. J. Fluoresc. 2022, 32, 2159–2172. [Google Scholar] [CrossRef]
- Pang, S.; Duan, L.; Liu, Z.; Song, X.; Li, X.; Wang, C. Co-Induction of a Glutathione-S-Transferase, a Glutathione Transporter and an ABC Transporter in Maize by Xenobiotics. PLoS ONE 2012, 7, e40712. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial Degradation of Pesticide Residues and an Emphasis on the Degradation of Cypermethrin and 3-Phenoxy Benzoic Acid: A Review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Deng, W.; Liu, S.; Yao, K. Microbial degradation of 3-phenoxybenzoic acid—A review. Wei Sheng Wu Xue Bao 2015, 55, 1081–1088. [Google Scholar]
- Zhang, J.; Zhu, W.; Zheng, Y.; Yang, J.; Zhu, X. The Antiandrogenic Activity of Pyrethroid Pesticides Cyfluthrin and β-Cyfluthrin. Reprod. Toxicol. 2008, 25, 491–496. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.-F.; Chen, W.-J.; Zhu, X.; Mishra, S.; Bhatt, P.; Chen, S. Efficient Biodegradation of Multiple Pyrethroid Pesticides by Rhodococcus Pyridinivorans Strain Y6 and Its Degradation Mechanism. Chem. Eng. J. 2023, 469, 143863. [Google Scholar] [CrossRef]
- Li, J.; Hu, K.; Hu, L.; Hou, X.; Li, Q.; Liu, A.; Chen, S.; Ao, X.; Hu, X.; He, L.; et al. Adsorption Behavior of 3-Phenoxybenzoic Acid by Lactobacillus Plantarum and Its Potential Application in Simulated Digestive Juices. Int. J. Mol. Sci. 2022, 23, 5809. [Google Scholar] [CrossRef] [PubMed]
- Akhila, S.D.; Ashwath, P.; Manjunatha, K.G.; Akshay, S.D.; Rao, R.; Reddy, D.; Vittal, R. Pesticide and Xenobiotic Metabolism in Aquatic Organisms. In Xenobiotics in Aquatic Animals: Reproductive and Developmental Impacts; Rather, M.A., Amin, A., Hajam, Y.A., Jamwal, A., Ahmad, I., Eds.; Springer Nature: Singapore, 2023; pp. 1–66. ISBN 978-981-9912-14-8. [Google Scholar]
