Analyzing Single and Combined Cultures of Plant Growth-Promoting Rhizobacteria Isolates from Afghanistan as a Potential Biofertilizer for Rice Growth and Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Genomic-DNA Extraction, Amplification, and Sequencing of the rpoB Gene
2.3. Compatibility Study
2.4. Indole-3-Acetic Acid (IAA) Production
2.5. Acetylene Reduction Assay (ARA)
2.6. Phosphate Solubilization Estimation and Organic Acid Production
2.7. Potassium Solubilization Estimation
2.8. Effects of Single and Combined Treatments of PGPR on Rice Growth
2.9. Nucleotide Sequence Accession Numbers
3. Results
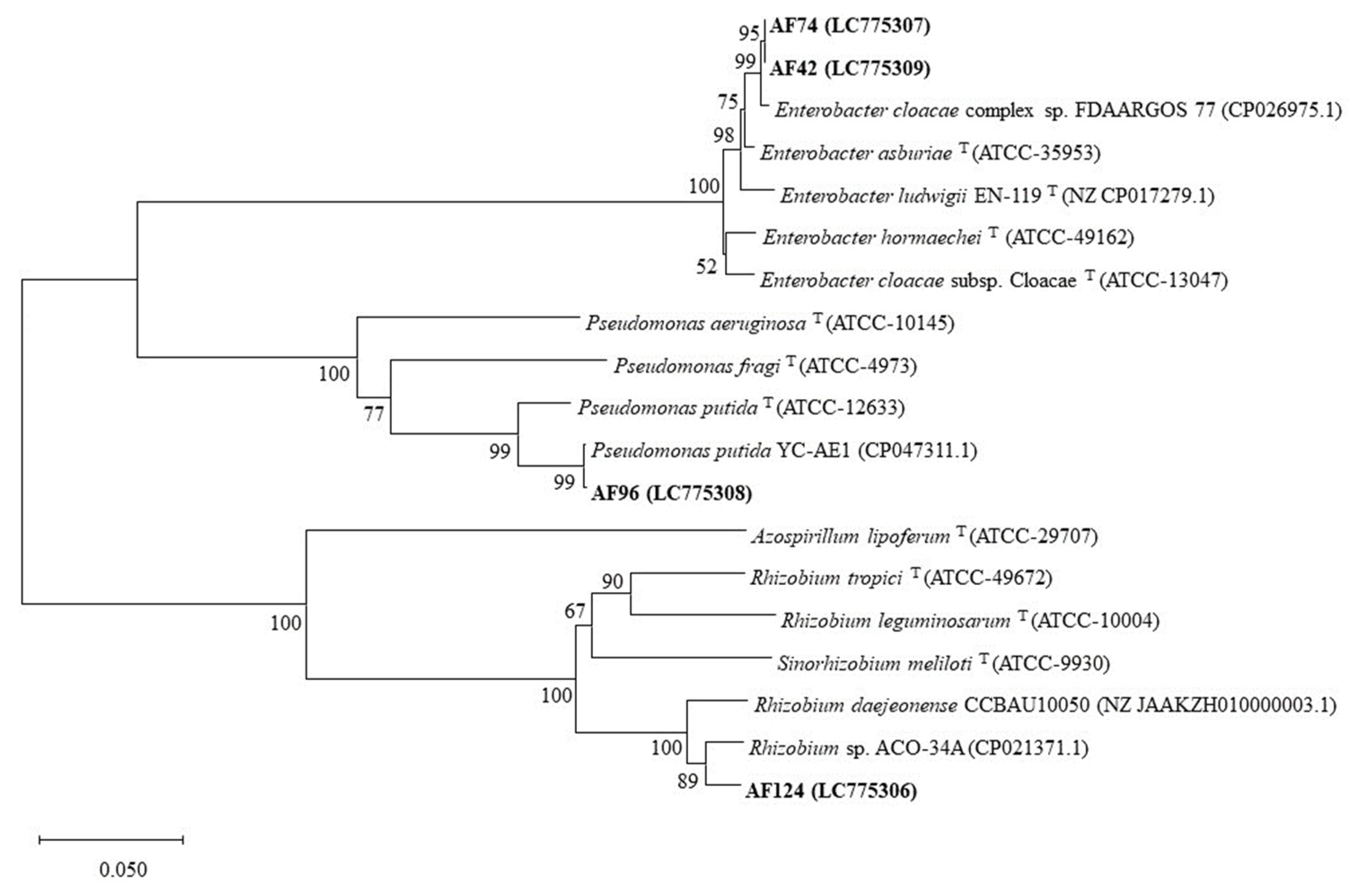
3.1. Genetic Characterization of the Selected Bacteria Based on the rpoB Gene
3.2. Antagonistic Effects of Bacterial Strains
3.3. Indole-3-acetic Acid Production
3.4. Acetylene Reduction Assay (ARA)
3.5. Phosphate Solubilization Assay and Organic Acids Production
3.6. Potassium Solubilization Activity
3.7. Plant Growth Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ch, R.; Chevallier, O.; McCarron, P.; McGrath, T.F.; Wu, D.; Kapil, A.P.; McBride, M.; Elliott, C.T. Metabolomic fingerprinting of volatile organic compounds for the geographical discrimination of rice samples from China, Vietnam and India. Food Chem. 2021, 334, 127553. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci. 2014, 1324, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Schoumans, O.F.; Chardon, W.J.; Bechmann, M.E.; Gascuel-Odoux, C.; Hofman, G.; Kronvang, B.; Rubæk, G.H.; Ulén, B.; Dorioz, J.M. Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: A review. Sci. Total Environ. 2014, 468, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Y. Evaluating the potential health and economic effects of nitrogen fertilizer application in grain production systems of China. J. Clean. Prod. 2020, 264, 121635. [Google Scholar] [CrossRef]
- Cordero, I.; Balaguer, L.; Rincón, A.; Pueyo, J.J. Inoculation of tomato plants with selected PGPR represents a feasible alternative to chemical fertilization under salt stress. J. Plant. Nutr. Soil Sci. 2018, 181, 694–703. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Kadmiri, M.I. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Wickramasinghe, W.; Girija, D.; Gopal, K.S.; Kesevan, S. Multi-phasic nitrogen fixing plant growth promoting rhizobacteria as biofertilizer for rice cultivation. Res. J. Agric. Sci. 2021, 12, 399–404. [Google Scholar]
- Beneduzi, A.; Moreira, F.; Costa, P.B.; Vargas, L.K.; Lisboa, B.B.; Favreto, R.; Baldani, J.I.; Passaglia, L.M.P. Diversity and plant growth promoting evaluation abilities of bacteria isolated from sugarcane cultivated in the South of Brazil. Appl. Soil Ecol. 2013, 63, 94–104. [Google Scholar] [CrossRef]
- Roriz, M.; Pereira, S.I.A.; Castro, P.M.L.; Carvalho, S.M.P.; Vasconcelos, M.W. Iron metabolism in soybean grown in calcareous soil is influenced by plant growth-promoting rhizobacteria—A functional analysis. Rhizosphere 2021, 17, 100274. [Google Scholar] [CrossRef]
