Abstract
Guar (Cyamopsis tetragonoloba) is an annual legume tolerant to drought. Guar meal (GM) is a protein- and carbohydrate-rich co-product generated after the mechanical separation of the endosperm from the germ and hull of guar seed. GM has received considerable interest in animal feed as an alternative to soybean meal (SM). In this study, we aimed to assess the nitrogen (N) balance indicators, performance, carcass traits, and main greenhouse gas (GHG) emissions resulting from enteric fermentation (E-CH4) and manure (M-CH4 and N2O). Two tests were performed: (i) a biological trial on 45 pigs (15 animals/group) and (ii) a digestibility test in metabolism cages (N = 15, 5 replicates/group). Three different diets were given to the pigs: one diet was based on 0% GM (SM diet); in the second, GM-50%, GM replaced 50% of the SM; and the third was GM-100%, in which GM fully replaced the SM. The GM and SM diets were analyzed for their proximate composition. A model based on prediction equations was used to estimate the GHGs. GM up to 10% in the diets of finishing pigs did not significantly impact growth performance or carcass traits, although a slight increase in neutral detergent fiber (NDF) was observed. GM up to 10% improved N digestibility (p < 0.0001), net protein utilization (p < 0.0001), the biological value of protein, coefficients of metabolizability, and the coefficient of the total tract’s apparent digestibility. Irrespective of its dietary proportion, GM decreased total nitrogen output (TNO, p = 0.11). A highly significant impact was noted for N2O and E-CH4 (for DM, p < 0.0001), as well as a significant impact for E-CH4, expressed as g CO2 Eq (p = 0.007), and g CO2 Eq. LU (livestock unit, p = 0.005), also reported as ADG (p = 0.024). Manure, M-CH4, was not significantly influenced. In conclusion, GM can replace up to 100% SM and is thus a valuable byproduct that does not alter animal performance and can positively impact N2O and E-CH4.
1. Introduction
The livestock sector is a notable consumer of natural resources. Classical diets for pigs are based on a mix of maize and soybean meal (SM) as the primary energy and protein-rich feedstuff. However, there is a growing discrepancy between production, availability, and demand [1,2]. Only non-genetically modified (non-GM) soybean is permitted in the European Union. With this background, supply chain disturbances and reduced packing plant capacity have caused considerable difficulties [3]. Romania is a country that relies on the importation of SM at a fluctuating price. In addition, the frequency of drought might lead to an increased gap between the feed supply and the nutritional needs of animals.
The identification of well-known feedstuffs and the use of locally accessible vegetable resources are required to address feed deficits. High priority has been given to the search for solutions to improve existing feed resources efficiently. The availability of non-conventional forage resources is increasing, although most of these resources need to be more palatable [4]. Guar (Cyamopsis tetragonoloba) is an atmospheric nitrogen (N)-fixing annual legume that is drought-tolerant and environmentally friendly. Guar meal (GM) is a co-product of the guar gum industry that is not genetically altered and is characterized by an elevated protein level (around 50%), as well as carbohydrates. GM is composed of germs and hulls that remain after the mechanical separation of the endosperm from the germ and guar seed hulls [5,6]. The nutritional value of GM was described in detail by Biel and Jaroszewska [7]. The protein concentrations in GM range from 33 to 60% and have favorable amino acid profiles. On the other hand, anti-nutritive components such as guar gum (mannan), saponins, and trypsin inhibitors limit GM’s usage in animal feed [8].
The anti-nutritional properties of GM, as well as its low palatability, have led to doubts about the use of this byproduct. However, thermic treatment and limiting the levels of inclusion can eliminate these drawbacks. According to Abdel-Wahab et al. [9], a reduction in the levels of certain anti-nutritional factors (e.g., β-mannan and saponin) can positively affect the health and performance of buffalo. In addition, this co-product is a natural feed ingredient as no chemicals or preservatives are used to obtain it. Previous studies highlighted changes in some growth, meat quality, and health parameters when using GM in poultry [10,11,12], cattle [13], sheep [14,15], goat [16], and buffalo [9]. Karpiesiuk et al. [2] and Hasan et al. [4] investigated the effects of using dietary GM supplementation as a cost-cutting technique on the performance and nutrient metabolism of pigs. Nonetheless, it remains critical to research the effects of using the GM co-products that remain after guar gum extraction on greenhouse gas (GHG) production.
Pig farming is growing steadily in terms of its complexity, industrialization, and intensification. The gases produced in the pig house impact not only the health and efficiency of the pig sector but also human health and quality of life. Relevant hazardous gases include gaseous N and its compounds such as nitrous oxide (N2O) and ammonia (NH3). Consequently, GHG emissions linked to global warming potential (GWP) have provoked public concern around the globe. Pork is one of the most commonly consumed meats [17]. According to Bälter et al. [18], food of animal origin emits more GHGs than feed of vegetable origin. Pig farming has a large impact on GHG production, especially enteric CH4 (E-CH4) and manure-based CH4 (M-CH4) and N2O in manure, while carbon dioxide (CO2) emissions are considered zero since plants reuse this gas through photosynthesis [19,20]. According to FAO [21], pigs produce lower E-CH4 emissions (avg. 11%) than ruminants (more than 90%), but there are higher CH4 emissions in their manure (more than 69% compared to ruminants, which produce less than 8%). The manure storage of N2O is more pronounced among chickens (avg. 66%) and pigs (avg. 20%) compared to that among ruminants (less than 7%) [21].
The reduction in GHG emissions via animal feed and manure management is a significant problem for sustainable pig farming. Biogenic CH4 is a volatile organic compound produced by bacteria in pigs’ digestive tracts (E-CH4) and feces through the anaerobic breakdown of organic matter [22,23]. The GWP of CH4 is 25 times greater than that of CO2 [24]. The microbial process in the N cycle and manure carbon content, along with the time required for storage and treatment, determines the amount of N2O emitted during storage and treatment [19,25]. The GWP of N2O is 298-fold greater than that of CO2. N2O originates only from manure [26] and accounts for about 26% of N2O production.
