Exogenous Enzymes as Zootechnical Additives in Monogastric Animal Feed: A Review
Abstract
1. Introduction
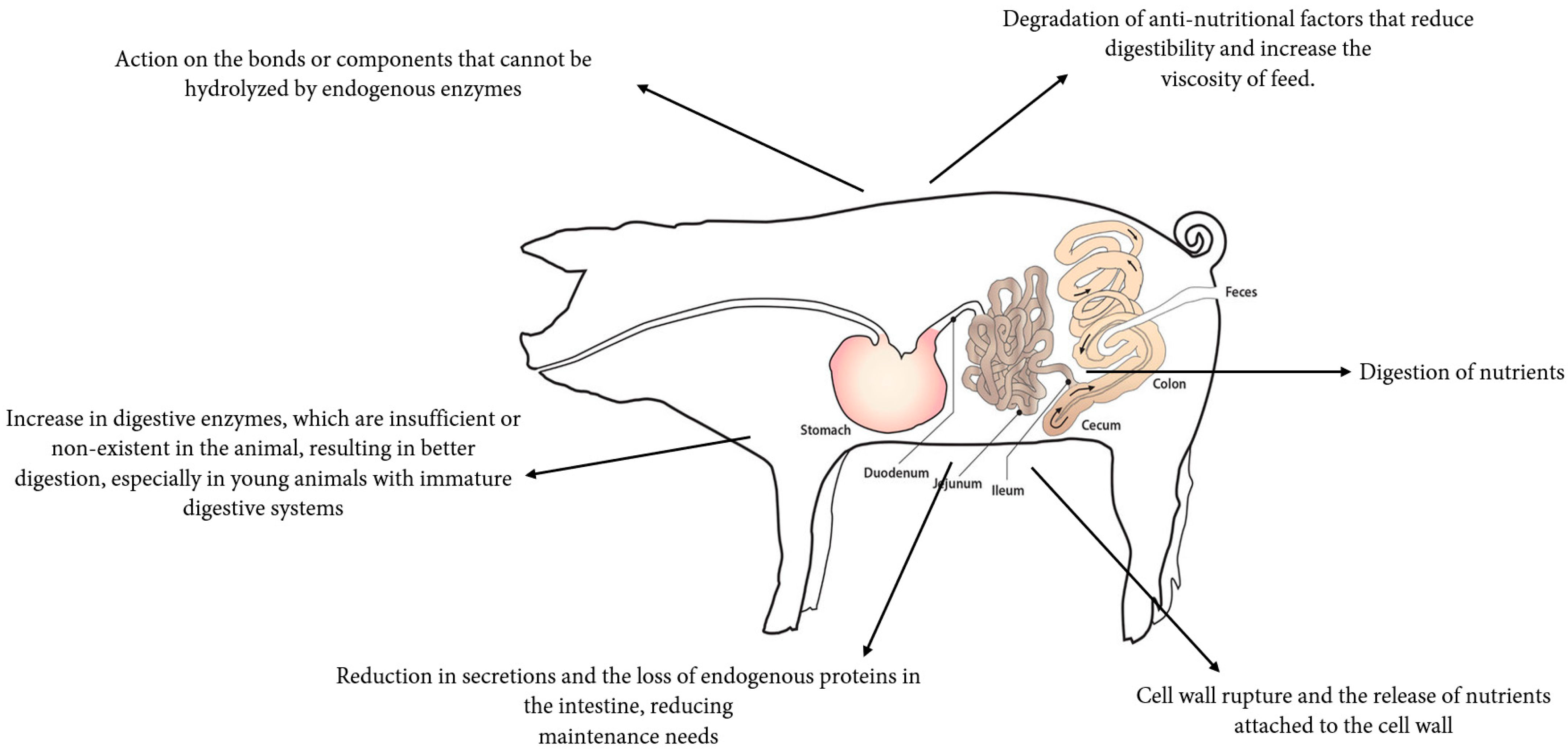
2. Exogenous Enzymes as Feed Additives
3. Application of Phytase Additive in Monogastric Animal Nutrition
4. Application of Xylanases Supplementation in Swine
5. Application of Xylanase Supplementation in Poultry
6. Functional Role and Mode of Action of Protease Supplementation in Swine and Poultry
7. The Application of Enzyme Cocktails in Swine and Poultry
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, J.R.N. Non-starch polysaccharides, protein and starch: From function and feed e highlights on sorghum. In Proceedings of the 17th Australian Poultry Science Symposium, Sydney, NSW, Australia, 7–9 February 2005; Volume 7, pp. 9–16. [Google Scholar]
- Selle, P.H.; Ravindran, V. Microbial phytase in poultry nutrition. Anim. Feed Sci. Technol. 2007, 135, 1–41. [Google Scholar] [CrossRef]
- Konietzny, U.; Greiner, R. Molecular and catalytic properties of phytate-degrading enzymes (phytases). Int. J. Food Sci. Technol. 2002, 37, 791–812. [Google Scholar] [CrossRef]
- Masey O’Neill, H.V.; Liu, N.; Wang, J.P.; Diallo, A.; Hill, S. Effect of xylanase on performance and apparent metabolisable energy in starter broilers fed diets containing one maize variety harvested in different regions of China. Asian Australas. J. Anim. Sci. 2012, 25, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Walk, C.L.; Bedford, M.R.; Santos, T.S.; Paiva, D.; Bradley, J.R.; Wladecki, H.; Honaker, C.; McElroy, A.P. Extraphosphoric effects of superdoses of a novel microbial phytase. Poult. Sci. 2013, 92, 719–725. [Google Scholar] [CrossRef]
- Son, J.H.; Ravindran, V. Feed enzyme technology: Present status and future developments. Recent Pat. Food Nutr. Agric. 2012, 3, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ugwuanyi, J.O. Enzymes for nutritional enrichment of agro-residues as livestock feed. In Agro-Industrial Wastes as Feedstock for Enzyme, Production; Gurpreet, S.D., Surinder, K., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 233–260. [Google Scholar] [CrossRef]
- Walsh, G.A.; Ronan, F.P.; Denis, R.H. Enzymes in the animal-feed industry. Trends. Biotechnol. 1993, 11, 424–430. [Google Scholar] [CrossRef]
- Ravindran, V. Feed enzymes: The science, practice, and metabolic realities. J. Appl. Poult. Res. 2013, 22, 628–636. [Google Scholar] [CrossRef]
- Ojha, B.K.; Singh, P.K.; Shrivastava, N. Enzymes in the Animal Feed Industry. In Enzymes in Food Biotechnology; Mohammed, K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 93–109. [Google Scholar]
- Elangovan, A.V.; Mandal, A.B.; Tyagi, P.K.; Toppo, S.; Johri, T.S. Effects of enzymes in diets with varying energy levels on growth and egg production performance of Japanese quail. J. Sci. Food Agric. 2004, 84, 2028–2034. [Google Scholar] [CrossRef]
- Shalash, S.M.; Sayed, M.A.; Hoda El-Gabry, E.; Nehad Ramadan, A.; Manal Mohamed, S. Nutritive value of distillers dried grains with soluble and broiler performance at starter period. Int. J. Poult. Sci. 2009, 8, 783–787. [Google Scholar] [CrossRef]
- Kocher, A.; Choct, M.; Ross, G.; Broz, J.; Chung, T.K. Effects of enzyme combinations on apparent metabolizable energy of corn-soybean meal-based diets in broilers. J. Appl. Poult. Res. 2003, 12, 275–283. [Google Scholar] [CrossRef]
- Yang, Z.B.; Yang, W.R.; Jiang, S.Z.; Zhang, G.G.; Zhang, Q.Q.; Siow, K.C. Effects of a thermotolerant multi-enzyme product on nutrient and energy utilization of broilers fed mash or crumbled corn-soybean meal diets. J. Appl. Poult. Res. 2010, 19, 38–45. [Google Scholar] [CrossRef]
- Hahn-Didde, D.; Purdum, S.E. The effects of an enzyme complex in moderate and low nutrient-dense diets with dried distillers grains with soluble in laying hens. J. Appl. Poult. Res. 2014, 23, 23–33. [Google Scholar] [CrossRef]
- Jain, J.; Sapna Singh, B. Characteristics and biotechnological applications of bacterial phytases. Process Biochem. 2016, 51, 159–169. [Google Scholar] [CrossRef]