- Montañez, A.; Blanco, A.R.; Barlocco, C.; Beracochea, M.; Sicardi, M. Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in vitro. Appl. Soil Ecol. 2012, 58, 21–28. [Google Scholar] [CrossRef]
- Filippi, M.C.C.; Da Silva, G.B.; Silva-Lobo, V.L.; Côrtes, M.V.C.B.; Moraes, A.J.G.; Prabhu, A.S. Leaf blast (Magnaporthe oryzae) suppression and growth promotion by rhizobacteria on aerobic rice in Brazil. Biol. Control 2011, 58, 160–166. [Google Scholar] [CrossRef]
- Brzezinska, M.S.; Kalwasińska, A.; Świątczak, J.; Żero, K.; Jankiewicz, U. Exploring the properties of chitinolytic Bacillus isolates for the pathogens biological control. Microb. Pathog. 2020, 148, 104462. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Mir, M.I.; Zulfajri, M.; Hanafiah, M.M.; Unnisa, S.A.; Mahboob, M. Plant growth promoting and antifungal asset of indigenous rhizobacteria secluded from saffron (Crocus sativus L.) rhizosphere. Microb. Pathog. 2021, 150, 104734. [Google Scholar] [CrossRef] [PubMed]
- Danish, S.; Zafar-ul-Hye, M.; Mohsin, F.; Hussain, M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS ONE 2020, 15, e0230615. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Sharma, A.K. Field evaluation of consortium of bacterial inoculants producing ACC deaminase on growth, nutrients and yield components of rice and wheat. J. Crop Sci. Biotechnol. 2021, 24, 293–305. [Google Scholar] [CrossRef]
- Beneduzi, A.; Peres, D.; da Costa, P.B.; Zanettini, M.H.B.; Passaglia, L.M.P. Genetic and phenotypic diversity of plant-growth-promoting bacilli isolated from wheat fields in southern Brazil. Res. Microbiol. 2008, 159, 244–250. [Google Scholar] [CrossRef]
- Yadav, J.; Verma, J.P.; Jaiswal, D.K.; Kumar, A. Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol. Eng. 2014, 62, 123–128. [Google Scholar]
- Cavite, H.J.M.; Mactal, A.G.; Evangelista, E.V.; Cruz, J.A. Growth and yield response of upland rice to application of plant growth-promoting rhizobacteria. J. Plant Growth Regul. 2021, 40, 494–508. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Vega, A.; Castro, P.M.L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 2020, 6, e05106. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Ali, S.; Babar, M. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. J. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Benjelloun, I.; Thami Alami, I.; El Khadir, M.; Douira, A.; Udupa, S.M. Co-inoculation of Mesorhizobium ciceri with either Bacillus sp. or Enterobacter aerogenes on chickpea improves growth and productivity in phosphate-deficient soils in dry areas of a Mediterranean region. Plants 2021, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Romano, J.D.; Kolter, R. Pseudomonas-Saccharomyces interactions: Influence of fungal metabolism on bacterial physiology and survival. J. Bacteriol. 