Taking into consideration the factors mentioned above, this study aimed to test two hypotheses: 1. The total substitution of SM with GM will affect the growth performance and carcass characteristics of pigs; 2. GM has the potential to reduce N from manure and the main GHG emissions (CH4 and N2O) in growing–finishing pigs.
2. Materials and Methods
The trials took place in the IBNA Balotesti experimental Biobase located in Ilfov county in the southeastern part of Romania. This area is located in the central-eastern part of the Walachia Plain and is characterized by a temperate–continental climate, with dry and hot summers and cold winters.
Two experiments were carried out following protocol 7976/12/12, authorized by the Ethical Committee of the IBNA Balotesti and pursuant to Romanian Law No. 199/2018, which complies with the EU Directive 2010/63/EU on animal research.
GM is a protein- and carbohydrate-rich co-product generated after the mechanical separation of the endosperm from the germ and hull of natural guar seed that is broken and heat-treated (roasted for 3 min at 120–130 °C) to improve digestibility and palatability. GM has high nutritional value and a similar or reduced cost to SM. Section 3.1 provides a detailed comparison between GM’s composition and that of soybean meal.
2.1. Animals and Housing
Experiment 1. Experimental design.
This study used a total of forty-eight healthy, crossbred finishing Topigs pigs ((female Large White × Hybrid (Large White × Pietrain) × male Talent (mainly Duroc)), (2 replicates/group; 8 pigs/pen), 69.73 ± 0.77 kg initial body weight (BW), 120 ± 5 days old, with a similar sex ratio (mixed, with 4 ♀ and 4 ♂ in each pen), and ear-tagged individually. The pigs were randomly assigned to three feeding groups for 35 days in a grow–finish shelter with strict environmental controls (21 °C; 60% relative humidity). The pigs received experimental diets for 35 days.
Experiment 2. N digestibility.
Following the procedure outlined in the law, using individual steel cages in an atmosphere-controlled room, a metabolic test was conducted over 21 days (7 days for accommodation) to assess N metabolism. A total of 15 barrows (Topigs hybrid pigs, BW avg. 89.6 kg ± 2 kg) were selected and split into three groups (5 replicates/group). Previous research has shown that three to six animals per group are statistically adequate for digestibility trials [27]. The pigs were individually housed and weighed. The digestibility trial weeks were divided into two balance periods (4 sampling days per period).
2.2. Treatments
To evaluate growth performance and carcass traits and estimate the main GHGs (CH4 and N2O), the GM and SM were analyzed for ether extract (EE), crude protein (CP), crude fiber (CF), amino acids, and minerals before they were included in the diets.
The composition and nutritional characteristics of the diets are outlined in Table 1. Throughout both experiments, the diets remained the same. Three feeding treatments that met the nutritional requirements of the Topigs hybrid were formulated: (1) The control group (SM); (2) GM-50%, where 50% of the SM was replaced with GM; and (3) GM-100%, in which the SM was completely replaced with GM. The diets included crystalline amino acids, DL-methionine and L-Lysine-HCl, to meet the requirements for all three diets, as well as calcium carbonate and monocalcium phosphate to provide the Ca and P requirements. The fiber level was about 3.8% higher in the GM-100% diet vs. that in the SM and GM-50% diets; NDF was +8.8% higher in GM-50% vs. the SM diet, with an increase of 17.06% in the GM-100% vs. SM diet.

Table 1.
Experimental diet composition based on two levels of GM that replaced 50% or 100% of the SM in the diets.
2.3. Measurement and Sampling
The BW of each pig was recorded on an electronic scale at the start and end of the biological and digestibility trials. The pigs were fasted overnight before being weighed, and blood was collected to minimize postprandial nutrient contents. The average daily gain (ADG) and feed conversion ratio (FCR) were computed based on BW. We used the equations of Diaz et al. to calculate the Kleiber ratio (KR), relative growth rate (RGR, %) based on ADG, and metabolic BW (MBW0.75), along with BW and age [28].
The carcass traits (backfat thickness, Longissimus dorsi area, and lean meat percentage) were determined on the left side using a PIGLOG 105 ultrasonic apparatus (SFK-Technology, Denmark) fitted with a formula for assessing meat percentage:
where LD is the Longissimus dorsi muscle. Fat-1 was measured 7 cm sideways behind the last rib from the middle dorsal line, whereas fat-2 was measured 10 cm from the last rib to the cranial section and 7 cm sideways from the middle dorsal line.
Y = 64.39 − 0.28 Fat-1 + 0.14 LD muscle thickness − 0.55 Fat-2
During the digestibility trial, samples were collected 4 days/week (two balance periods) from 08.00 h to 09.00 h to determine the N contents of the feces and urine. After weighing the total urine collected, 10% aliquots of urine were subsampled and stored at 4 °C for analysis. H2SO4 at a concentration of 25% was added to each urine container to decrease the pH and diminish nitrogenous component volatilization. In the same way, after weighing the total feces samples, a quantity of about 10% ± 0.4 g was subsampled and stored at 4 °C prior to the N analyses. The samples were processed according to the procedure outlined by Hăbeanu et al. [29]. In the same manner, a blank digest was used. The samples were digested in the presence of catalyzers with H2SO4 and then distilled and titrated. Class A glassware was employed for transvasation, dilution, and storage. The total N production was estimated after measuring N intake and excretion.