- Dersjant-Li, Y.; Awati, A.; Chulze, H. Phytase in non-ruminant animal nutrition: A critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 2015, 95, 878–896. [Google Scholar] [CrossRef]
- Ghazi, S.; Rooke, J.A.; Galbraith, H.; Bedford, M.R. The potential for the improvement of the nutritive value of soya-bean meal by different proteases in broiler chicks and broiler cockerels. Br. Poult. Sci. 2002, 43, 70–77. [Google Scholar] [CrossRef]
- Marsman, G.J.; Gruppen, H.; Van der Poel, A.F.; Kwakkel, R.P.; Verstegen, M.W.; Voragen, A.G. The effect of thermal processing and enzyme treatments of soybean meal on growth performance, ileal nutrient digestibilities, and chyme characteristics in broiler chicks. Poult. Sci. 1997, 76, 864–872. [Google Scholar] [CrossRef]
- Nortey, T.N.; Patience, J.F.; Sands, J.S.; Yanza, Y.R.; Amnah, S. Effects of xylanase supplementation on digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat by-products from dry milling in grower pigs. J. Anim. Sci. 2008, 86, 3450–3464. [Google Scholar] [CrossRef]
- Yin, Y.L.; McEvoy, J.D.G.; Schulze, H. Apparent digestibility (ileal and overall) of nutrients and endogenous nitrogen losses in growing pigs fed wheat (var. Soissons) or its byproducts without or with xylanase supplementation. Livest. Prod. Sci. 2000, 62, 119–132. [Google Scholar] [CrossRef]
- Debnath, D.; Pal, A.K.; Sahu, N.P.; Jain, K.K.; Yengkokpam, S.; Mukherjee, S.C. Effect of dietary microbial phytase supplementation on growth and nutrient digestibility of Pangasius pangasius (Hamilton) fingerlings. Aquac. Res. 2005, 36, 180–187. [Google Scholar] [CrossRef]
- Debnath, D.; Sahu, N.P.; Pal, A.K.; Jain, K.K.; Yengkokpam, S.; Mukherjee, S.C. Mineral status of Pangasius pangasius (Hamilton) fingerlings in relation to supplemental phytase; absorption, wholebody and bone mineral content. Aquac. Res. 2005, 36, 326–335. [Google Scholar] [CrossRef]
- Iqbal, T.H.; Lewism, K.O.; Cooper, B.T. Phytase activity in the human and rat smallintestine. Gut 1994, 35, 1233–1236. [Google Scholar] [CrossRef]
- Walz, O.P.; Pallauf, J. Microbial phytase combined with amino acid supplementation reduces P and N excretion of growing and finishing pigs without loss of performance. Int. J. Food Sci. Technol. 2002, 37, 835–848. [Google Scholar] [CrossRef]
- Esteve-Garcia, E.; Perez-Vendrell, A.M.; Broz, J. Phosphorus equivalence of a consensusphytase produced by Hansenula polymorpha in diets for young turkeys. Arch. Anim. Nutr. 2005, 59, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Adeola, O.; Cowieson, A.J. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve non-ruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef] [PubMed]
- Augspurger, N.I.; Webel, D.M.; Lei, X.G.; Baker, D.H. Efficacy of an E. coli phytase expressed in yeast for releasing phytate-bound phosphorus in young chicks and pigs. J. Anim. Sci. 2003, 81, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Simons, P.C.; Versteegh, H.A.; Jongbloed, A.W.; Kemme, P.A.; Slump, P.; Bos, K.D.; Wolters, M.G.; Beudeker, R.F.; Verschoor, G.J. Improvement of phosphorus availability by microbial phytase in broilers and pigs. Br. J. Nutr. 1990, 64, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Touchburn, S.P.; Chavez, E.R. Implications of phytic acid and supplemental microbial phytase in poultry nutrition: A review. World’s Poult. Sci. J. 1998, 54, 27–47. [Google Scholar] [CrossRef]
- Peter, C.M.; Baker, D.H. Microbial phytase does not improve protein-amino acid utilization in soybean meal fed to young chickens. J. Nutr. 2001, 131, 1792–1797. [Google Scholar] [CrossRef]
- Cowieson, A.J. Factors that affect the nutritional value of maize for broilers. Anim. Feed Sci. Techno. 2005, 119, 293–305. [Google Scholar] [CrossRef]
- Kemme, P.A.; Schlemmer, U.; Mroz, Z.; Jongbloed, A.W. Monitoring thestepwise phytate degradation in theupper gastrointestinal tract of pigs. J. Sci. Food Agric. 2006, 86, 612–622. [Google Scholar] [CrossRef]