2005, 187, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.G.; Kumar, K.V.K.; Zhou, L.W.; Reddy, M.S.; Kloepper, J.W. Combined use of PGPRs and reduced rates of Azoxystrobin to improve management of sheath blight of rice. Plant Dis. 2021, 105, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, X.; Zhu, T.H.; Liu, G.H.; Mao, C. Co-inoculation with phosphate-solubilzing and nitrogen-fixing bacteria on solubilization of rock phosphate and their effect on growth promotion and nutrient uptake by walnut. Eur. J. Soil Biol. 2012, 50, 112–117. [Google Scholar] [CrossRef]
- Marimuthu, S.; Subbian, P.; Ramamoorthy, V.; Samiyappan, R. Synergistic effect of combined application of Azospirillum and Pseudomonas fluorescens with inorganic fertilizers on root rot incidence and yield of cotton/Synergistische Wirkung der kombinierten Anwendung von Azospirillum und Pseudomonas fluorescens mit anorganischen Düngern auf die Wurzelfäule und den Ertrag von Baumwolle. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 2002, 109, 569–577. [Google Scholar]
- Anandham, R.; Sridar, R.; Nalayini, P.; Poonguzhali, S.; Madhaiyan, M. Potential for plant growth promotion in groundnut (Arachis hypogaea L.) cv. ALR-2 by co-inoculation of sulfur-oxidizing bacteria and Rhizobium. Microbiol. Res. 2007, 162, 139–153. [Google Scholar] [CrossRef]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol. Res. 2020, 239, 126569. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef]
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Kumar, A. Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol. Eng. 2013, 51, 282–286. [Google Scholar] [CrossRef]
- Sánchez, A.C.; Gutiérrez, R.T.; Santana, R.C.; Urrutia, A.R.; Fauvart, M.; Michiels, J.; Vanderleyden, J. Effects of co-inoculation of native Rhizobium and Pseudomonas strains on growth parameters and yield of two contrasting Phaseolus vulgaris L. genotypes under Cuban soil conditions. Eur. J. Soil Biol. 2014, 62, 105–112. [Google Scholar] [CrossRef]
- Pastor-Bueis, R.; Jimenez-Gomez, A.; Barquero, M.; Mateos, P.F.; Gonzalez-Andres, F. Yield response of common bean to co-inoculation with Rhizobium and Pseudomonas endophytes and microscopic evidence of different colonised spaces inside the nodule. Eur. J. Agron. 2021, 122, 126187. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Ohkama-Ohtsu, N.; Sarhadi, W.A.; Kojima, K.; Rallos, R.V.; Ramirez, M.D.A.; Yamaya, H.; Sekimoto, H.; Yokoyama, T. Isolation and screening of indigenous plant growth-promoting rhizobacteria from different rice cultivars in Afghanistan soils. Microbes Environ. 2019, 34, 347–355. [Google Scholar] [CrossRef]
- Döbereiner, J.; Marriel, I.E.; Nery, M. Ecological distribution of Spirillum lipoferum Beijerinck. Can. J. Microbiol. 1976, 22, 1464–1473. [Google Scholar] [CrossRef] [PubMed]