- Wassan, J.T.; Wang, H.; Zheng, H. Developing a New Phylogeny-Driven Random Forest Model for Functional Metagenomics. IEEE Trans. NanoBioscience 2023, 22, 763–770. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. The Soil Microbiome—From Metagenomics to Metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef]
- Akbar, S.; Sultan, S.; Kertesz, M. Determination of Cypermethrin Degradation Potential of Soil Bacteria Along with Plant Growth-Promoting Characteristics. Curr. Microbiol. 2015, 70, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Huang, Y.; Lin, Z.; Bhatt, P.; Chen, S. New Insights into the Microbial Degradation and Catalytic Mechanism of Synthetic Pyrethroids. Environ. Res. 2020, 182, 109138. [Google Scholar] [CrossRef]
- Bhatt, P.; Huang, Y.; Zhang, W.; Sharma, A.; Chen, S. Enhanced Cypermethrin Degradation Kinetics and Metabolic Pathway in Bacillus Thuringiensis Strain SG4. Microorganisms 2020, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Godínez, L.J.; Aguirre-Noyola, J.L.; Martínez-Romero, E.; Arteaga-Garibay, R.I.; Ireta-Moreno, J.; Ruvalcaba-Gómez, J.M.; Plants, A. Look at Plant-Growth-Promoting Bacteria. Plants 2023, 12, 1668. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Olszewski, J.; Kucharski, J. Soil Bacterial Community and Soil Enzyme Activity Depending on the Cultivation of Triticum aestivum, Brassica napus, and Pisum sativum ssp. arvense. Diversity 2019, 11, 246. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, H.; Tan, X.; Wang, F.; Jia, H.; Megharaj, M.; He, W. Long-Term As Contamination Alters Soil Enzyme Functional Stability in Response to Additional Heat Disturbance. Chemosphere 2019, 229, 471–480. [Google Scholar] [CrossRef]
