Abstract
Reed canary grass (RCG) is a native perennial grass with a wide range of uses that naturally occurs in moist habitats. The conducted research indicates the possibilities of RCG cultivation outside natural, humid habitats in monoculture on sandy soils in temperate climates to obtain biomass and seeds. The influence of two factors was analysed: (1) fertilisation with compost from urban greenery in doses of 0, 10, and 20 Mg·ha−1 and (2) mineral nitrogen fertilisation in doses of 0, 40, 80, and 120 kg·ha−1. Compost fertilisation (10 and 20 Mg·ha−1) increased dry matter yields in all years of the study, by 12.1% and 41.0%, respectively. Also, nitrogen fertilisation in doses of 40, 80, and 120 kg·ha−1 increased dry matter yield by 26.8%, 41.6%, and 65.0%, respectively. When harvesting RCG plants for energy biomass at their stage of full maturity, a significant seed yield of 242 to 600 kg·ha−1 can also be obtained in the first three years, while in the fourth year of use, the seed yield was almost three times lower (90–158 kg·ha−1). The obtained results indicate that, in sandy soils, the use of compost fertilisation in RCG cultivation can partially or entirely replace mineral fertilisation and ensure high and stable yields. An additional benefit may be the achievement of a high seed yield in the initial years of cultivation. The use of organic fertilisers and the independence from mineral fertilisers can significantly increase the profitability of bioenergy crops.
1. Introduction
Alterations in the EU’s agricultural policy have resulted in a gradual decline in the extent of meadows and pastures in Poland. This decline is occurring in favour of agricultural land, urbanized regions, and forests [1,2]. In Poland, there is an observable trend of the reduction in the number of cattle on individual farms [2]. This shift is giving rise to a concerning alteration in the utilization of meadows and pastures. As a result, grasslands are being lost, often at the expense of areas that impose more significant environmental pressures, such as arable land, infrastructure investments, or transportation zones [1]. The transformation of meadows, for instance, into arable land, results in a reduced carbon sequestration in the soil, increased emissions of greenhouse gases, chemical and physical soil erosion, and a diminished biodiversity. The disappearance of grasslands in damp and low-lying areas leads to a decreased water retention, a heightened risk of flooding, and constraints on natural water purification possibilities [3,4].
The prevailing energy policy of the EU, along with the prevailing geopolitical situation, provides an opportunity to halt the decline in grassland areas and harness these spaces for energy purposes. Implementing suitable cultivation of grasslands and utilizing perennial native species of grasses are crucial to efficiently generate biomass, especially for energy production [5]. One of the primary objectives of the EU policy concerning climate change mitigation and enhanced energy security is the reduction in pollutant emissions from the combustion of fossil fuels by decreasing the reliance on fossil fuels for energy production in Europe [6,7,8,9]. Recent events, such as the conflict in Ukraine, have underscored the critical importance of diversifying energy sources for the energy security of Central and Eastern European countries. Furthermore, this underscores the emphasis on developing renewable energy sources (RESs) [8].
In 2021, the proportion of energy derived from renewable sources in the EU was 21.8%, while in Poland, it was 15.6%. The EU has set ambitious targets for the future, as outlined in the Directive 2018/2001 [9], which mandates a minimum of 32% renewable energy in the EU by 2030. However, the current geopolitical situation and the imperative to reduce dependence on Russian fossil fuels have necessitated an expedited energy transition. Consequently, the new goal for 2030 is to reach 42.5% of energy from renewable sources in the EU [10]. Solid biofuels are the most significant contributors among RESs in the EU, accounting for 41% of total renewable energy in 2021, with an additional 14% coming from liquid biofuels and biogas [10]. In Poland, the majority of energy production from renewable sources in 2021 was attributed to solid biofuels, representing 69.3%, while liquid biofuels and biogas together constituted 10.6% [11].
In Poland, biomass is currently viewed as the most promising avenue of renewable energy, owing to its wide availability and cost-effectiveness due to favourable climatic and habitat conditions. Biomass, along with solar and wind energy, exhibits a substantial growth potential [12,13,14]. Biomass can be utilised as a solid fuel, including wood chips and pellets, or it can be processed into gaseous and liquid fuels [12,15]. While energy production from agriculture has traditionally played a minor role in Poland, the goals outlined in legislative directives like Directive 2018/2001, coupled with EU and Polish strategies and the prevailing geopolitical landscape, have ignited growing interest in harnessing biomass from plant cultivation for energy production [13,15].