Blood samples (~6 mL) were taken via jugular venipuncture in heparin tubes (2 replicates per animal). Then, the samples were centrifuged for 15 min at 3000 rpm (iFuge D06, Neuation Technologies Pvt. Ltd., Gujarat, India), and the plasma obtained was placed in Eppendorf safe-lock tubes and stored in a freezer at −20 °C until urea nitrogen (PUN) analysis (Spotchem EZ SP-4430 chemistry analyzer).
2.4. Analytical Laboratory Procedure
The proximate composition of the GM, SM, and diets was assessed at IBNA Balotesti in duplicate for dry matter (DM), CP, EE, CF, and their fractions compared to neutral detergent fiber (NDF) and acid detergent fiber (ADF), using the procedures outlined in Commission Regulation (EC) no. 152 (OJEU, 2009). Briefly, the semiautomatic traditional Kjeldahl technique, using a Kjeltek auto 1030 Tecator (SR EN ISO 5983-2:2009 [30] AOAC 2001.11 [31]), was employed to determine the CP. CF extraction was performed using an intermediate filtration method (standard SR EN ISO 6865:2002 [32]). Van Soest extractions were used to assess NDF and ADF according to SR EN ISO 16472:2006 [33] and SR EN ISO 13906:2008 [34]. A Raw Fiber Extractor FIWE 6 (Velp Scientifica, Usmate Velate, MB, Italy) was used for the analysis.
The amino acid composition was determined in duplicate via high-performance liquid chromatography, using HPLC Surveyor Plus Thermo Electron equipment (Waltham, MA, USA) fitted with a HyperSil BDS C18 column (Thermo Electron, Waltham, MA, USA) using silica sized at 250 mm × 4.6 mm × 5 μm, as previously described by Varzaru et al. [35] and Gheorghe et al. [36].
In the digestibility trial, from the total quantity of collected feces, 10% ± 0.4 g subsamples were taken for analysis.
The samples were digested with H2SO4 in the presence of catalyzers to assess the N concentration, followed by distillation and titration. The semiautomatic Kjeldahl method was carried out on a Kjeltec Auto 1030 Analyzer (Hillerod, Denmark). The reagents for mineral concentration were supplied by Merck (Darmstadt, Germany). Class A glassware was used for transvasation, dilution, and storage. Stock solutions traceable to standard reference material (NIST) were used for calibration. N retained (NR), total N output (TNO), and N digestibility were determined by measuring N intake (on a DM basis) and N excretions according to the methods described by Hăbeanu et al. [29] and using equations described by Hlatini et al. [27]. The coefficient of the total tract apparent digestibility (CTTAD), the coefficient of metabolizability (CAM), the biological value of protein (BVP), and the net protein utilization were calculated using the following equations:
CTTAD = (N intake − fecal N output)/N intake
CAM = N intake − N fecal output − N urinary output/N intake
BVP = N retained/N digested
NPU = N retained/N intake.
Blood samples were taken from the jugular vein, placed in heparin tubes (in duplicate), and then centrifuged for 15 min at 3000 rpm to separate the plasma. We used a chemical analyzer (Spotchem EZ SP-4430) to test the N and urea N in the plasma (PUN).
2.5. CH4 (E-CH4 and M-CH4) and N2O Emissions
In this study, to obtain models for estimating GHGs, the input and experimental output data were incorporated into prediction equations established by the IPCC [19,37] and prior works. The TNO assessed in our feeding trial was integrated into the equation for N2O prediction proposed by Philippe and Nick [26]:
where 0.2% is the conversion factor of the N excreted into N2O (IPCC, 2006) [19] in manure storage pits under animal housing, and 44/28 is the ratio of the molar mass of N2O compared to that of N.
N2O = TNO ∗ 0.2/100 ∗ 44/28
EvaPig® software, version 2.0.3.2, created by the French National Institute for Agricultural Research, Metex Nvistago, and the French Association of Zootechnie, was used for compound feed verification.
In this work, to determine the E-CH4, expressed as the CO2 equivalent (g Eq.CO2 * day−1), we applied Philippe and Nick’s equation [26]:
where dRes are digestible residues.
E-CH4 = 0.012 ∗ dRes ∗ DM intake
The IPCC’s [19,37] equations were used to estimate manure CH4 emissions:
where VS means solid volatile excretions per day resulting from organic solid manure substances, calculated using the IPCC’s [37] Tier 1 method with the following equation:
CH4, manure = VS ∗ B0 ∗ MCF ∗ fSMD
VS = VSt ∗ BW ∗ 1000−1 (kg * day−1).
Here, VSt = 4.9, where B0 denotes the maximum CH4 production in the pigs’ manure (0.45 m3 CH4 * kg−1 VS * 0.67, which is the factor used for converting m3 into kg; IPCC [37]); MCF is the CH4 conversion factor for the waste management system used, depending on the climate region, expressed in %; MCF (4% for solid storage in a dry, temperate region; IPCC [37]); and fSMD is the fraction of manure used by each management system. In our study, the solid storage system applied to the entire amount of manure (fSMD = 1).
2.6. Statistical Analyses
IBM SPSS (2011, version 20) was used to describe the experimental data recorded and predicted statistically. A completely randomized experimental design was used to assign the animals to three dietary treatments, with each group having a similar sex ratio of 24 ♀:24 ♂ (mixed, with 4 ♀ and 4 ♂ in each pen). In our model, in the first experiment, the diet was considered a fixed factor, the pen was the experimental unit for measuring intake, and the pigs served as the experimental units for determining the other variables assessed. In the balance trial, each pig was considered an experimental unit. A Shapiro–Wilk test was used to verify data distribution. The impact of diet was regarded as statistically significant if the p-value was less than 0.05. The means determined to be significant were separated using an LSD test. Replicate effects were not included in the study as the p-value was higher than 0.05. A Pearson test was used to determine the measure of correlation. A strong correlation was defined as a coefficient value > 0.7, and a moderate correlation was defined as a value below 0.69 and up to 0.5 (Akoglu cited by [38]); higher values were defined as significantly affected.