- Yadav, R.; Tripathi, P.; Singh, R.P.; Khare, P. Assessment of Soil Enzymatic Resilience in Chlorpyrifos Contaminated Soils by Biochar Aided Pelargonium Graveolens L. Plantation. Environ. Sci. Pollut. Res. 2023, 30, 7040–7055. [Google Scholar] [CrossRef]
- Rahul, R.; Sharma, P.; Singh, A.; Singh, J.; Kumar, M. Soil Enzymes and Their Role in Soil Health Improvement. In Advances in Agricultural and Industrial Microbiology: Volume 1: Microbial Diversity and Application in Agroindustry; Nayak, S.K., Baliyarsingh, B., Mannazzu, I., Singh, A., Mishra, B.B., Eds.; Springer Nature: Singapore, 2022; pp. 39–61. ISBN 9789811689185. [Google Scholar]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil Enzyme Activity: A Brief History and Biochemistry as a Basis for Appropriate Interpretations and Meta-Analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Kumar, M.; Ashraf, S.; Ansari, R.A.; Zuhaib, M.; Jamil, A.; Dhakar, N.; Kasim, R.; Rizvi, A. Implementation of Trichoderma Spp. for Conservation of Soil Health. In Phytobiont and Ecosystem Restitution; Kumar, V., Kumar, M., Prasad, R., Eds.; Springer: Singapore, 2018; pp. 319–328. ISBN 9789811311871. [Google Scholar]
- Aioub, A.A.A.; Li, Y.; Qie, X.; Zhang, X.; Hu, Z. Reduction of Soil Contamination by Cypermethrin Residues Using Phytoremediation with Plantago Major and Some Surfactants. Environ. Sci. Eur. 2019, 31, 26. [Google Scholar] [CrossRef]