- Mohkam, M.; Nezafat, N.; Berenjian, A.; Mobasher, M.A.; Ghasemi, Y. Identification of Bacillus probiotics isolated from soil rhizosphere using 16S rRNA, recA, rpoB gene sequencing and RAPD-PCR. Probiotics Antimicrob. Proteins 2016, 8, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Haque, S.; Singh, H.; Verma, J.; Vibha, K.; Singh, R.; Jawed, A.; Tripathi, C.K.M. Isolation, screening, and identification of novel isolates of Actinomycetes from India for antimicrobial applications. Front. Microbiol. 2016, 7, 1921. [Google Scholar] [CrossRef] [PubMed]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, J.; Guo, J. Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J. Microbiol. Biotechnol. 2006, 22, 983–990. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kato, H.; Ishikawa, M. A simple analytical method for index of soil nitrogen availability by extracting in phosphate buffer solution. Jpn. J. Soil Sci. Plant Nutr. 1989, 60, 160–163. [Google Scholar]
- Truog, E. Determination of readily available phosphorus in soils. J. Am. Soc. Agron. 1930, 22, 874–882. [Google Scholar] [CrossRef]
- Kamewada, K.; Shibata, K. Simple extraction method for measuring exchangeable cations in soils that is not required measuring cation exchange capacity. Jpn. J. Soil Sci. Plant Nutr. 1997, 68, 61–64. [Google Scholar]
- Aliyat, F.Z.; Maldani, M.; El Guilli, M.; Nassiri, L.; Ibijbijen, J. Phosphate-solubilizing bacteria isolated from phosphate solid sludge and their ability to solubilize three inorganic phosphate forms: Calcium, Iron, and Aluminum phosphates. Microorganisms 2022, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Vyas, P.; Gulati, A. Organic acid production in vitro and plant growth promotion in maize under controlled environment by phosphate-solubilizing fluorescent Pseudomonas. BMC Microbiol. 2009, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Zuluga, M.Y.A.; De Oliveira, A.L.M.; Valentinuzzi, F.; Jayme, N.S.; Montrisi, S.; Fattorini, R.; Cesco, S.; Pii, Y. An insight into the role of the organic acids produced by Enterobacter sp. strain 15S in solubilizing tricalcium phosphate: In situ study on cucumber. BMC Microbiol. 2023, 23, 184. [Google Scholar] [CrossRef]
- Pollmann, S.; Düchting, P.; Weiler, E.W. Tryptophan-dependent indole-3-acetic acid biosynthesis by ‘IAA-synthase’proceeds via indole-3-acetamide. Phytochemistry 2009, 70, 523–531. [Google Scholar] [CrossRef]
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P. A brief appraisal of ethylene signaling under abiotic stress in plants. Plant Signal. Behav. 2020, 15, 1782051-7. [Google Scholar] [CrossRef]
- Singh, P.; Dutta, P.; Chakrabarty, D. miRNAs play critical roles in response to abiotic stress by modulating cross-talk of phytohormone signaling. Plant Cell Rep. 2021, 40, 1617–1630. [Google Scholar] [CrossRef]