- Rapp, C.; Lantzsch, H.J.; Drochner, W. Hydrolysis of phytic acid by intrin-sic plant and supplemented microbialphytase (Aspergillus niger) in the stom-ach and small intestine of minipigs fittedwith re-entrant cannulas 3. Hydrolysisof phytic acid (IP6) and occurrence ofhydrolysis products (IP5, IP4, IP3andIP2). J. Anim. Physiol. Anim. Nutr. 2001, 85, 420–430. [Google Scholar] [CrossRef]
- Gautier, A.E.; Walk, C.L.; Dilger, R.N. Effects of a high level of phytase on broiler performance, bone ash, phosphorus utilization, and phytate dephosphorylation to inositol. Poult. Sci. 2018, 97, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Dadalt, J.C.; Gallardo, C.; Polycarpo, G.V.; Budino, F.E.L.; Rogiewicz, A.; Berto, D.A. Trindade Neto MA. Ileal amino acid digestibility of broken rice fed to post weaned piglets with or without multicarbohydrase and phytase supplementation. Asian-Australas. J. Anim. Sci. 2016, 29, 483–1489. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. Effects of dietary seaweed extract supplementation in sows and post-weaned pigs on performance, intestinal morphology, intestinal microflora and immune status. Br. J. Nutr. 2011, 106, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R.; Pethick, D.W.; Hopwood, D.E.; Hampson, D.J. Nutritional influences on some major enteric bacterial diseases of pigs. Nutr. Res. Rev. 2002, 15, 333–371. [Google Scholar] [CrossRef] [PubMed]
- Lan, R.; Li, T.; Kim, I.H. Effects of xylanase supplementation on growth performance, nutrient digestibility, blood parameters, fecal microbiota, fecal score and fecal noxious gas emission of weaning pigs fed corn-soybean meal-based diet. Anim. Sci. J. 2017, 88, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Yang, P.; Wang, Y. Cloning expression and characterization of protease-resistance xylanase from Strepromyces fradiae var. k11. J. Microbiol. Biotechnol. 2008, 18, 410–416. [Google Scholar]
- Woyengo, T.A.; Sands, J.S.; Guenter, W.; Nyachoti, C.M. Nutrient digestibility and performance responses of growing pigs fed phytase- and xylanase-supplemented wheat-based diets. J. Anim. Sci. 2008, 86, 848–857. [Google Scholar] [CrossRef]
- Diebold, G.; Mosenthin, R.; Sauer, W.C.; Dugan, M.E.R.; Lien, K.A. Supplementation of xylanase and phospholipase to wheatbased diets for weaner pigs. J. Anim. Physiol. Anim. Nutr. 2005, 89, 316–332. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Kornegay, E.T.; Radcliffe, S.; Denbow, D.M.; Veit, H.P.; Larsen, C.T. Comparison of genetically engineered microbial and plant phytase for young broilers. Poult. Sci. 2000, 79, 709–717. [Google Scholar] [CrossRef]
- Kiarie, E.; Owusu-Asiedu, A.; Peron, A.; Simmins, P.H.; Nyachoti, C.M. Efficacy of xylanase and β-glucanase blend in mixed grains and grain co-products-based diets for fattening pigs. Livest. Sci. 2012, 148, 129–133. [Google Scholar] [CrossRef]
- Casas, G.A.; Stein, H.H. Effects of microbial phytase on the apparent and standardized total tract digestibility of phosphorus in rice coproducts fed to growing pigs. J. Anim. Sci. 2015, 93, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Pustjens, A.M.; Vries, S.; Gerrits, W.J.J.; Kabel, M.A.; Schols, H.A. Residual carbohydrates from in vitro digested processed rapeseed (Brassica napus) meal. J. Agric. Food Chem. 2012, 60, 8257–8263. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.F.; Peng, J.; Tang, T.J.; Liu, Z.L.; Dai, J.J.; Jin, L.Z. Xylanase supplementation improved digestibility and performance of growing pigs fed Chinese double-low rapeseed meal inclusion diets: In vitro and in vivo studies. Asian-Australas. J. Anim. Sci. 2007, 20, 1721–1728. [Google Scholar] [CrossRef]