- Aioub, A.A.A.; Zuo, Y.; Aioub, A.A.A.; Hu, Z. Biochemical and Phytoremediation of Plantago Major L. to Protect Tomato Plants from the Contamination of Cypermethrin Pesticide. Environ. Sci. Pollut. Res. 2021, 28, 43992–44001. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Fulekar, M.H. Cadmium Phytoremediation Potential of Deenanath Grass (Pennisetum Pedicellatum) and the Assessment of Bacterial Communities in the Rhizospheric Soil. Environ. Sci. Pollut. Res. 2022, 29, 2936–2953. [Google Scholar] [CrossRef] [PubMed]
- Bhanse, P.; Kumar, M.; Singh, L.; Awasthi, M.K.; Qureshi, A. Role of Plant Growth-Promoting Rhizobacteria in Boosting the Phytoremediation of Stressed Soils: Opportunities, Challenges, and Prospects. Chemosphere 2022, 303, 134954. [Google Scholar] [CrossRef] [PubMed]
- Myresiotis, C.K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Biodegradation of Soil-Applied Pesticides by Selected Strains of Plant Growth-Promoting Rhizobacteria (PGPR) and Their Effects on Bacterial Growth. Biodegradation 2012, 23, 297–310. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhatt, K.; Huang, Y.; Lin, Z.; Chen, S. Esterase Is a Powerful Tool for the Biodegradation of Pyrethroid Insecticides. Chemosphere 2020, 244, 125507. [Google Scholar] [CrossRef] [PubMed]
- Aponte, H.; Meli, P.; Butler, B.; Paolini, J.; Matus, F.; Merino, C.; Cornejo, P.; Kuzyakov, Y. Meta-Analysis of Heavy Metal Effects on Soil Enzyme Activities. Sci. Total Environ. 2020, 737, 139744. [Google Scholar] [CrossRef]
- Bernard, L.; Basile-Doelsch, I.; Derrien, D.; Fanin, N.; Fontaine, S.; Guenet, B.; Karimi, B.; Marsden, C.; Maron, P.-A. Advancing the Mechanistic Understanding of the Priming Effect on Soil Organic Matter Mineralisation. Funct. Ecol. 2022, 36, 1355–1377. [Google Scholar] [CrossRef]