- Hakim, S.; Naqqash, T.; Nawaz, M.S.; Laraib, I.; Siddique, M.J.; Zia, R.; Mirza, M.S.; Imran, A. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 2021, 5, 617157. [Google Scholar] [CrossRef]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A. Bacterial plant biostimulants: A sustainable way towards improving growth, productivity, and health of crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Alvarenga, D.O.; Rousk, K. Indirect effects of climate change inhibit N2 fixation associated with the feathermoss Hylocomium splendens in subarctic tundra. Sci. Total Environ. 2021, 795, 148676. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; Rodés, R.; de la Fuente, E.; Fernández, L. Does the routine heat treatment of sugarcane stem pieces for xylem pathogen control affect the nitrogenase activity of an N2-fixing endophyte in the cane? Funct. Plant Biol. 2001, 28, 907–912. [Google Scholar] [CrossRef]
- Chinachanta, K.; Shutsrirung, A. Screening for P-and K-solubilizing, and siderophore producing capacity of rhizobacteria from Khao Dawk Mali 105 Aromatic Rice. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 858, pp. 1–13. [Google Scholar]
- Pienkos, P.; Bodmer, S.; Tabita, F.R. Oxygen inactivation and recovery of nitrogenase activity in cyanobacteria. J. Bacteriol. 1983, 153, 182–190. [Google Scholar] [CrossRef]
- Habibi, S.; Djedidi, S.; Prongjunthuek, K.; Mortuza, M.F.; Ohkama-Ohtsu, N.; Sekimoto, H.; Yokoyoma, T. Physiological and genetic characterization of rice nitrogen fixer PGPR isolated from rhizosphere soils of different crops. Plant Soil 2014, 379, 51–66. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Mechanisms used by plant growth-promoting bacteria. In Bacteria in Agrobiology: Plant Nutrient Management; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–46. [Google Scholar]
- Basak, B.B.; Biswas, D.R. Co-inoculation of potassium solubilizing and nitrogen fixing bacteria on solubilization of waste mica and their effect on growth promotion and nutrient acquisition by a forage crop. Biol. Fertil. Soils 2010, 46, 641–648. [Google Scholar] [CrossRef]
- Berde, C.V.; Gawde, S.S.; Berde, V.B. Potassium solubilization: Mechanism and functional impact on plant growth. Soil Microbiomes Sustain. Agric. 2021, 27, 133–148. [Google Scholar]
- Meena, V.S.; Maurya, B.; Verma, J.P. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef]
- Ranawat, B.; Mishra, S.; Singh, A. Enterobacter hormaechei (MF957335) enhanced yield, disease and salinity tolerance in tomato. Arch. Microbiol. 2021, 203, 2659–2667. [Google Scholar] [CrossRef]
- Bonik, A.; Dangar, T.K. Application of rice (Oryza sativa L.) root endophytic diazotrophic Azotobacter sp. strain AviZ (MCC 3432) can increase rice yield under green house and field condition. Microbiol. Res. 2019, 219, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Game, B.C.; Ilhe, B.M.; Pawar, V.S. Effect of Azotobacter, phosphate solubilizing bacteria and potash mobilizing bacteria inoculation on productivity of wheat (Triticum aestivum L.). Interm. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2800–2807. [Google Scholar] [CrossRef]