The group size was established based on Charan and Kantharia [39], where E = Total number of animals − Total number of groups, and E = the degree of freedom of the analysis of variance. In our study, the E value was 45, which is higher than 20. Therefore, including more animals would not increase the probability of obtaining significant findings.
3. Results
3.1. Feedstuff Chemical Composition
Data on the chemical composition of protein-rich sources showed that GM has higher levels of analyzed CP (+15.4%), EE (+43%), and limitative amino acids (AA) (+34% lysine and +42% Met + Cyst) (Table 2). The lysine level was found to be higher in both feedstuffs. The diets were formulated to include 50 g or 100 g GM/kg feed. The addition of GM into the diet increased the NDF and ADF levels. These fiber fractions potentially impacted the responses of the animals.

Table 2.
Comparison of the compositions of SM and GM.
Throughout the experiments, we did not observe any health problems or the refusal of feed among the animals.
3.2. Growth Performance in the Biological Trial
Irrespective of the GM inclusion level, the carcass traits and growth performance were not significantly affected (Figure 1).
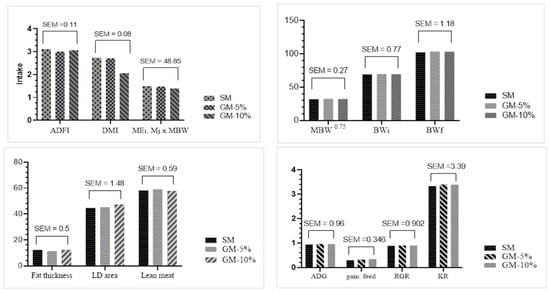
Figure 1.
Intake and descriptive statistics of the growth parameters and carcass traits of the fattening pigs fed two levels of a GM diet that replaced 50% or 100% SM (SM diet). p > 0.05, with no significant difference between the mean. The measurements were performed with PIGLOG 105 on live animals to determine their carcass traits. The number of observations was 48. Abbreviations: average daily feed intake (ADFI, Kg); dry matter intake (DMI, Kg); body weight (BW, Kg); metabolic BW (MBW0.75); average daily gain (ADG, Kg); relative growth rate (RGR, %); Kleiber ratio (KR, Kg); LD, Longissimus dorsi; fat thickness (mm); LD area (mm); lean meat (%); standard error of the mean (SEM).
3.3. N Digestibility
Table 3 presents the intake data, mean values, and SEMs for the balance indicators. While N, fiber, and ADF decreased (p > 0.05), the NDF fraction increased in the group fed GM, regardless of the percentage of inclusion. This result was reflected in the fecal N composition, with a significant decrease observed in the experimental groups compared to the SM group. However, no influence on urinary N was observed (p > 0.05). Therefore, although a decrease in TNO was determined, this decrease was not significant. A significantly higher impact was obtained for N excretion in the % intake, N digestibility, NPU, CAM, and CTTAD indicators.

Table 3.
N metabolism of the fattening pigs fed two levels of a GM diet that replaced 50% or 100% of the SM diet.
3.4. CH4 (E-CH4 and M-CH4) and N2O Emissions Estimated
Table 4 outlines the level of the main GHGs ge nerated via the enteric fermentative process and in the manure. When expressed as DMI bases, N2O decreased significantly in the GM-50% and GM-100% groups vs. the SM-fed group. A significant decrease was observed for E-CH4, expressed as the g of CO2 Eq * day−1 (9% lower in the GM-50%-fed group and 21% lower in the GM-100%-fed group compared with the SM diet) and reported as the livestock unit (LU), considered to be 500 kg LW (live weight) based on Philippe and Nick [26] (10% and 22% lower in the GM-50% and GM-100% groups compared to the SM group). The highest influence (p < 0.0001) was obtained when evaluated using the DMI bases (8% and 15% lower in the experimental groups vs. that in the SM group). Conversely, the dietary addition of GM did not significantly influence M-CH4.

Table 4.
Mean GHG level (g CO2 Eq. N2O, E-CH4, and M-CH4) ± SEM for the fattening pigs fed two levels of GM that replaced 50% or 100% of the SM.
3.5. Relationship between Input and Output Parameters
The Pearson coefficients from Table 5 show a strong correlation between specific input and output parameters. For example, the level of dietary GM is strongly positively correlated with the % N digestibility and NPU and negatively correlated with the N excretion of the % intake (p < 0.0001) and has a high-to-moderate correlation with E-CH4 (r = −0.48; p < 0.01). However, for the other input parameters, such as the ADFI, DMI, fiber, and the fractions of ADF and NDF, the N intake presents a strong positive relationship with the TNO, NR, N2O, and E-CH4 (p < 0.0001) and a negative moderate correlation with BVFP (with r ranging between 0.47 and 0.6 except for NDF).

Table 5.
Pearson correlations between input and output parameters.
4. Discussion
GM is a concentrated protein source derived as a co-product of galactomannan extraction from guar seed. GM could be considered an appropriate feedstuff for animal feeding since the basic components of animal diets are frequently used in human nutrition. Furthermore, GM is nutritionally comparable to SM and is a reasonably inexpensive and readily accessible feed material that is also processed in large quantities for gum extraction.
This study showed the potential of GM to replace classical SM feedstuff, with a focus on nitrogen metabolism due to the relationship between TNO and N2O. For the first time, we report data predicting GHG emissions (N2O and CH4) resulting from the use of GM.