- Yanez, J.L.; Beltranena, E.; Cervantes, M. Effect of phytase and xylanase supplementation or particle size on nutrient digestibility of diets containing distillers dried grains with solubles co-fermented from wheat and corn in ileal-cannulated grower pigs. J. Anim. Sci. 2011, 89, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.K.; Bergstrom, J.R.; Tokach, M.D.; DeRouchey, J.M.; Goodband, R.D.; Nelssen, J.L.; Dritz, S.S. Efficacy of commercial enzymes in diets containing various concentrations and sources of dried distillers grains with solubles for nursery pigs. J. Anim. Sci. 2010, 88, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Widyaratne, G.P.; Patience, J.F.; Zijlstra, R.T. Effect of xylanase supplementation of diets containing wheat distiller’s dried grains with soluble on energy, amino acid and phosphorus digestibility and growth performance of grower-finisher pigs. Can. J. Anim. Sci. 2009, 89, 91–95. [Google Scholar] [CrossRef]
- Jacela, J.Y.; Dritz, S.S.; DeRouchey, J.M.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L. Effects of supplemental enzymes in diets containing distillers dried grains with solubles on finishing pig growth performance. Prof. Anim. Sci. 2010, 26, 412–424. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kumar, B.; Verma, P. A detailed overview of xylanases: An emerging biomolecule for current and future prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Petry, A.L.; Huntley, N.F.; Bedford, M.R.; Patience, J.F. Xylanase increased the energetic; contribution of fiber and improved the oxidative status, gut barrier integrity, and growth performance of growing pigs fed insoluble corn-based fiber. J. Anim. Sci. 2020, 98, skaa233. [Google Scholar] [CrossRef]
- Gorenz, B.; Iseri, V.; Rubach, J.; Dilger, R.N. Xylanase supplementation of pelleted wheat-based diets increases growth efficiency and apparent metabolizable energy and decreases viscosity of intestinal contents in broilers. Poult. Sci. 2022, 101, 102220. [Google Scholar] [CrossRef] [PubMed]
- Inayah, S.R.; Mutia, R.; Jayanegara, A.; Yanza, Y.R.; Amnah, S. Effects of Xylanase Supplementation on the Performance, Nutrient Digestibility, and Digestive Organ Profiles of Broiler Chickens: A Meta-analysis. World Poult. Res. 2022, 12, 199–211. [Google Scholar] [CrossRef]
- McCormick, K.; Walk, C.L.; Wyatt, C.L.; Adeola, O. Phosphorus utilization response of pigs and broiler chickens to diets supplemented with antimicrobials and phytase. Anim. Nutr. 2017, 3, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, T.; Satoh, K.; Kadoma, Y.; Murakami, Y.; Unten, S.; Atsumi, T.; Sakagami, H.; Fujisawa, S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002, 22, 2711–2717. [Google Scholar]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Hruby, M.; Faurschou Isaksen, M. The effect of conditioning temperature and exogenous xylanase addition on the viscosity of wheat-based diets and the performance of broiler chickens. Br. Poult. Sci. 2005, 46, 717–724. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, S.F. Physiological effects of dietary amino acids on gut health and functions of swine. Front. Vet. Sci. 2019, 6, 169. [Google Scholar] [CrossRef]
- Attia, Y.A.; Bovera, F.; Wang, J.; Al-Harthi, M.A.; Kim, W.K. Multiple amino acid supplementations to low-protein diets: Effect on performance, carcass yield, meat quality and nitrogen excretion of finishing broilers under hot climate conditions. Animals 2020, 10, 973. [Google Scholar] [CrossRef]
- Siegert, W.; Zuber, T.; Sommerfeld, V.; Krieg, J.; Feuerstein, D.; Kurrle, U.; Rodehutscord, M. Prececal amino acid digestibility and phytate degradation in broiler chickens when using different oilseed meals, phytase and protease supplements in the feed. Poult. Sci. 2019, 98, 5700–5713. [Google Scholar] [CrossRef]
- Waldroup, P.; Jiang, Q.; Fritts, C. Effects of supplementing broiler diets low in crude protein with essential and nonessential amino acids. Int. J. Poult. Sci. 2005, 4, 425–431. [Google Scholar]
- Berres, J.; Vieira, S.; Dozier Iii, W.; Cortês, M.; De Barros, R.; Nogueira, E.; Kutschenko, M. Broiler responses to reduced-protein diets supplemented with valine, isoleucine, glycine, and glutamic acid. J. Appl. Poult. Res. 2010, 19, 68–79. [Google Scholar] [CrossRef]