- Odukkathil, G.; Vasudevan, N. Toxicity and Bioremediation of Pesticides in Agricultural Soil. Rev. Environ. Sci. Biotechnol. 2013, 12, 421–444. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.-F.; Liu, L.-Y.; Zhu, F.-J.; Ma, W.-L. National-Scale Monitoring of Historic Used Organochlorine Pesticides (OCPs) and Current Used Pesticides (CUPs) in Chinese Surface Soil: Old Topic and New Story. J. Hazard. Mater. 2023, 443, 130285. [Google Scholar] [CrossRef]
- Zhai, Q.; Chen, X.; Zhang, M.; Zhang, C.; Zhang, Z.; Pan, H.; Zhang, H.; Sun, F. Immobilization of Klebsiella jilinsis Strain 2N3 by Corn Straw Biochar Enhanced the Degradation of Nicosulfuron and Restores the Soil Microbiome Function and Composition. Appl. Soil Ecol. 2023, 189, 104917. [Google Scholar] [CrossRef]
- Ravi, R.K.; Hiranmai, R.Y. Bioremediation: Efficient Technology to Combat Pesticide Pollutants in Environment. In Strategies and Tools for Pollutant Mitigation: Avenues to a Cleaner Environment; Aravind, J., Kamaraj, M., Prashanthi Devi, M., Rajakumar, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 151–172. ISBN 978-3-030-63575-6. [Google Scholar]
- Jiang, W.; Gao, J.; Cheng, Z.; Zhai, W.; Liu, D.; Zhou, Z.; Wang, P. The Influence of Oxytetracycline on the Degradation and Enantioselectivity of the Chiral Pesticide Beta-Cypermethrin in Soil. Environ. Pollut. 2019, 255, 113215. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Butarewicz, A.; Łozowicka, B. Soil Biological Activity as an Indicator of Soil Pollution with Pesticides—A Review. Appl. Soil Ecol. 2020, 147, 103356. [Google Scholar] [CrossRef]
- Arora, S.; Sahni, D. Pesticides Effect on Soil Microbial Ecology and Enzyme Activity—An Overview. J. Appl. Nat. Sci. 2016, 8, 1126–1132. [Google Scholar] [CrossRef]