In previous research, dietary guar meal was not sufficiently studied as an alternative to traditional soybean meal [4]. Previous studies attempted to identify the ideal levels of dietary GM that could be used without adversely impacting performance [40], and few studies have investigated the impact of guar inclusion on carcass characteristics [41]. Anti-nutritional factors are widely recognized to restrict the amount of GM that can be included in the diet. For example, feeding animals can produce certain issues due to anti-trypsin and very viscous non-starch polysaccharides such as galactomannan polysaccharides, which raise the viscosity of the digesta and limit digestive enzyme activity as well as decrease nutritional digestibility [42]. These problems can be reduced by processing the meal; the anti-trypsin factor can be inhibited by toasting and by reducing the level of dietary inclusion. Pigs, chickens, and laboratory animals (rats and mice) have all been proven to suffer adverse effects from galactomannan, which is present in residual guar gum from meal [43]. Guar gum has been shown to inhibit rat and pig glucose absorption, which negatively impacts growth performance. However, research indicates that eating the gum’s galactomannans, as found in guar meal, may enhance gut health by reducing the colonization of pathogenic bacteria in the gut. Furthermore, GM contains trypsin inhibitors involved in protein digestion.
In the present study, performance and carcass traits did not yield a significant decrease when including GM in the diet. Even though GM increased the NDF fraction in the diet, we predicted that the pancreas’ considerable increase in total enzymatic activity with age would yield an improvement in feed intake and nutrient digestibility. In the literature, most studies focused on broiler chickens. Lee et al. [44] observed a decrease in the BW and feed efficiency of chickens fed with high concentrations of GM, likely due to the existence of residual guar gum in the GM. According to Owusu-Asiedu et al. [45], the ADG and ADFI in pigs decreased with cellulose and guar gum during the first 7 days of being fed a high-NSP diet. Increased non-starch polysaccharide (NSP) content in the pigs’ diets directly affected growth rate and voluntary feed intake. Pigs and their microbiota may adjust to high-fiber diets over a longer period of time. A higher percentage of energy was also digested in the large intestine because of increased dietary NSP, which similarly decreased the total-tract energy digestibility and voluntary feed intake. In 2018, Karpiesiuk et al. [2] partially replaced SM with GM (25, 50, and 75%) in a pig-fattening diet. Pigs in the group fed diets containing 25% GM protein obtained the best performance, as evidenced by the lowest feed conversion ratio and the highest growth rate. On the other hand, Hasan et al. [4] observed a negative impact on ADFI and ADG when using GuarPro F-71 in young pigs’ diets as a 75% replacement for SM, which showed a linear decrease as guar inclusion increased but not a decrease in feed efficiency, in conflict with our results and those of Heo et al. [46]. GM diets had no noticeable impact on ADFI in growing–finishing pigs [47]. The ADFI of Yorkshire Landrace pigs decreased when GM was added to their diets. There is a lack of information in the literature about how GM affects the quality of swine meat. Karpiesiuk et al. [41] added GM with 50% and 75% protein to pig diets and reported that the addition of GM may have negatively impacted performance; however, meat quality was not affected (unpublished data).
Milczarek et al. [40] proposed using a dietary GM level of 4% to obtain good performance and improve the carcass composition of broiler chickens, as well as the physico-chemical qualities of their muscles. Conversely, in terms of carcass composition, dressing percentage, and carcass muscularity, chickens fed diets with a proportion of GM higher than 12% performed noticeably worse.
Nitrogen (N or its gaseous form, N2) and carbon (C) are components vital to life. Firstly, N2 must be transformed by nitrifying bacteria to enter the feed in food chains as part of the N cycle and be used by plants and animals as a nutrient. Some of the consumed N is lost through organic or inorganic excretion. In anaerobic environments, nitrification and denitrification processes produce manure-based nitrous oxide (N2O). Oxygen encourages the formation of N2O [20,23,26].
The farm’s manure storage releases both N2O and CH4. C is the fourth most frequent element in the Earth’s crust. Global energy and the C cycle are driven by methanogenesis. The second-most common GHG after CO2, CH4 is produced by animals through enteric fermentation and their manure.
This increase is responsible for around 20% of the current trend in global warming. Presently, between 500 and 600 GT of the world’s yearly CH4 emissions come from various habitats [47]. CH4 remains in the atmosphere for nine to fifteen years and is over 25 times more effective than CO2 at retaining atmospheric heat [48]. The raising of livestock plays a substantial role in the build-up of CH4 in the atmosphere. As our understanding of GHGs continues to evolve, feeding factors remain incompletely explored.
To decrease the main greenhouse gases, various dietary approaches were investigated. These approaches included high fiber contents, weight increases, feed efficiency, rates of protein and fat deposition, slaughter weight, and carcass lean yield [49,50,51]. Kpogo et al. [49], for example, previously investigated the impact of multicarbohydrase enzymes on GHG in the diet using wheat millrun as a co-product. Even though adding 30% wheat millrun to pig diets increases their fiber content, the authors did not observe a significant effect on GHGs. Furthermore, adding multienzymes to wheat millrun diets did not significantly affect emissions. In 2022, Hăbeanu et al. [38] highlighted that the intestinal microbial community influences pigs’ growth and intestinal health. Feeding pigs a higher level of fiber led to higher levels of the total bacteria identified, which influenced the total volatile fatty acids (VFAs). The authors noted an important decrease in E-CH4 in pigs fed high-fiber diets featuring the addition of mustard and grapeseed cake.
The findings of the present study did not agree with those obtained by Kpogo et al. [49], which showed that N digestibility was not enhanced. Conversely, Chen et al. [52] found that utilizing a cocktail of non-starch polysaccharide enzymes in a corn–miscellaneous meal diet improved nutrient digestibility; nonetheless, the authors did not observe a significant influence on N2O and CH4 emissions throughout the experiment. Our data regarding E-CH4 are less comprehensive than those obtained by Hăbeanu et al. [38]. However, if we take into consideration the data reported to LU, our data are similar to those obtained by Philippe et al. [17,26].
There is no evidence in the literature to suggest a low correlation between DMI, feed intake, N intake, and other parameters on M-CH4 emissions.