- Zulkifli, I.; Akmal, A.; Soleimani, A. Effects of low-protein diets on acute phase proteins and heat shock protein 70 responses, and growth performance in broiler chickens under heat stress condition. Poult. Sci. 2018, 97, 1306–1314. [Google Scholar] [CrossRef]
- Amer, S.A.; Naser, M.A.; Abdel-Wareth, A.A.; Saleh, A.A.; Elsayed, S.A.; Abdel Fattah, D.M.; Metwally, A.E. Effect of dietary supplementation of alpha-galactosidase on the growth performance, ileal digestibility, intestinal morphology, and biochemical parameters in broiler chickens. BMC Vet. Res. 2020, 16, 144. [Google Scholar] [CrossRef]
- Saeed, M.; Ayaşan, T.; Alagawany, M.; El-Hack, M.; Abdel-Latif, M.; Patra, A. The role of ß-mannanase (Hemicell) in improving poultry productivity, health and environment. Braz. J. Poult. Sci. 2019, 21. [Google Scholar] [CrossRef]
- Olukosi, O.; Cowieson, A.; Adeola, O. Age-related influence of a cocktail of xylanase, amylase, and protease or phytase individually or in combination in broilers. Poult. Sci. 2007, 86, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.; Vieira, S.; Angel, C.; Favero, A.; Maiorka, A. Performance and nutrient utilization of broilers fed diets supplemented with a novel monocomponent protease. J. Appl. Poult. Res. 2011, 20, 322–334. [Google Scholar] [CrossRef]
- Leinonen, I.; Williams, A.G. Effects of dietary protease on nitrogen emissions from broiler production: A holistic comparison using Life Cycle Assessment. J. Sci. Food Agric. 2015, 95, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.; Abdollahi, M.; Zaefarian, F.; Pappenberger, G.; Ravindran, V. The effect of a mono-component exogenous protease and graded concentrations of ascorbic acid on the performance, nutrient digestibility and intestinal architecture of broiler chickens. Anim. Feed Sci. Technol. 2018, 235, 128–137. [Google Scholar] [CrossRef]
- Pereira, L.F.P.; Adeola, O. Energy and phosphorus values of sunflower meal and rice bran for broiler chickens using the regression method. Poult. Sci. 2016, 95, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, C.; Vieira, S.; Rios, H.; Simões, C.; Sorbara, J. Energy and nutrient utilisation of broilers fed soybean meal from two different Brazilian production areas with an exogenous protease. Anim. Feed Sci. Technol. 2016, 221, 267–273. [Google Scholar] [CrossRef]
- Borda-Molina, D.; Zuber, T.; Siegert, W.; Camarinha-Silva, A.; Feuerstein, D.; Rodehutscord, M. Effects of protease and phytase supplements on small intestinal microbiota and amino acid digestibility in broiler chickens. Poult. Sci. 2013, 98, 2906–2918. [Google Scholar] [CrossRef] [PubMed]
- Boguhn, J.; Broz, J.; Rodehutscord, M. Amino acid digestibility of soybean meal and DDGS without and with supplementation of a protease in turkeys. In Proceedings of the 18th European Symposium on Poultry Nutrition, Çeşme/Izmir, Turkey, 31 October–4 November 2011; pp. 542–544. [Google Scholar]
- Manangi, M.; Sands, J.; Coon, C. Effect of phytase on ileal amino acid digestibility, nitrogen retention and AMEn for broilers fed diets containing low and high phytate phosphorus. Int. J. Poult. Sci. 2009, 8, 929–938. [Google Scholar] [CrossRef][Green Version]
- Angel, C.; Saylor, W.; Vieira, S.; Ward, N. Effects of a monocomponent protease on performance and protein utilization in 7-to 22-day-old broiler chickens. Poult. Sci. 2011, 90, 2281–2286. [Google Scholar] [CrossRef]
- Huisman, J.; Jansman, A.J.M. Dietary effects and some analytical aspects of antinutritional factors in peas (Pisum sativum), common beans (Phaseolus vulgaris) and soyabeans (Glycine max) in monogastric farm animals. Nutr. Abstr. Rev. 1991, 61, 90121. [Google Scholar]
- Raemaekers, R.H. Crop Production in Tropical Africa; DGIC: Brussels, Belgium, 2001; pp. 809–828. [Google Scholar]
- Nsoh, A. Growth Performance, Blood Profile and Carcass Characteristics of Growing Pigs Fed Diets Containing Varying Levels of Soybean Milk Residue (SBMR). Available online: http://hdl.handle.net/123456789/5322 (accessed on 10 August 2013).