- Llorente, B.E.; Alasia, M.A.; Larraburu, E.E. Biofertilization with Azospirillum brasilense improves in vitro culture of Handroanthus ochraceus, a forestry, ornamental and medicinal plant. New Biotechnol. 2016, 33, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Thakur, M.C.; Gontia, I.; Albrecht, V.; Stoffels, M.; Schmid, M.; Hartmann, A. Isolation, partial identification and application of diazotrophic rhizobacteria from traditional Indian rice cultivars. Eur. J. Soil Biol. 2009, 45, 62–72. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Forestry and Fisheries. Available online: http://www.maff.go.jp/e/ (accessed on 13 October 2023).
| Site | pH(1:2.5) (Soil:H2O) | Available N (mg/Kg DS) | Available P2O5 (mg/Kg DS) | Available K2O (mg/Kg DS) |
|---|---|---|---|---|
| Honmachi paddy field | 6.47 ± 0.1 | 88.5 ± 4.2 | 170.9 ± 6.8 | 183 ± 9.1 |
| PGPR Treatment | Bacterial Species | IAA (µg mL−1) | ARA (nmol tube−1) |
|---|---|---|---|
| Control | N.D. | N.D. | |
| Single | AF124 (Rhizobium daejeonense) | 29.9 ± 1.7 a | 944.0 ± 74.0 a |
| AF74 (Enterobacter cloacae) | 30.1 ± 2.6 a | 7.2 ± 2.4 hg | |
| AF96 (Pseudomonas putida) | 11.9 ± 1.0 d | N.D. | |
| AF42 (Enterobacter cloacae) | 28.7 ± 1.1 ba | 9.0 ± 1.0 hg | |
| Dual | AF124 + AF74 | 21.0 ± 2.0 cb | 417.5 ± 39.1 d |
| AF124 + AF96 | 18.0 ± 0.9 c | 134.1 ± 27.1 e | |
| AF124 + AF42 | 17.2 ± 1.2 c | 10.0 ± 1.8 hg | |
| AF74 + AF96 | 25.2 ± 1.0 b | 9.3 ± 1.5 hg | |
| AF74 + AF42 | 24.2 ± 1.1 b | 11.0 ± 1.0 g | |
| AF96 + AF42 | 24.9 ± 1.0 b | 4.5 ± 0.9 h | |
| Triple | AF124 + AF74 + AF96 | 17.4 ± 0.4 c | 233.1 ± 45.1 ed |
| AF124 + AF74 + AF42 | 16.2 ± 0.3 c | 552.5 ± 63.3 c | |
| AF124 + AF96 + AF42 | 16.2 ± 0.4 c | 696.5 ± 71.2 b | |
| AF74 + AF96 + AF42 | 23.5 ± 0.6 cb | 18.0 ± 2.2 f | |
| Quadruple | AF124 + AF74 + AF96 + AF42 | 18.8 ± 0.2 c | 291.5 ± 41.4 ed |
| PGPR Treatment | Organic Acid Production (μmol) | Solubilized P | pH of P-Medium | Solubilized K | pH of K-Medium | ||||
|---|---|---|---|---|---|---|---|---|---|
| Bacterial Species | Shikimic Acid | Oxalic Acid | Malic Acid | Tartaric Acid | (µg mL−1) | (µg mL−1) | |||
| Negative control | N.D. | N.D. | N.D. | N.D. | N.D. | 6.6 ± 0.1 a | 13.5 ± 1.8 e | 6.3 ±0.0 a | |
| Single | AF124 (Rhizobium daejeonense) | N.D. | N.D. | N.D. | N.D. | 5.8 ± 1.9 b | 5.9 ± 0.1 b | 29.0 ± 1.0 e | 6.0 ±0.2 a |
| AF74 (Enterobacter cloacae) | 0.98 ± 0.18 a | 4.31 ± 0.27 ab | 121.8 ± 1.3 b | 60.2 ± 0.8 ab | 69.8 ± 1.5 a | 5.6 ± 0.0 c | 37.0 ± 2.0 de | 4.4 ±0.1 b | |
| AF96 (Pseudomonas putida) | N.D. | N.D. | 165.5 ± 5.7 b | N.D. | 24.1 ± 4.2 b | 5.8 ± 0.1 b | 40.5 ± 1.5 de | 4.4 ±0.0 b | |
| AF42 (Enterobacter cloacae) | 0.99 ± 0.29 a | 4.90 ± 0.46 a | N.D. | 75.2 ± 17.2 a | 62.6 ± 6.1 a | 5.6 ± 0.0 c | 46.0 ± 4.0 de | 4.6 ±0.1 b | |
| Dual | AF124 + AF74 | 0.71 ± 0.10 a | 4.47 ± 0.79 ab | N.D. | 37.2 ± 8.2 bc | 62.9 ± 2.1 a | 5.6 ± 0.1 c | 70.0 ± 10.9 dc | 4.9 ±0.0 b |