5. Conclusions
The results of this study did not support our first hypothesis, which predicted that the performance of the pigs would decrease if GM completely replaced SM. Replacing 100% of the SM in the diet with GM can positively alter certain indicators of N metabolism. For N2O (based DM) and E-CH4, the positive effects of dietary GM were greater when utilizing a GM-100% diet. This result supports our second hypothesis, although the impact of the anaerobic process in the manure on M-CH4 was less pronounced. This particular type of GHG decreases if organic matter is more digestible.
Author Contributions
Conceptualization, G.M., M.H. and M.M.; methodology, N.L., A.G., D.P. and M.D.; software, A.G. and I.M.; validation, L.V., C.G.N. and D.P.; formal analysis, A.G. and M.D.; investigation, G.M., N.L. and M.D.; resources, M.H., G.M. and M.M.; data curation, L.V. and D.P.; writing—original draft preparation, M.H. and G.M.; writing—review and editing, C.G.N., I.M. and M.M.; visualization, D.P., N.L. and A.G.; supervision, L.V., D.P. and M.M.; project administration, G.M. and M.H.; funding acquisition, M.H., G.M. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ADER 9.1.4./2019 and a Grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI-UEFISCDI, project number ERANET-ICT-AGRI-FOOD- Solution4Farmineg, within PNCDI III (financial support through the partners of the Joint Call of the Cofund ERA-Nets SusCrop (Grant N° 771134), FACCE ERA-GAS (Grant N° 696356), ICT-AGRI-FOOD (Grant N° 862665) and SusAn (Grant N° 696231).
Institutional Review Board Statement
All experimental procedures complied with the protocol authorized by the Ethical Committee of the National Research–Development Institute for Biology and Animal Nutrition and with respect to Romanian Law No. 199/2018, which complies with the EU Directive 2010/63/EU on animal research, in accordance with protocol number 7976/12/22.
Data Availability Statement
The raw data presented in this paper are available from the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pach, F.; Nagel, F. Replacing the substitute—Guar meal as an alternative for non-genetically modified soybean meal in the nutrition of rainbow trout (Oncorhynchus mykiss, Walbaum, 1792). Aquac. Nutr. 2017, 24, 666–672. [Google Scholar] [CrossRef]
- Karpiesiuk, K.; Kozera, W.; Bugnacka, D.; Woźniakowska, A.; Jarocka, B. The effect of partial replacement of soybean meal protein with guar (Cyamopsis tetragonoloba) meal protein on the cost-effectiveness of pig fattening. Ann. Warsaw Univ. Life Sci. SGGW Land Reclam. 2018, 57, 341–348. [Google Scholar] [CrossRef]
- Helm, E.T.; Patience, J.F.; Romoser, M.R.; Johnson, C.D.; Ross, J.W.; Gabler, N.K. Evaluation of increased fiber, decreased amino acids, or decreased electrolyte balance as dietary approaches to slow finishing pig growth rates. J. Anim. Sci. 2021, 99, skab164. [Google Scholar] [CrossRef]
- Hasan, M.S.; Humphrey, R.M.; Yang, Z.; Crenshaw, M.A.; Brett, J.; Liao, S.F. Effects of dietary inclusion of GuarPro F-71 on the growth performance and nutrient metabolism in young growing pigs. J. Appl. Anim. Nutr. 2020, 8, 143–149. [Google Scholar] [CrossRef]
- Sandhu, P.P.; Bains, K.; Singla, G.; Sangwan, R.S. Nutritional and functional properties of defatted, debittered and off-flavour free high protein guar (Cyamopsis tetragonoloba) meal flour. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 89, 695–701. [Google Scholar] [CrossRef]
- Janampet, R.S.; Malavath, K.K.; Neeradi, R.; Chedurupalli, S.; Thirunahari, R. Effect of feeding guar meal on nutrient utilization and growth performance in Mahbubnagar local kids. Vet. World 2016, 9, 1043–1046. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A. Evaluation of Chemical Composition of Cluster Bean (Cyamopsis tetragonoloba L.) Meal as an Alternative to Soybean Meal. Anim. Nutr. Feed Technol. 2017, 17, 457–467. [Google Scholar] [CrossRef]
- Sharma, S.L.; Singh, P.; Patil, A.K.; Sharma, J. Effect of feeding compressed complete feed block containing guar meal on blood biochemical profile of crossbred calves. J. Anim. Res. 2015, 5, 575–578. [Google Scholar] [CrossRef]
- Abdel-Wahab, W.; Sayed, S.K.; Sabek, R.A.M.; Abbas, M.S.; Sobhy, H.M. Effect of using Guar Korma Meal as a New Source of Protein on Productive Performance of Buffalos. Asian J. Anim. Sci. 2016, 10, 300–306. [Google Scholar] [CrossRef][Green Version]
- Ahmed, H.A.; Abou-Elkhair, R.M. Potential Application of Guar Meal in Broiler Diet. Asian J. Anim. Vet. Adv. 2016, 11, 280–287. [Google Scholar] [CrossRef][Green Version]
- Reddy, E.T.; Reddy, V.R.; Preetham, C.; Rao, S.V.R.; Rao, D.S. Effect of dietary inclusion of graded levels of toasted guar meal on performance, nutrient digestibility, carcass traits, and serum parameters in commercial broiler chickens. Trop. Anim. Health Prod. 2017, 49, 1400–1414. [Google Scholar] [CrossRef]
- Peng, P.; Tang, X.; Deng, D.; Fang, R. The Nutritive Value of Guar Meal and its Effect on Growth Performance of Meat Ducks. Pakistan J. Zool. 2020, 52, 1001–1006. [Google Scholar] [CrossRef]
- Salehpour, M.; Qazvinian, K.; Cadavez, A.P.V. Effects of feeding different levels of guar meal on performance and blood metabolites in Holstein lactating cows. Sci. Papers. Ser. D Anim. Sci. 2012, LV, 73–77. Available online: https://animalsciencejournal.usamv.ro/index.php/scientific-papers/88-a13 (accessed on 18 October 2023).