- Yin, Y.L.; Baidoo, S.K.; Jin, L.Z.; Liu, Y.G.; Schulze, H.; Simmins, P.H. The effect of different carbohydrase and protease supplementation on apparent (ileal and overall) digestibility of nutrients of five hulless barley varieties in young pigs. Livest. Prod. Sci. 2001, 71, 109–120. [Google Scholar] [CrossRef]
- Yin, Y.L.; Deng, Z.Y.; Huang, H.L.; Li, T.J.; Zhong, H.Y. The effect of arabinoxylanase and protease supplementation on nutritional value of diets containing wheat bran or rice bran in growing pig. J. Anim. Feed Sci. 2004, 13, 445–461. [Google Scholar] [CrossRef]
- Omogbenigun, F.O.; Nyachoti, C.M.; Slominski, B.A. Dietary supplementation with multienzyme preparations improves nutrient utilization and growth performance in weaned pigs. J. Anim. Sci. 2004, 82, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.K.; Ingale, S.L.; Kim, J.S.; Kim, Y.W.; Kim, K.H.; Lohakare, J.D.; Chae, B.J. Effects of exogenous enzyme supplementation to corn-and soybean meal-based or complex diets on growth performance, nutrient digestibility, and blood metabolites in growing pigs. J. Anim. Sci. 2012, 90, 3041–3048. [Google Scholar] [CrossRef]
- Han, J.A.; BeMiller, J.N. Effects of protein on crosslinking of normal maize, waxy maize, and potato starches. Carbohydr. Polym. 2008, 73, 532–540. [Google Scholar] [CrossRef]
- Bedford, M.R. Exogenous enzymes in monogastric nutrition their current value and future benefits. Anim. Feed Sci. Technol. 2000, 86, 1–13. [Google Scholar] [CrossRef]
- Bedford, M.R.; Schulze, H. Exogenous enzymes for pigs and poultry. Nutr. Res. Rev. 1998, 11, 91–114. [Google Scholar] [CrossRef]
- Eltahan, H.M.; Cho, S.; Rana, M.M.; Elkomy, A.E.; Wadaan, M.A.; Alagawany, M.; Eltahan, H.M. Dietary exogenous phytase improve egg quality, reproductive hormones, and prolongs the lifetime of the aging Hy-line brown laying hens fed non-phytate Phosphorus. Poult. Sci. 2023, 102, 10289. [Google Scholar] [CrossRef]
- Lelis, G.R.; Albino, L.F.T.; Calderano, A.A. Diet supplementation with phytase on performance of broiler chickens. Braz. J. Anim. Sci. 2012, 41, 929–933. [Google Scholar] [CrossRef]
- Gonzalez-Vega, J.C.; Walk, C.L.; Stein, H.H. Effect of phytate, microbial phytase, fiber, and soybean oil on calculated values for apparent and standardized total tract digestibility of calcium and apparent total tract digestibility of phosphorus in fish meal fed to growing pigs. J. Anim. Sci. 2015, 93, 4808–4818. [Google Scholar] [CrossRef] [PubMed]
- Maison, T.; Liu, Y.; Stein, H.H. Apparent and standardized total tract digestibility by growing pigs of phosphorus in canola meal from North America and 00-rapeseed meal and 00-rapeseed expellers from Europe without and with microbial phytase. J. Anim. Sci. 2015, 93, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vega, J.C.; Walk, C.L.; Stein, H.H. Effects of microbial phytase on apparent and standardized total tract digestibility of calcium in calcium supplements fed to growing pigs. J. Anim. Sci. 2015, 93, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.X.; Kim, I.H. Effects of supplementation of high-dosing Trichoderma reesei phytase in the corn-wheat-soybean meal-based diets on growth performance, nutrient digestibility, carcass traits, faecal gas emission, and meat quality in growing-finishing pigs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.E.; Zhou, F.X.; Dutra Jr, W.M.; Kim, S.W. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim. Nut. 2019, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Yun, H.M.; Kim, I.H. Influence of low- or high-density corn and soybean meal-based diets and protease supplementation on growth performance, apparent digestibility, blood characteristics and noxious gas emission of finishing pigs. Anim. Feed Sci. Tech. 2016, 216, 281–287. [Google Scholar] [CrossRef]
- Liu, X.; Yin, J.; Kim, I.H. Effect of protease derived from Pseudoalteromonas arctica supplementation on growth performance, nutrient digestibility, meat quality, noxious gas emission and blood profiles in finishing pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1926–1933. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, Y.; Liu, Y.; Yu, B.; He, J.; Zheng, P.; Yu, J. Effects of a Novel Protease on Growth Performance, Nutrient Digestibility and Intestinal Health in Weaned Piglets. Animals 2022, 12, 2803. [Google Scholar] [CrossRef]
- Xie, P.; Huang, H.L.; Dong, X.Y.; Zou, X.T. Evaluation of extruded or unextruded double-low rapeseed meal and multienzymes preparation in pigs’ nutrition during the finishing phase of production. Ital. J. Anim. Sci. 2012, 11, e34. [Google Scholar] [CrossRef]