| AF124 + AF96 | N.D. | N.D. | 245.7 ± 39.1 a | N.D. | 21.3 ± 2.8 b | 5.8 ± 0.1 c | 52.5 ± 1.5 dc | 4.4 ± 0.3 b | |
| AF124 + AF42 | 0.69 ± 0.00 a | 3.24 ± 0.19 bc | 132.4 ± 5.3 b | 15.4 ± 1.7 cd | 66.5 ± 6.0 a | 5.6 ± 0.0 c | 91.1 ± 6.0 bc | 4.3 ± 0.1 b | |
| AF74 + AF96 | 0.83 ± 0.01 a | 3.22 ± 0.10 bc | 122.1 ± 1.8 b | 36.3 ± 4.0 bc | 61.8 ± 4.6 a | 5.6 ± 0.1 c | 86.0 ± 12.0 bc | 4.2 ± 0.0 b | |
| AF74 + AF42 | 0.73 ± 0.01 a | 4.36 ± 0.35 ab | 122.9 ± 5.5 b | 20.9 ± 2.0 cd | 64.3 ± 5.2 a | 5.6 ± 0.0 c | 72.5 ± 15.4 cd | 4.5 ± 0.1 b | |
| AF96 +AF42 | 0.71 ± 0.03 a | N.D. | 113.3 ± 3.4 b | 16.7 ± 1.2 cd | 55.8 ± 2.8 a | 5.6 ± 0.1 c | 90.5 ± 2.6 bc | 4.2 ± 0.1 b | |
| Triple | AF124 + AF74 + AF96 | 0.75 ± 0.01 a | 2.74 ± 0.03 c | 122.9 ± 3.8 b | 22.1 ± 1.1 cd | 61.6 ± 4.6 a | 5.6 ± 0.0 c | 114.0 ± 8.1 b | 4.3 ± 0.1 b |
| AF124 + AF74 + AF42 | 0.65 ± 0.01 a | 3.06 ± 0.14 bc | 118.7 ± 3.9 b | 25.3 ± 2.3 cd | 63.9 ± 4.3 a | 5.6 ± 0.1 c | 104.3 ± 12.9 bc | 4.4 ± 0.0 b | |
| AF124 + AF96 + AF42 | 0.80 ± 0.02 a | 2.70 ± 0.05 c | 132.9 ± 12.7 b | 7.8 ± 0.3 cd | 58.6 ± 1.5 a | 5.6 ± 0.1 c | 88.1 ± 3.0 bc | 4.3 ± 0.1 b | |
| AF74 + AF96 + AF42 | 0.75 ± 0.03 a | 3.34 ± 0.18 bc | 120.8 ± 2.6 b | 26.0 ± 1.4 cd | 61.2 ± 4.2 a | 5.6 ± 0.0 c | 113.5 ± 7.5 b | 4.2 ± 0.1 b | |
| Quadruple | AF124 + AF74 + AF96 + AF42 | 0.76 ± 0.04 a | 3.05 ± 0.05 bc | 133.6 ± 3.5 b | 23.7 ± 1.3 cd | 63.7 ± 3.0 a | 5.6 ± 0.1 c | 173.1 ± 39.0 a | 4.3 ± 0.1 b |
| PGPR Treatment | Bacterial Species | Shoot Height (cm) | Root Length (cm) | Shoot Dry Weight (mg plant−1) | Root Dry Weight (mg plant−1) |
|---|---|---|---|---|---|
| Control | 49.1 ± 5.0 a | 7.3 ± 1.0 d | 277.5 ± 8.4 c | 69.7 ± 5.5 e | |
| Single | AF124 (Rhizobium daejeonense) | 50.0 ± 2.0 a | 12.7 ± 0.5 abc * | 406.1 ± 8.9 ab * | 130.2 ± 2.8 abcd * |
| AF74 (Enterobacter cloacae) | 53.3 ± 1.7 a | 10.7 ± 0.8 abcd | 463.3 ± 9.6 a ** | 139.0 ± 7.2 abc * | |
| AF96 (Pseudomonas putida) | 51.3 ± 1.3 a | 11.1 ± 1.0 abcd | 338.0 ± 28.5 bc | 100.8 ± 6.7 cde | |
| AF42 (Enterobacter cloacae) | 50.3 ± 1.8 a | 11.0 ± 1.1 abcd * | 379.1 ± 27.6 abc ** | 113.3 ± 8.8 bcde * | |
| Dual | AF124 + AF74 | 54.3 ± 3.7 a | 8.7 ± 0.5 cd | 427.1 ± 2.8 ab ** | 150.3 ± 11.1 ab ** |
| AF124 + AF96 | 56.6 ± 2.9 a | 11.3 ± 1.7 abcd | 404.3 ± 4.9 ab ** | 133.4 ± 3.6 abcd * | |
| AF124 + AF42 | 55.8 ± 1.7 a | 11.0 ± 0.5 abcd * | 368.3 ± 35.7 abc | 148.6 ± 17.1 abc ** | |
| AF74 + AF96 | 53.0 ± 3.2 a | 10.3 ± 0.5 abcd | 336.3 ± 31.5 bc | 85.8 ± 12.2 de | |
| AF74 + AF42 | 55.3 ± 4.1 a | 12.3 ± 1.5 abc * | 337.8 ± 23.4 bc | 132.2 ± 9.8 abcd * | |
| AF96 +AF42 | 56.1 ± 1.5 a | 10.7 ± 0.6 abcd | 428.0 ± 23.3 ab * | 164.4 ± 2.8 a ** | |
| Triple | AF124 + AF74 + AF96 | 55.0 ± 1.5 a | 11.3 ± 0.4 abcd * | 466.5 ± 32.0 a ** | 110.1 ± 5.1 bcde * |
| AF124 + AF74 + AF42 | 59.1 ± 1.3 a | 14.8 ± 0.1 a ** | 429.8 ± 10.1 ab * | 146.6 ± 8.7 abc ** | |