In the European climate, various plant species, including grasses grown on arable and marginal lands or degraded soils, serve as a valuable source of biomass for energy purposes [16]. Selecting the right plant species that can thrive in specific soil and environmental conditions in a changing climate is crucial for producing a substantial amount of biomass that can be easily converted into energy. Perennial grasses (PGs) are known for their ability to quickly adapt to local conditions, and the areas they occupy can be readily converted for arable land use [16,17]. PGs have a wide range of applications, primarily serving as animal fodder and sources of fibre and for renewable energy production. They are employed in the production of biofuels, biopolymers, biopharmaceuticals, biodegradable products, and materials for gardening, animal husbandry, and fertilisation [17,18,19,20]. The ecosystem services offered by PGs are also of great significance. They contribute to carbon sequestration and the dynamics of soil organic carbon, provide beneficial effects to soil organisms, protect against soil erosion, and enhance biodiversity, including an increase in pollinator populations and biological pest control [17,21,22]. In comparison to annual crops, PGs require a less frequent cultivation, have a longer lifespan (over ten years), have reduced chemical and nutrient demands, sequester 20–30 times more carbon dioxide, and enhance soil carbon content, porosity, and water-holding capacity. Moreover, PGs contain 6–18 times more energy than that required for their cultivation and transportation for energy processing [19,20].
Due to these exceptional properties, PGs can be utilised in areas where annual crops may not yield the expected results and are economically unviable. They are a fundamental component of multifunctional agriculture [18]. As noted by Zahorec et al. [18], Valentine et al. [23], and Nanda et al. [24], bioenergy production will play a crucial role in achieving renewable energy sources (RESs) and mitigating CO2 emissions. Furthermore, to maintain food security by preserving crops for food production, a shift from 1st generation biofuels (derived from food crops) to 2nd generation biofuels (sourced from non-food crops like PGs) is necessary. The cultivation and processing of PGs for biofuels also present an opportunity to promote rural development.
Commonly grown grass species for energy purposes are species introduced into Europe and Poland with a C4 photosynthesis cycle, i.e., silvergrass, prairie cordgrass, switchgrass, among other native tall grass species for the production of solid biomass, are valuable species with a high biomass yield, i.e., reed canary grass (RCG), Dactylis glomerata, Phragmites australis, Arrhenatherum elatiuscan, or Bromus inermis [25,26,27]. The growth cycle of indigenous grass species with a C3 photosynthesis process differs from that of the previously mentioned introduced species. Consequently, the cultivation techniques for these native species need to be suitably adjusted to align with their economic uses. As these native grass species reach their full morphological development, especially during their maturation phase, they exhibit their highest dry matter content, which is crucial for biomass production [25,27]. Additionally, these native grasses produce seeds that can be harvested, allowing for their reuse in subsequent sowings or for sale. Native grass species are frequently characterised by their greater resilience to changing environmental conditions. They exhibit superior winter survival rates and are more prolific in reproduction when compared to other introduced energy plants, such as mallow. This translates to reduced expenses in establishing a crop [22,28].
RCG is a tall (1.0–3 m high), native perennial grass species from areas of North America, Asia, and Europe that grows well on most kinds of soils and provides high yields ranging from 6 Mg·ha−1 to 20 Mg·ha−1 DM when adequately cultivated on fertile soil, and high yields of RCG are obtained on organic soils [18,20,21,26,29,30,31]. RCG, naturally occurring in wet areas (floodplains and shores) of temperate climates, tolerates flooding better than other cool season grasses, and nevertheless, it is more drought resistant than many other species, grows at temperatures of 5–30 °C, tolerates low temperatures well (even −20 °C), and requires a minimum growing season of 111 days [18,21,28,29,31]. RCG has an extensive root system, with roots reaching depths of over 3 m. The branches are relatively short and measure about 1 cm in thickness. In favourable conditions, RCG forms dense masses of broad foliage, and its panicles span from 7 to 40 cm. However, RCG’s small seeds (approximately 1000 seeds per gram) can be challenging to produce consistently due to its poor seed production and its seeds’ susceptibility to seed shattering [18,22]. The typical lifespan of a RCG plant is estimated to be around ten years [18,32]. RCG has historically been employed as fodder in North America and Northern Europe, but its usage as animal feed has been limited due to the presence of poisonous alkaloids. In contemporary times, it finds a greater utility in the production of various materials and energy [18,28,33]. Perennial grasses can also serve other purposes, such as water purification in cases of nutrient-contaminated waters, soil remediation, and stabilising areas like escarpments and slopes [18,22].