- Emiola, I.A.; Opapeju, F.O.; Slominski, A.; Nyachoti, C.M. Growth performance and nutrient digestibility in pigs fed wheat distillers dried grains with solubles-based diets supplemented with a multicarbohydrase enzyme. J. Anim. Sci. 2009, 87, 2315–2322. [Google Scholar] [CrossRef]
- Prandini, A.; Sigolo, S.; Moschini, M.; Faeti, V.; Marchetto, G.; Marino, A.; Casa, G.D. Effect of Italian heavy pig diets based on different barley varieties with or without non-starch polysaccharides degrading enzymes on growth performance, carcass characteristics and fresh thigh quality. Ital. J. Anim. Sci. 2016, 15, 428–436. [Google Scholar] [CrossRef]
- Nguyen, D.; Upadhaya, S.D.; Lei, X.J.; Yin, J.; Kim, I.H. Influence of dietary protease supplementation to corn–soybean meal-based high- and low-energy diets on growth performance, nutrient digestibility, blood profiles, and gas emission in growing pigs. Can. J. Anim. Sci. 2019, 99, 482–488. [Google Scholar] [CrossRef]
- Balasubramanium, B.; Park, J.H.; Shanmugam, S.; Kim, I.H. Influences of enzyme blend supplementation on growth performance, nutrient digestibility, fecal microbiota and meat-quality in grower-finisher pigs. Animals 2020, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Nortey, T.N.; Patience, J.F.; Simmins, P.H.; Trottier, N.L.; Zijlstra, R.T. Effects of individual or combined xylanase and phytase supplementation on energy, amino acid, and phosphorus digestibility and growth performance of grower pigs fed wheat-based diets containing wheat millrun. J. Anim. Sci. 2007, 85, 1432–1443. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, Y.; Lu, F.; Han, Z.; Wang, T. Effects of different levels of supplementary alpha-amylase on digestive enzyme activities and pancreatic amylase mRNA expression of young broilers. Asian-Australas. J. Anim. Sci. 2008, 21, 97–102. [Google Scholar] [CrossRef]
- Yegani, M.; Korver, D.R. Effects of corn source and exogenous enzymes on growth performance and nutrient digestibility in broiler chickens. Poult. Sci. 2013, 92, 1208–1220. [Google Scholar] [CrossRef]
- Ferket, P.R.; Sell, J.L. Effect of severity of early protein restriction on large turkey toms.: 1. Performance characteristics and leg weakness. Poult. Sci. 1989, 68, 676–686. [Google Scholar] [CrossRef]
- Hester, P.Y.; Krueger, K.K.; Jackson, M. The effect of restrictive and compensatory growth on the incidence of leg abnormalities and performance of commercial male turkeys. Poult. Sci. 1990, 69, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Pintar, J.; Homen, B.; Gazic, K.; Janjecic, Z.; Sikiric, M.; Cerny, T. Effects of supplemental phytase on nutrient excretion and retention in broilers fed different cereal-based diets. Czech Anim. Sci. 2005, 50, 40–46. [Google Scholar] [CrossRef]
- Min, Y.; Choi, Y.; Choe, K.Y.; Jeong, Y.; Kim, D.; Kim, J.; Jung, H.; Song, M. Effects of dietary mixture of protease and probiotics on growth performance, blood constituents, and carcass characteristics of growing-finishing pigs. J. Anim. Sci. Technol. 2019, 61, 272–277. [Google Scholar] [CrossRef] [PubMed]

| Enzymes | Substrates | Effect | Example | References |
|---|---|---|---|---|
| Phytase | Phytates | Phytase degrades phytate bonds, liberating trapped nutrients, which leads to enhanced livestock efficiency. Additionally, it increases phosphorus absorption, reducing the risk of soil and water contamination through excreta. Moreover, phytase supplementation increases amino acid availability. | Histidine acid phytase (pH 5.0) mainly applied to feed for poultry or pigs. | Ojha et al. [10] |
| Proteases | Proteins | Certain proteases have been found to enhance the apparent ileal nitrogen digestibility and apparent nitrogen retention in both broiler chicks and broiler cockerels. When added exogenously, proteases can further enhance the digestibility of proteins in feed ingredients by solubilizing and hydrolyzing dietary proteins. As a result, levels of antinutritional factors decrease. These proteases can originate from animal, vegetable, or microbial sources. | Proteases isolated from microorganisms such as Aspergillus niger and Bacillus spp. Chymosin, pepsin A Bromelain, papain, ficine, aminopeptidase, bacillolysin 1, dipeptidyl peptidase III, chymotrypsin, subtilisin, trypsin. | Ghazi et al. [18]; Marsman et al. [19]. |