| AF124 + AF96 + AF42 | 57.1 ± 3.0 a | 9.6 ± 0.3 bcd | 383.0 ± 15.3 abc * | 129.0 ± 12.5 abcd * | |
| AF74 + AF96 + AF42 | 55.7 ± 2.3 a | 10.5 ± 1.3 abcd | 372.0 ± 23.1 abc * | 124.8 ± 11.2 abcd * | |
| Quadruple | AF124 + AF74 + AF96 + AF42 | 55.3 ± 2.0 a | 13.7 ± 0.6 ab * | 405.2 ± 9.8 ab * | 130.0 ± 8.6 abcd * |
| IAA (µg mL−1) | ARA (nmol tube−1) | Solubilized P (µg mL−1) | Solubilized K (µg mL−1) | Shoot Dry Weight (mg plant−1) | Root Dry Weight (mg plant−1) | ||
|---|---|---|---|---|---|---|---|
| Single inoculation | IAA (µg mL−1) | ||||||
| ARA (nmol tube−1) | 0.40 | ||||||
| Solubilized P (µg mL−1) | 0.62 | −0.45 | |||||
| Solubilized K (µg mL−1) | 0.64 | −0.18 | 0.75 * | ||||
| Shoot dry weight (mg plant−1) | 0.91 ** | 0.27 | 0.67 | 0.54 | |||
| Root dry weight (mg plant−1) | 0.94 ** | 0.40 | 0.57 | 0.57 | 0.97 ** | ||
| Dual inoculation | IAA (µg mL−1) | ||||||
| ARA (nmol tube−1) | 0.12 | ||||||
| Solubilized P (µg mL−1) | 0.83 * | 0.14 | |||||
| Solubilized K (µg mL−1) | 0.87 * | −0.02 | 0.91 ** | ||||
| Shoot dry weight (mg plant−1) | 0.61 | 0.56 | 0.43 | 0.55 | |||
| Root dry weight (mg plant−1) | 0.57 | 0.33 | 0.56 | 0.63 | 0.87 * | ||
| Triple inoculation | IAA (µg mL−1) | ||||||
| ARA (nmol tube−1) | 0.24 | ||||||
| Solubilized P (µg mL−1) | 0.93 * | 0.52 | |||||
| Solubilized K (µg mL−1) | 0.95 ** | 0.33 | 0.97 ** | ||||
| Shoot dry weight (mg plant−1) | 0.70 * | 0.45 | 0.86 * | 0.87 * | |||
| Root dry weight (mg plant−1) | 0.79 * | 0.67 * | 0.90 * | 0.82 * | 0.69 * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibi, S.; Yokoyama, T.; Haidari, M.D.; Torii, A.; Yasuda, M.; Ohkama-Ohtsu, N. Analyzing Single and Combined Cultures of Plant Growth-Promoting Rhizobacteria Isolates from Afghanistan as a Potential Biofertilizer for Rice Growth and Development. Agriculture 2023, 13, 2252. https://doi.org/10.3390/agriculture13122252
Habibi S, Yokoyama T, Haidari MD, Torii A, Yasuda M, Ohkama-Ohtsu N. Analyzing Single and Combined Cultures of Plant Growth-Promoting Rhizobacteria Isolates from Afghanistan as a Potential Biofertilizer for Rice Growth and Development. Agriculture. 2023; 13(12):2252. https://doi.org/10.3390/agriculture13122252
Chicago/Turabian StyleHabibi, Safiullah, Tadashi Yokoyama, Mohammad Daud Haidari, Akihiro Torii, Michiko Yasuda, and Naoko Ohkama-Ohtsu. 2023. "Analyzing Single and Combined Cultures of Plant Growth-Promoting Rhizobacteria Isolates from Afghanistan as a Potential Biofertilizer for Rice Growth and Development" Agriculture 13, no. 12: 2252. https://doi.org/10.3390/agriculture13122252
APA StyleHabibi, S., Yokoyama, T., Haidari, M. D., Torii, A., Yasuda, M., & Ohkama-Ohtsu, N. (2023). Analyzing Single and Combined Cultures of Plant Growth-Promoting Rhizobacteria Isolates from Afghanistan as a Potential Biofertilizer for Rice Growth and Development. Agriculture, 13(12), 2252. https://doi.org/10.3390/agriculture13122252