- Sim, J.X.F.; Drigo, B.; Doolette, C.L.; Vasileiadis, S.; Karpouzas, D.G.; Lombi, E. Impact of Twenty Pesticides on Soil Carbon Microbial Functions and Community Composition. Chemosphere 2022, 307, 135820. [Google Scholar] [CrossRef]
- Riah, W.; Laval, K.; Laroche-Ajzenberg, E.; Mougin, C.; Latour, X.; Trinsoutrot-Gattin, I. Effects of Pesticides on Soil Enzymes: A Review. Environ. Chem. Lett. 2014, 12, 257–273. [Google Scholar] [CrossRef]
- Babaniyi, B.R.; Thompson, S.O.; Ogundele, O.D.; Oluwole, O.F. Effects of Agrochemicals on Soil Microbial Enzymes. In Ecological Interplays in Microbial Enzymology; Maddela, N.R., Abiodun, A.S., Prasad, R., Eds.; Environmental and Microbial Biotechnology; Springer Nature: Singapore, 2022; pp. 353–377. ISBN 978-981-19015-5-3. [Google Scholar]
- Gunjal, A.B.; Waghmode, M.S.; Patil, N.N.; Nawani, N.N. Chapter 9—Significance of Soil Enzymes in Agriculture. In Smart Bioremediation Technologies; Bhatt, P., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 159–168. ISBN 978-0-12-818307-6. [Google Scholar]
- Chettri, D.; Sharma, B.; Verma, A.K.; Verma, A.K. Significance of Microbial Enzyme Activities in Agriculture. In Microbiological Activity for Soil and Plant Health Management; Soni, R., Suyal, D.C., Bhargava, P., Goel, R., Eds.; Springer: Singapore, 2021; pp. 351–373. ISBN 9789811629228. [Google Scholar]
- Wu, G.; Miyata, T.; Kang, C.Y.; Xie, L.H. Insecticide toxicity and synergism by enzyme inhibitors in 18 species of pest insect and natural enemies in crucifer vegetable crops. Pest Manag. Sci. 2007, 63, 500–510. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, J.; Baćmaga, M.; Tomkiel, M. Response of Microorganisms and Enzymes to Soil Contamination with a Mixture of Terbuthylazine, Mesotrione, and S-Metolachlor. Environ. Sci. Pollut. Res. 2017, 24, 1910–1925. [Google Scholar] [CrossRef]