- Singh, N.; Arya, R.S.; Sharma, T.; Dhuria, R.K.; Garg, D.D. Effect of feeding of cluster bean (Cyamopsis tetragonoloba) straw based complete feed in loose and compressed form on rumen and haemato-biochemical parameters in Marwari sheep. Vet. Pract. 2008, 9, 110–115. Available online: https://www.semanticscholar.org/paper/Effect-of-feeding-of-clusterbean-(Cyamopsis-straw-Singh-Arya/8acff1d26ddd2a510cdd5ca763a6b1345d6ede8e (accessed on 18 October 2023).
- Abo Omar, J.; Zaazaa, A.; Sheqwarah, M.; Shanab, B.A.; Qaisi, W.; Abdallah, J. Effects of Guar (Cyamopsis tetragonoloba) Residues on the Performance and Nutrients Digestibility in Finishing Awassi Lambs. Open J. Anim. Sci. 2021, 11, 96–104. [Google Scholar] [CrossRef]
- Pachauri, V.C.; Upadhyaya, R.S. Nutritive Value of Cluster Bean (Cyamopsis tetragonoloba) Hay as Affected by Supplementation of Oat Grain in Goats. Indian J. Anim. Sci. 1986, 56, 154–155. Available online: https://www.scirp.org/%28S%28351jmbntvnsjt1aadkozje%29%29/reference/referencespapers.aspx?referenceid=2918065 (accessed on 18 October 2023).
- Philippe, F.X.; Laitat, M.; Canart, B.; Vandenheede, M.; Nicks, B. Comparison of ammonia and greenhouse gas emissions during the fattening of pigs, kept either on fully slatted floor or on deep litter. Livestock Sci. 2007, 111, 144–152. [Google Scholar] [CrossRef]
- Bälter, K.; Sjörs, C.; Sjölander, A.; Gardner, C.; Hedenus, F.; Tillander, A. Is a diet low in greenhouse gas emissions a nutritious diet?—Analyses of self-selected diets in the LifeGene study. Arch. Public Health 2017, 75, 17. [Google Scholar] [CrossRef]
- IPCC 2006 Guidelines for National Greenhouse Gas Inventories. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/. (accessed on 10 June 2023).
- Hăbeanu, M.; Lefter, N.A.; Toma, S.M.; Idriceanu, L.; Gheorghe, A.; Surdu, I. Nitrous oxide prediction in manure from pigs given mustard x grapeseed oil cakes as a replacement for sunflower meal. Arch. Zootech. 2021, 24, 47–57. [Google Scholar] [CrossRef]
- FAO. Global Livestock Environmental Assessment Model (GLEAM). Rome (Italy): Food and Agriculture Organization of the United Nations (FAO). 2017. Available online: www.fao.org/gleam/en (accessed on 20 January 2023).
- Liu, Z.; Powers, W.; Liu, H. Greenhouse gas emissions from swine operations: Evaluation of the Intergovernmental Panel on Climate Change approaches through meta-analysis. J. Anim. Sci. 2014, 91, 4017–4032. [Google Scholar] [CrossRef]
- Hăbeanu, M.; Gheorghe, A.; Lefter, A.N.; Untea, A.; Idriceanu, L.; Ranta, M.F. Assessment of certain nitrogen metabolism indicators, enteric CH4 and CO2 emitted through manure related to different diets in barrow. Arch. Zootech. 2020, 23, 129–142. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Allen, P.; Bastviken, D.; Conrad, R.; Gudasz, C.; St-Pierre, A.; Thanh-Duc, N.; del Giorgio, P.A. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 2014, 507, 488–491. [Google Scholar] [CrossRef]
- Broucek, J. Nitrous oxide production from cattle and swine manure. J. Anim. Behav. Biometeorol. 2017, 5, 13–19. [Google Scholar] [CrossRef]
- Philippe, F.X.; Nicks, B. Review on greenhouse gas emissions from pig houses: Production of carbon dioxide, methane and nitrous oxide by animals and manure. Agric. Ecosyst. Environ. 2014, 199, e10–e25. [Google Scholar] [CrossRef]
- Hlatini, V.A.; Ncobela, C.N.; Chimonyo, M. Nitrogen balance response to varying levels of dietary protein in slow-growing Windsnyer pigs. S. Afr. J. Anim. Sci. 2020, 50, 644–653. [Google Scholar] [CrossRef]
- Diaz, J.A.C.; Berry, D.P.; Rebeiz, N.; Metzler-Zebeli, B.U. Feed efficiency metrics in growing pigs. J. Anim. Sci. 2017, 95, 3037–3046. [Google Scholar] [CrossRef]
- Hăbeanu, M.; Lefter, N.A.; Gheorghe, A.; Untea, A.; Ropotă, M.; Grigore, D.M.; Varzaru, I.; Toma, S.M. Evaluation of Performance, Nitrogen Metabolism and Tissue Composition in Barrows Fed an n-3 PUFA-Rich Diet. Animals 2019, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- SR EN ISO 5983-2:2009; Animal Feeding Stuffs—Determination of Nitrogen Content and Calculation of Crude Protein Content. International Organization for Standardization: Genève, Switzerland, 2009.
- AOAC 2001.11; Protein (Crude) in Animal Feed, Forage (Plant Tissue), Grain, and Oilseeds. Block Digestion Method Using Copper Catalyst and Steam Distillation into Boric Acid. AOAC International: Rockville, MD, USA, 2001.
- SR EN ISO 6865:2002; Animal Feeding Stuffs—Determination of Crude Fibre Content—Method with Intermediate Filtration. International Organization for Standardization: Genève, Switzerland, 2002.