| Carbohydrases | Carbohydrates (fiber and/or starch) | Exogenous enzymes, such as carbohydrases and proteases, improve the digestibility of plant biomass, leading to an increase in energy availability. This beneficial effect extends to both poultry and pig diets. | Xylanases and β-glucanases (degrade cell walls, used in poultry), β-mannanases Pectinases α-galactosidases α-amylase (improves digestibility of starch, body weight gain has been observed in poultry) | Nortey et al. [20]; Yin et al. [21]. |
| Enzyme | Level | Animals | Effects | Reference |
|---|---|---|---|---|
| Phytase | 1000 FYT/kg | Laying hens | Enhanced overall shell quality and a beneficial influence on reproductive hormones to sustain and support continuous egg production. | Eltahan et al. [88] |
| Phytase | 250 ftu/kg and 500 ftu/kg | Broiler chickens | Reduction in nutritional levels improves bird performance | Lelis et al. [89] |
| Phytase | 50 g/ton diet | Weaned pig | Improved ATTD of energy and protein; improved standard ileal digestibilty (SID) of histine | Pluske et al. [38] |
| Phytase | 500 units/kg diet | Growing pigs | Increased the ATTD and STTD of Ca and ATTD of P | Gonzalez-Vega et al. [90] |
| Phytase | 1500 units/kg diet | Growing pigs | Increased digestibility of P in Canola meal, 00-rapeseed meal and 00-rapeseed expellers | Maison et al. [91]. |
| Phytase | 500 units/kg 250 units/kg diet | Growing pigs | Fecal output, as well as the output of Ca in feces, was reduced. Increased ATTD of Ca and P and increased STTD of Ca. Endogenous loss of Ca decreased. Daily P output reduced | Gonzalez-Vega et al. [92] |
| Phytase | 1500 FTU/kg diet | Growing-finishing pigs | Increased body weight and the ATTD of P but no effects on meat quality | Dang and Kim et al. [93] |
| Xylanase | 4000 unit/kg diet | Growing pigs | No effect on nutrient digestibility | Yanez et al. [48] |
| Xylanase | 4000 unit/kg diet | Growing- finishing pigs | Improved apparent ileal digestibilty (AID) of energy and threonine in wheat but no improvement in growth performance | Widyaratne et al. [50] |
| Xylanase | 45,000 XU/kg | Weaning Pigs | Enhanced growth performance and gut morphology, reduced digesta viscosity, and reduced intestinal oxidative stress | Duarte et al. [94] |
| Protease | 125 g/ton | Finishing pigs | Improved growth performance and ATTD of nutrients and reduced stress-related hormones | Upadhaya et al. [95] |
| Protease | 1 to 3 g/kg feed | Finishing pigs | Linear reduction in feed conversion during overall experimental period; linear increase in nutrient digestibility; linear reduction in serum total protein concentration | Liu et al. [96] |
| Protease | 150–300 mg/kg | Weaned Piglets | Promoted nutrient absorption, improved small intestine morphology and enhanced digestive enzyme activity | Zhu et al. [97] |
| Phytase, Xylanase | 250 and 500; 200 and 4000 units/kg diet respectively | Growing pigs | No effect of phytase on AID of amino acids, and xylanase improved AID of some AA | Woyengo et al. [41] |
| Cellulase + xylanase + beta-glucanase + protease | 10,000, 6000, 5000 and 12,000 units/g respectively | Finishing Pigs | No effect on growth performance. Improved IgG and reduced malondialdehyde levels in serum in extruded RSM | Xie et al. [98] |
| Xylanase + glucanase + cellulase | 2200, 1100 and 1200 unit/kg diet respectively | Finishing pigs | Increased AID of DM, organic matter, energy, threonine, proline and serine | Emiola et al. [99] |
| Multienzyme (beta-glucanase and beta-xylanase) | 1 g/kg feed | Growing-finishing pigs | No effect on growth performance and carcass characteristics for both barley types | Prandini et al. [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sureshkumar, S.; Song, J.; Sampath, V.; Kim, I. Exogenous Enzymes as Zootechnical Additives in Monogastric Animal Feed: A Review. Agriculture 2023, 13, 2195. https://doi.org/10.3390/agriculture13122195
Sureshkumar S, Song J, Sampath V, Kim I. Exogenous Enzymes as Zootechnical Additives in Monogastric Animal Feed: A Review. Agriculture. 2023; 13(12):2195. https://doi.org/10.3390/agriculture13122195
Chicago/Turabian StyleSureshkumar, Shanmugam, Junho Song, Vetriselvi Sampath, and Inho Kim. 2023. "Exogenous Enzymes as Zootechnical Additives in Monogastric Animal Feed: A Review" Agriculture 13, no. 12: 2195. https://doi.org/10.3390/agriculture13122195
APA StyleSureshkumar, S., Song, J., Sampath, V., & Kim, I. (2023). Exogenous Enzymes as Zootechnical Additives in Monogastric Animal Feed: A Review. Agriculture, 13(12), 2195. https://doi.org/10.3390/agriculture13122195