- Caglayan, C.; Taslimi, P.; Türk, C.; Gulcin, İ.; Kandemir, F.M.; Demir, Y.; Beydemir, Ş. Inhibition Effects of Some Pesticides and Heavy Metals on Carbonic Anhydrase Enzyme Activity Purified from Horse Mackerel (Trachurus Trachurus) Gill Tissues. Environ. Sci. Pollut. Res. 2020, 27, 10607–10616. [Google Scholar] [CrossRef] [PubMed]

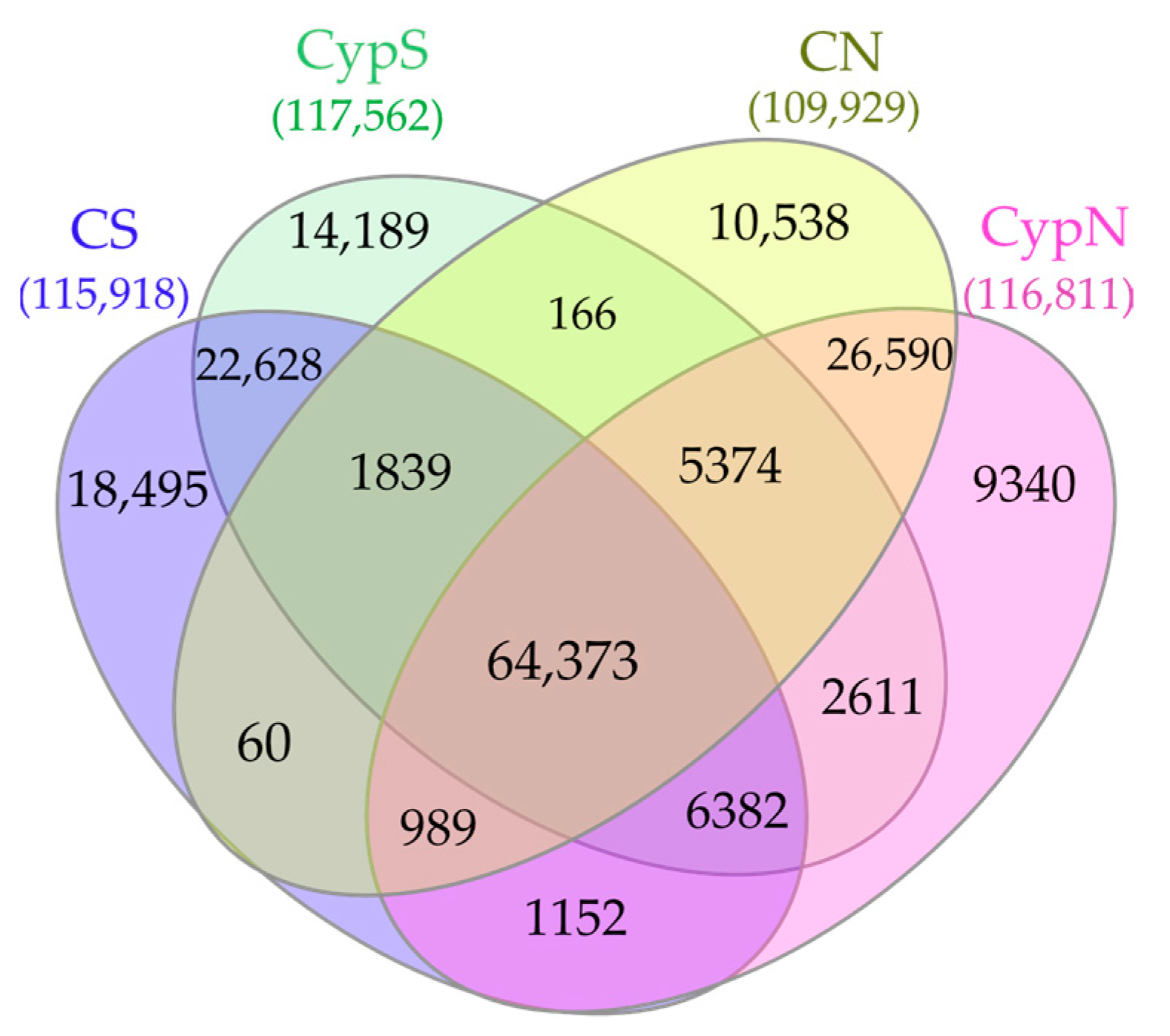
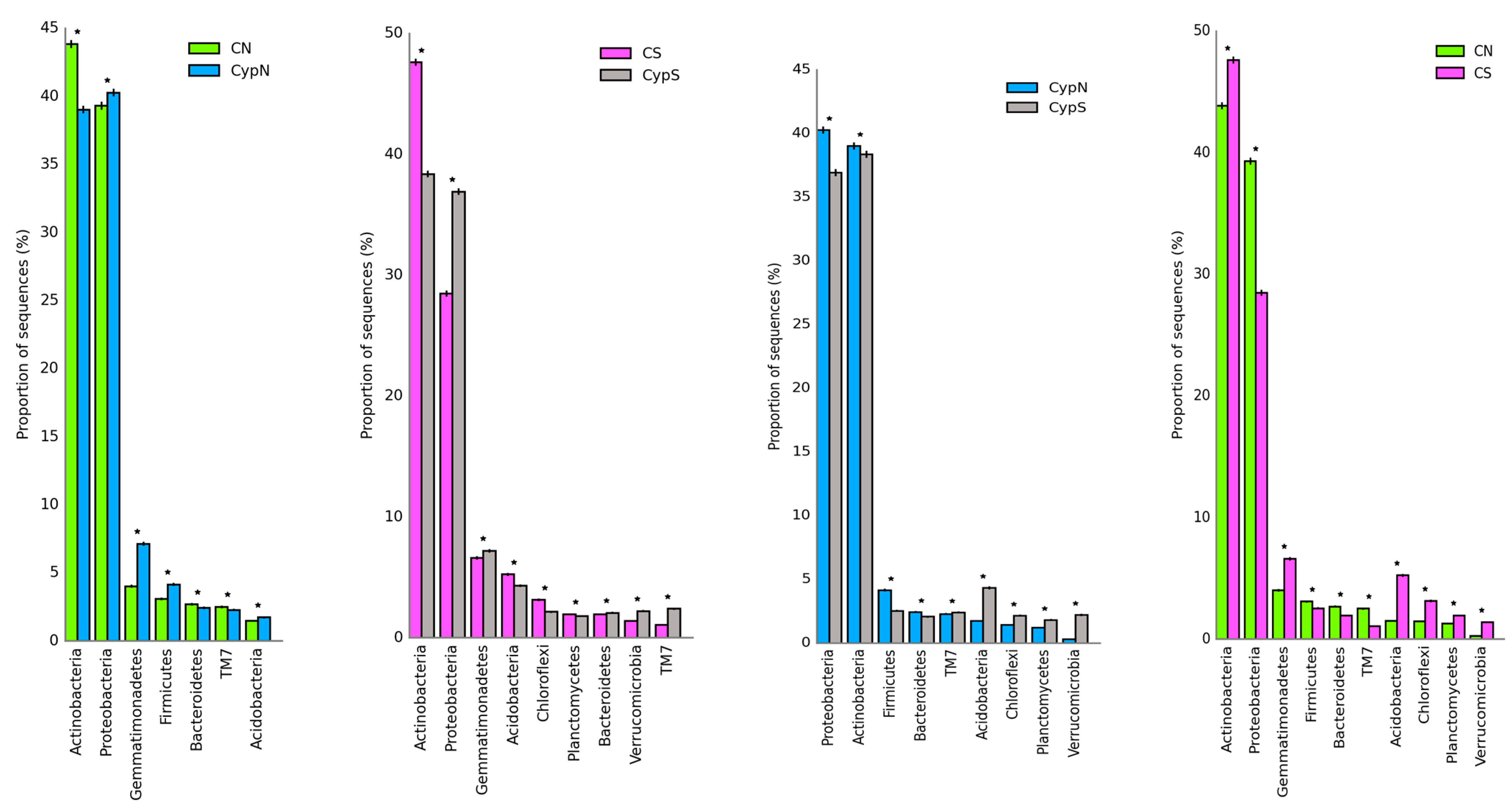
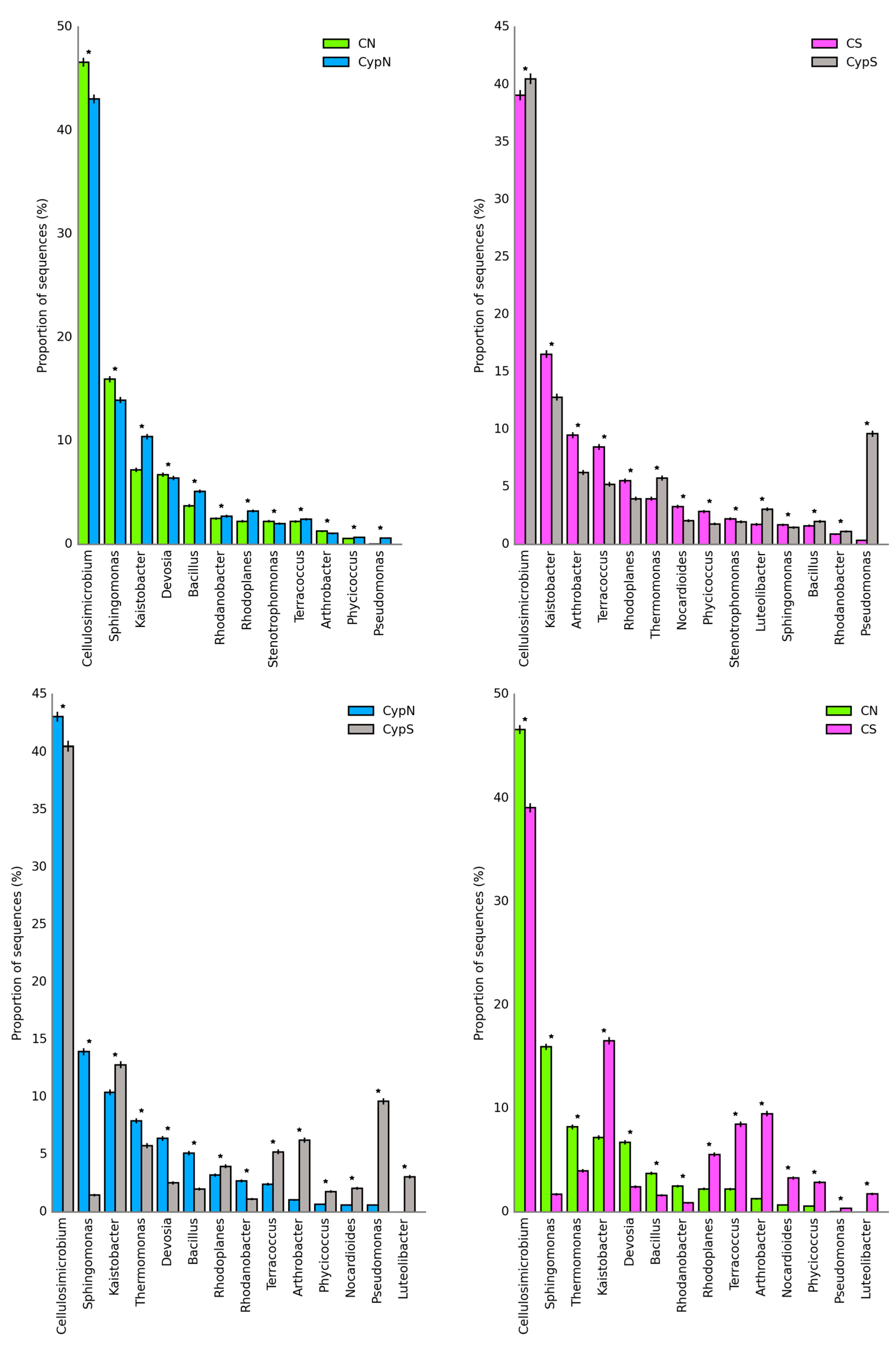

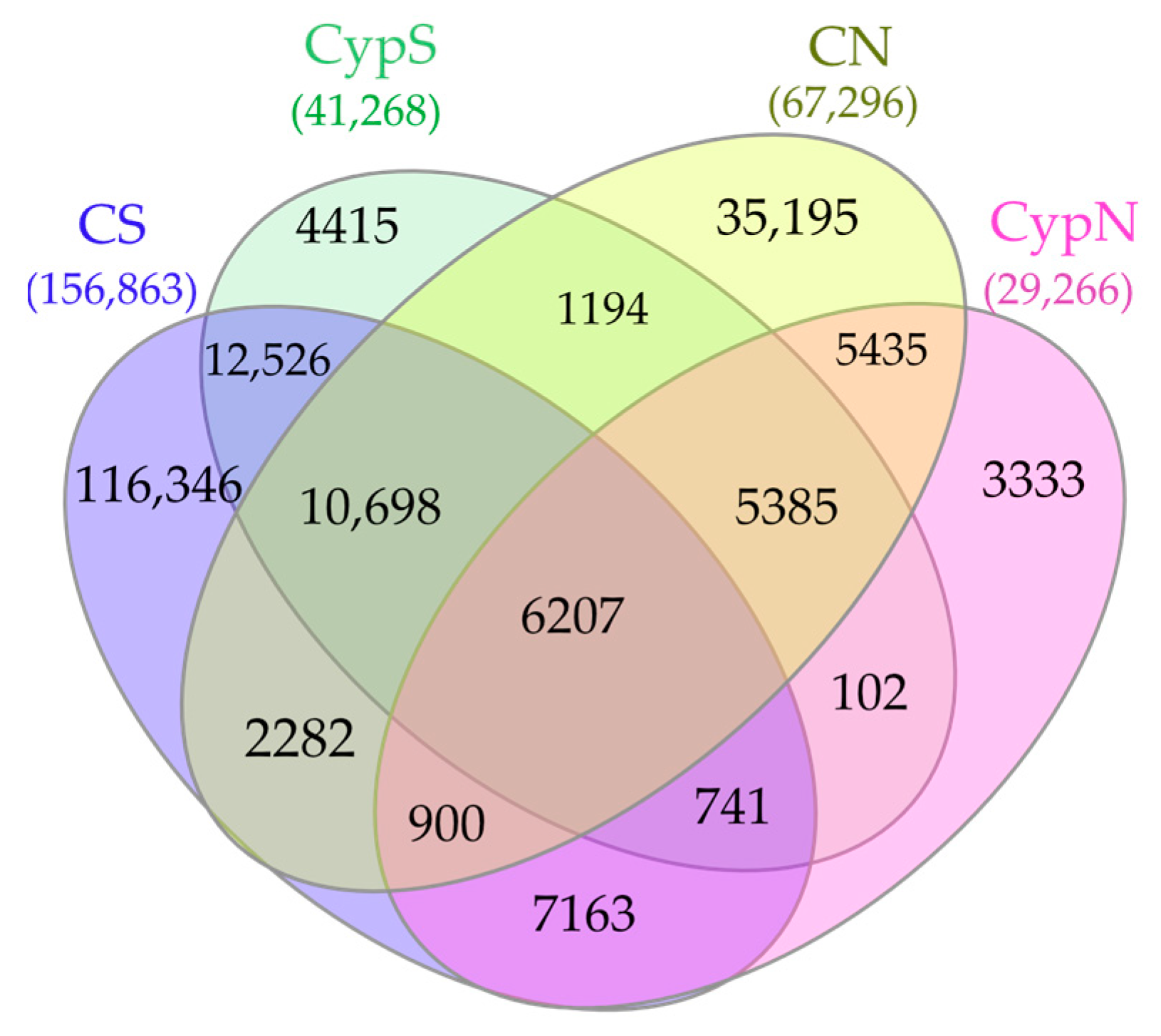
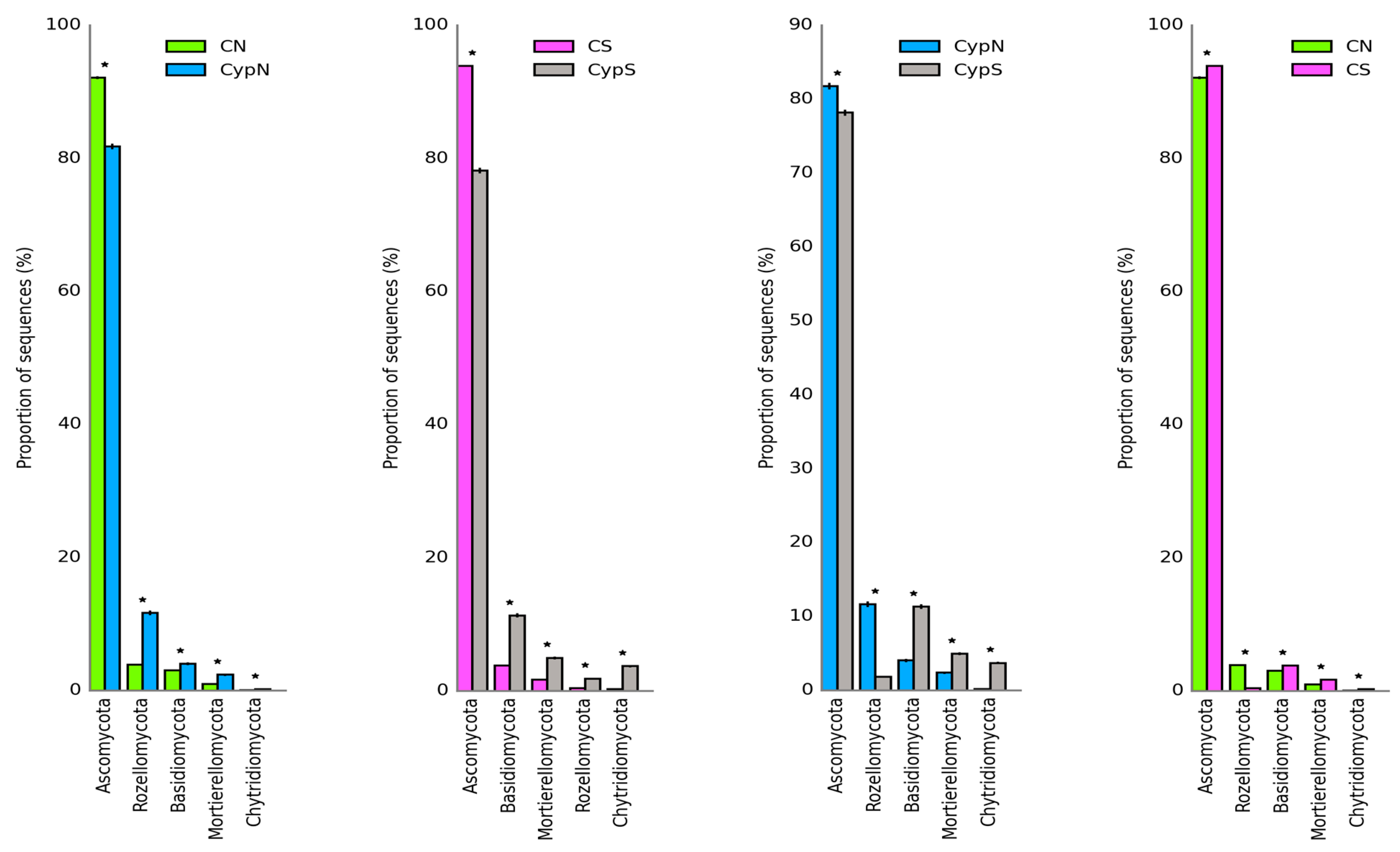
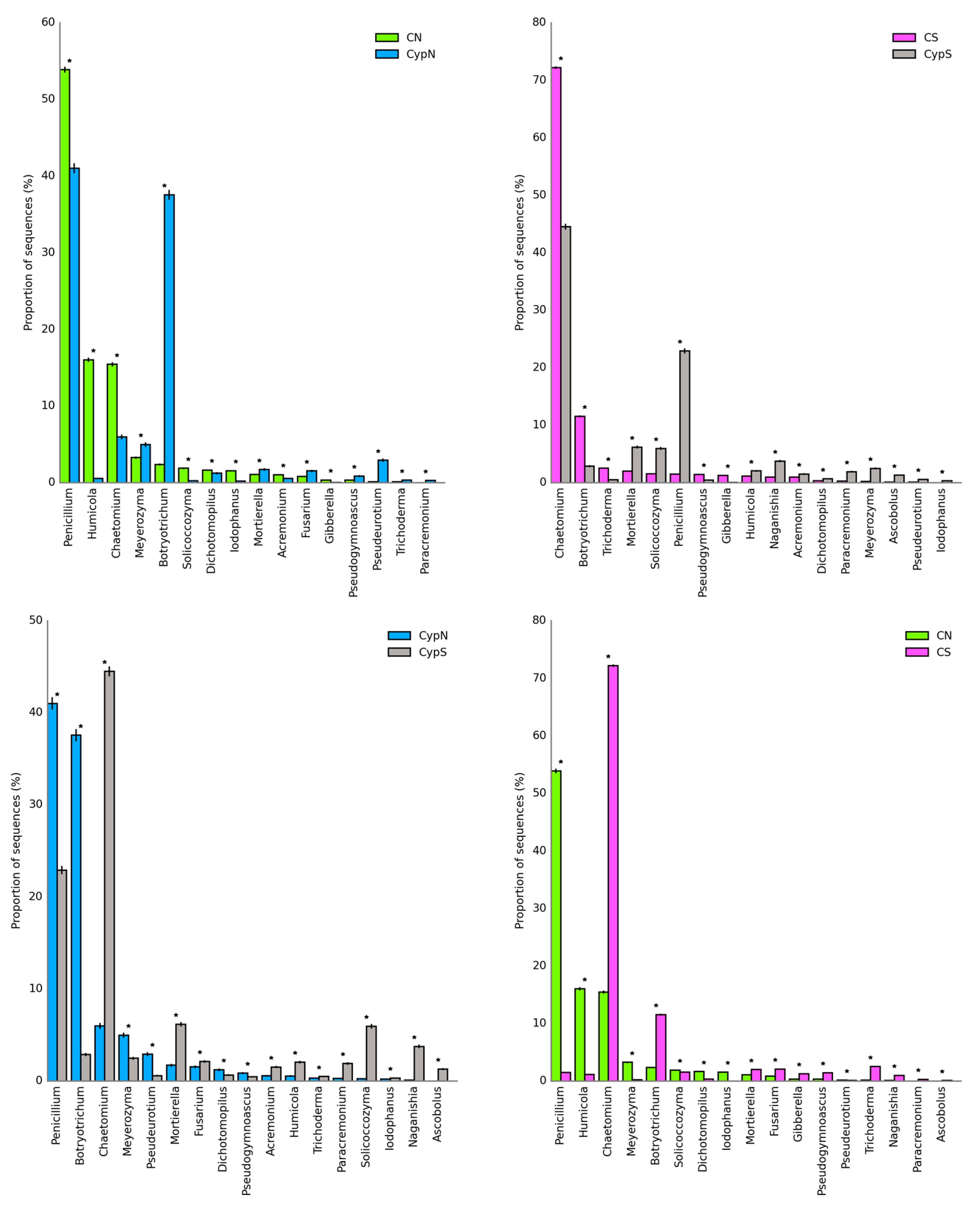

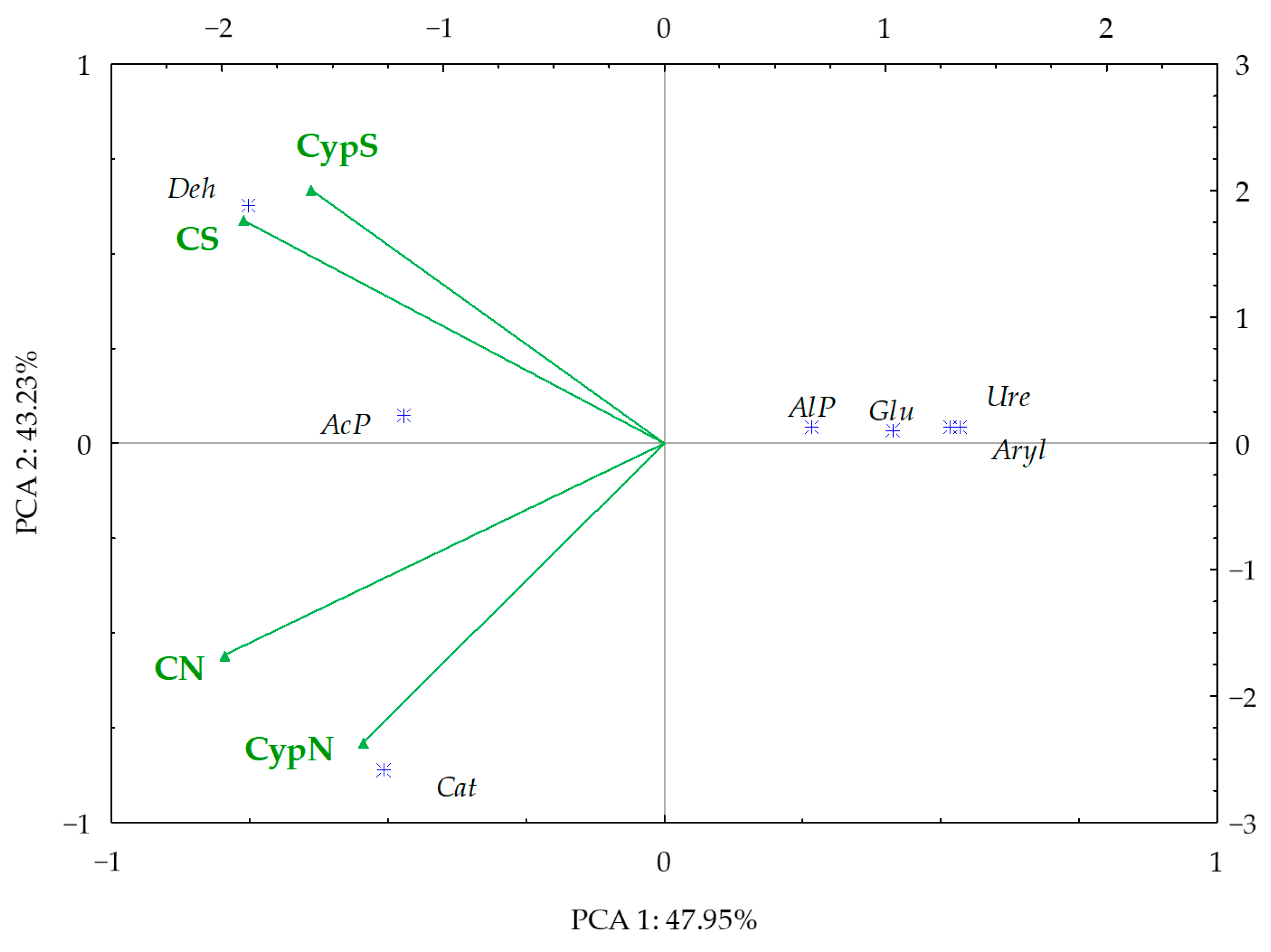

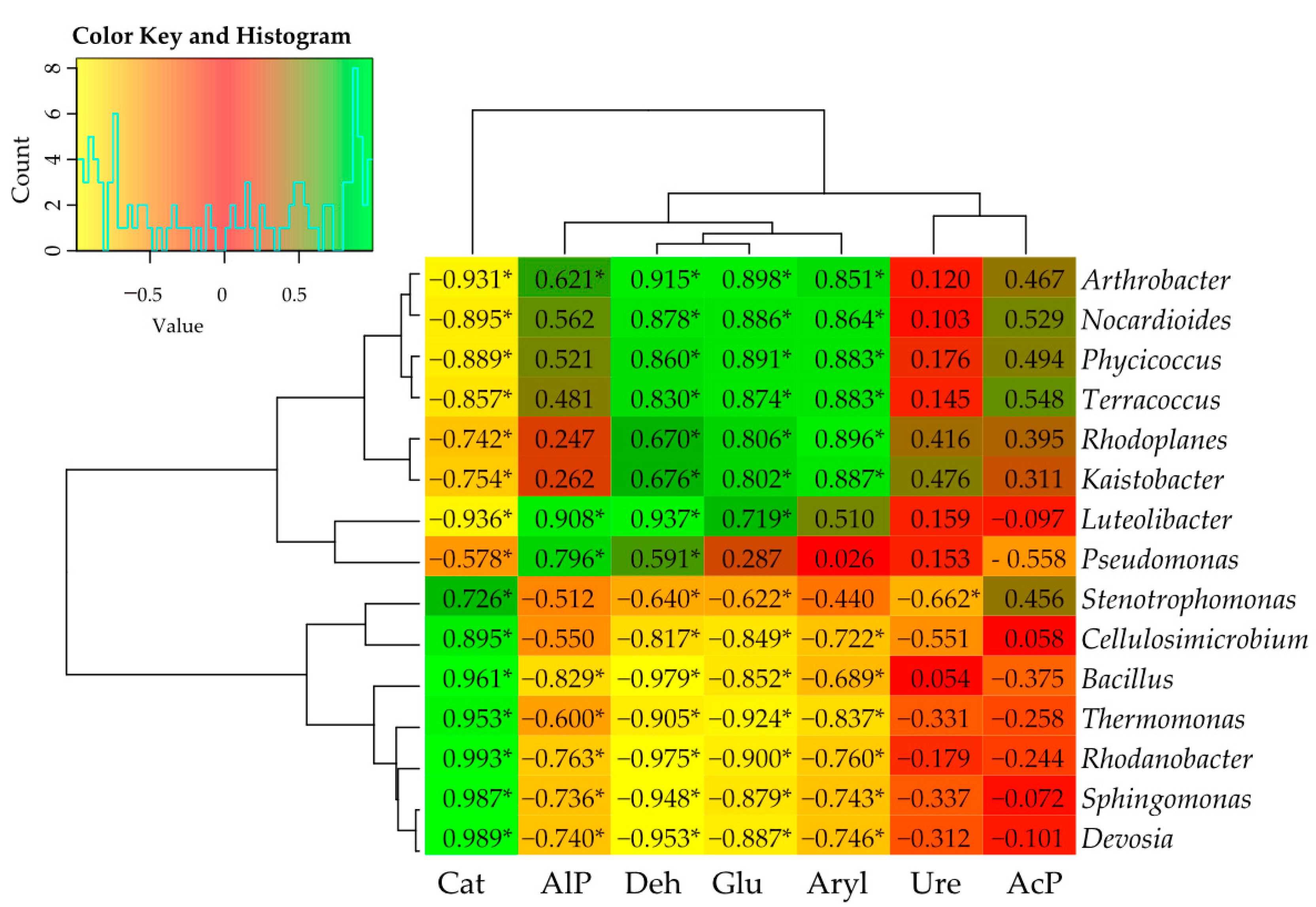
| Object | Shannon | Simpson | Shaneven | Margalefa | Richness | Pielou J | Brillouin |
|---|---|---|---|---|---|---|---|
| CS | 4.313 b | 0.960 a | 0.698 b | 41.423 a | 484 a | 0.698 b | 4.302 b |
| CypS | 4.446 a | 0.964 a | 0.719 a | 41.404 a | 484 a | 0.719 a | 4.434 a |
| CN | 3.834 d | 0.929 c | 0.639 c | 34.724 b | 404 b | 0.639 c | 3.825 d |
| CypN | 4.074 c | 0.951 b | 0.678 b | 34.800 b | 407 b | 0.678 b | 4.065 c |
| Object | Shannon | Simpson | Shaneven | Margalefa | Richness | Pielou J | Brillouin |
|---|---|---|---|---|---|---|---|
| CS | 2.249 c | 0.643 c | 0.395 c | 24.910 a | 299 a | 0.395 c | 2.244 c |
| CypS | 3.048 a | 0.855 a | 0.594 a | 15.808 b | 169 b | 0.594 a | 3.037 a |
| CN | 2.603 b | 0.848 b | 0.541 b | 10.974 d | 123 c | 0.541 b | 2.597 b |
| CypN | 2.588 b | 0.829 b | 0.544 b | 11.182 c | 116 d | 0.544 b | 2.578 b |