- SR EN ISO 16472:2006; Animal Feeding Stuffs—Determination of Amylase-Treated Neutral Detergent Fibre Content (aNDF). International Organization for Standardization: Genève, Switzerland, 2006.
- SR EN ISO 13906:2008; Animal Feeding Stuffs—Determination of Acid Detergent Fibre (ADF) and Acid Detergent Lignin (ADL) Contents. International Organization for Standardization: Genève, Switzerland, 2008.
- Vărzaru, I.; Untea, A.E.; Martura, T.; Olteanu, M.; Panaite, T.D.; Schitea, M.; Van, I. Development and validation of an RP-HPLC method for methionine, cysteine and lysine separation and determination in corn samples. Rev. Chim. 2013, 64, 673–679. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=22a2bd1ce31687e77ed00ae6401420434eedb30e (accessed on 18 October 2023).
- Gheorghe, A.; Lefter, N.A.; Idriceanu, L.; Ropotă, M.; Hăbeanu, M. Effects of dietary extruded linseed and Lactobacillus acidophilus on growth performance, carcass traits, plasma lipoprotein response, and caecal bacterial populations in broiler chicks. Ital. J. Anim. Sci. 2020, 19, 822–832. [Google Scholar] [CrossRef]
- 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Available online: https://www.ipcc-nggip.iges.or.jp/public/2019rf/index.html (accessed on 10 June 2023).
- Hăbeanu, M.; Lefter, N.A.; Toma, S.M.; Dumitru, M.; Cismileanu, A.; Surdu, I.; Gheorghe, A.; Dragomir, C.; Untea, A. Changes in ileum and cecum volatile fatty acids and their relationship with microflora and enteric methane in pigs fed different fiber level. Agriculture 2022, 12, 451. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Milczarek, A.; Pachnik, M.; Osek, M.; and Swinarska, R. Rearing Performance and Carcass Composition of Broiler Chickens Fed Rations Containing Guar Meal at Graded Levels. Agriculture 2022, 12, 1385. [Google Scholar] [CrossRef]
- Karpiesiuk, K.; Kozera, W.; Daszkiewicz, T.; Lipiński, K.; Kaliniewicz, J.; Okorski, A.; Pszczółkowska, A.; Żak, G.; Matusevičius, P. The effect of dietary supplementation with guar (Cyamopsis tetragonoloba) meal protein on the quality and chemical composition of pig carcasses. Ann. Anim. Sci. 2023, 23, 1095–1104. [Google Scholar] [CrossRef]
- Parrini, S.; Aquilani, C.; Pugliese, C.; Bozzi, R.; Sirtori, F. Soybean Replacement by Alternative Protein Sources in Pig Nutrition and Its Effect on Meat Quality. Animals 2023, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.; Eeckhaut, V.; Goossens, E.; Ducatelle, R.; Van Nieuwerburgh, F.; Poulsen, K.; Sampaio Baptista, A.A.; Loureiro Bracarense, A.P.F.R.; Van Immersee, F. Guar gum as galactomannan source induces dysbiosis and reduces performance in broiler chickens and dietary b-mannanase restores the gut homeostasis. Poult. Sci. 2023, 102, 102810. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Connor-Appleton, S.; Bailey, C.A.; Cartwright, A.L. Effects of guar meal by-product with and without β-mannanase Hemicell on broiler performance. Poult. Sci. 2005, 84, 1261–1267. [Google Scholar] [CrossRef]
- Owusu-Asiedu, A.; Patience, J.F.; Laarveld, B.; Van Kessel, A.G.; Simmins, P.H.; Zijlstra, R.T. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci. 2006, 84, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Heo, P.S.; Lee, S.W.; Kim, D.H.; Lee, G.Y.; Kim, K.H.; Kim, Y.Y. Various levels of guar meal supplementation on growth performance and meat quality in growing-finishing pigs (Abstract). J. Anim. Sci. 2009, 87 (Suppl. 2), 144. Available online: https://www.feedipedia.org/node/4984 (accessed on 18 October 2023).
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R719–R736. Available online: https://www.cell.com/current-biology/pdf/S0960-982230623-7.pdf (accessed on 18 October 2023). [CrossRef]
- Ma, T.; Deng, K.; Diao, O. Prediction of methane emission from sheep based on data measured in vivo from open-circuit respiratory studies. Asian-Australas J. Anim. Sci. 2019, 32, 1389–1396. [Google Scholar] [CrossRef]
- Kpogo, A.L.; Jose, J.; Panisson, J.C.; Agyekum, A.K.; Predicala, B.Z.; Alvarado, A.C.; Agnew, J.M.; Sprenger, C.J.; Beaulieu, A.D. Greenhouse gases and performance of growing pigs fed wheat-based diets containing wheat millrun and a multi-carbohydrase enzyme. J. Anim. Sci. 2021, 99, skab213. [Google Scholar] [CrossRef]
- Le Goff, G.; Le Groumellec, L.; van Milgen, J.; Dubois, S.; Noblet, J. Digestibility and metabolic utilisation of dietary energy in adult sows: Influence of addition and origin of dietary fibre. Br. J. Nutr. 2002, 87, 325–335. [Google Scholar] [CrossRef]
- Jarret, G.; Martinez, J.; Dourmad, J.Y. Effect of biofuel co-products in pig diets on the excretory patterns of N and C and on the subsequent ammonia and methane emissions from pig effluent. Animals 2011, 5, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, D.; Zhang, l.; Zhong, R.; Liu, Z.; Liu, L.; Chen, L.; Zhang, H. Supplementation of non-starch polysaccharide enzymes cocktail in a corn-miscellaneous meal diet improves nutrient digestibility and reduces carbon dioxide emissions in finishing pigs. Animals 2020, 10, 232. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).