| Object | Deh µmol TFF | Cat mol O2 | Ure mmol N-NH4 | AcP | AlP | Aryl | Glu |
|---|---|---|---|---|---|---|---|
| mmol PNP | |||||||
| CS | 4.41 a ± 0.28 | 0.17 c ± 0.01 | 0.06 b ± 0.00 | 3.89 a ± 0.10 | 0.63 b ± 0.01 | 0.12 a ± 0.01 | 0.34 a ± 0.01 |
| CypS | 4.61 a ± 0.31 | 0.15 c ± 0.00 | 0.06 b ± 0.00 | 0.73 c ± 0.01 | 0.74 a ± 0.01 | 0.10 b ± 0.00 | 0.33 ab ± 0.01 |
| CN | 1.39 b ± 0.05 | 3.98 a ± 0.03 | 0.05 c ± 0.03 | 3.03 b ± 0.05 | 0.59 c ± 0.03 | 0.09 b ± 0.01 | 0.29 c ± 0.02 |
| CypN | 0.75 c ± 0.06 | 3.88 b ± 0.03 | 0.07 a ± 0.03 | 0.53 d ± 0.01 | 0.50 d ± 0.02 | 0.09 b ± 0.00 | 0.30 bc ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowik, A.; Wyszkowska, J.; Zaborowska, M.; Kucharski, J. Impact of Cypermethrin (Arpon G) on Soil Health and Zea mays Growth: A Microbiological and Enzymatic Study. Agriculture 2023, 13, 2261. https://doi.org/10.3390/agriculture13122261
Borowik A, Wyszkowska J, Zaborowska M, Kucharski J. Impact of Cypermethrin (Arpon G) on Soil Health and Zea mays Growth: A Microbiological and Enzymatic Study. Agriculture. 2023; 13(12):2261. https://doi.org/10.3390/agriculture13122261
Chicago/Turabian StyleBorowik, Agata, Jadwiga Wyszkowska, Magdalena Zaborowska, and Jan Kucharski. 2023. "Impact of Cypermethrin (Arpon G) on Soil Health and Zea mays Growth: A Microbiological and Enzymatic Study" Agriculture 13, no. 12: 2261. https://doi.org/10.3390/agriculture13122261
APA StyleBorowik, A., Wyszkowska, J., Zaborowska, M., & Kucharski, J. (2023). Impact of Cypermethrin (Arpon G) on Soil Health and Zea mays Growth: A Microbiological and Enzymatic Study. Agriculture, 13(12), 2261. https://doi.org/10.3390/agriculture13122261