Using the RCG as an energy resource requires the knowledge of the variability of the above-ground biomass production in temperate climate conditions and light soils, which are predominant in Poland (about 60% of the area) and are primarily potential areas for energy crops [29,34,35]. Due to its yield, extensive root system, and drought resistance, RCG may be a promising energy crop for cultivation on light and marginal soils in temperate climate conditions [18,22,32]. The cultivation of RCG requires fertilisation with NPK in the establishment year and subsequent years to optimise its yield [14,22]. In the case of RCG cultivation, nitrogen fertilisation is recommended [18,22,28,29], to which plants react strongly and improve the quality and size of their yield, which are, among others, related to morphological features such as the number, length, and thickness of shoots. Particularly in sandy soils, where there are biophysical growth limitations related to low availability of nutrients and water, fertilisation is required to obtain appropriate efficiency and stability of RCG yields [18,22]. In addition to the critical mineral nitrogen fertilisation of RCG, in the case of cultivation on light soils, it is essential to improve their sorption and retention properties, and organic fertilisation is also crucial in the case of energy crops cultivated on sandy and marginal soils [18]. Due to the decreasing availability of natural fertilisers in the Baltic countries [36], the usage of compost from urban greenery may be a solution.
RCG reaches its maximum biomass when its seeds have ripened, and these plants exhibit strong winter hardiness [33]. Energy can be derived from RCG through various methods, including direct combustion, the production of biofuels and biogas, or thermochemical conversion [20,22]. The calorific value of RCG is higher than 17,000 MJ·kg−1 and is suitable for use in the energy sector. Moreover, RCG is useful for cellulose material production because it has a high concentration of cellulose and hemicellulose, which is a desired trait for a biofuel [18,20,22,25]. Delayed harvesting of PGs is recommended to improve the chemical properties of biomass in terms of its usefulness in the combustion process and in terms of the ash composition (lowering the content of ash, P, Cl, K, Ca, S, and Mg). The content of silica and the mentioned elements depends on the soil on which RCG is cultivated. The highest concentrations are recorded in heavy mineral soils [18,22]. RCG has a high adaptability to environmental conditions and is suitable for upscaling on marginality areas in temperate climates [18,22,31]. A recent study conducted by Ferdini et al. in 2023 [37] indicated that, due to climate change and global warming, the potential cultivation area for RCG is expected to significantly expand in Central Europe and the Baltic countries. These changes may lead to increased yields and a reduced yield variability, making RCG a promising bioenergy crop in this region, including Poland. While sowing is one of the most cost-effective methods for establishing energy crops, obtaining seeds, particularly for perennial grasses like RCG, can be challenging. A higher vegetative yield is often associated with a reduced seed yield [38]. Nonetheless, improved habitat conditions, including higher organic matter content and nutrient availability, have a positive impact on RCG seed’s germination capacity and yield [39].
The purpose of this study was to assess the possibility of obtaining the biomass for energy purposes and seeds of RCG cultivated in conditions of transitional temperate climate and varied pre-sowing fertilisation with compost and mineral nitrogen on light mineral soils, which occupy the largest area of agricultural land in Poland and can be easily used for harvest than organic soils. The research hypothesis is that the appropriate cultivation of RCG on light mineral soil in the conditions of Central European climate and fertilisation allows to obtain biomass suitable for energy use, and the additional benefit of this will be obtaining seeds for further sowing and establishment of RCG crops.
2. Materials and Methods
2.1. Experiment Design
The subject of the study contained results of field experiment implementation from the years 2012–2015. The experiment was established on 7 September 2011, by applying a random sub-blocks design (split-plot) in triplicate with a single plot area of 12 m2. Seeds of RCG were sown with pure sowing using a seed drill in the amount of 20 kg·ha−1 with a row spacing of 18 cm and a depth of 2 cm. The experiment was established in a post after oat (Avena sativa L.) was harvested there for seeds. Before sowing the plants, the following agrotechnical operations were carried out: After oat harvest, post-harvest tillage and medium ploughing were performed. Before ploughing, compost fertilisation was applied in the amount accepted in the first study factor. The compost was made from waste from the urban greenery care in Szczecin, which included a green mass of cut plants, leaves (trees and shrubs), and other plant waste (small branches, root remains, and moss).
The examined factors included factor I with compost doses of 0, 10, and 20 Mg∙ha−1 and factor II with nitrogen doses of 0, 40, 80, and 120 kg∙ha−1. Immediately before sowing the plants (in 2011), mineral fertilisation with nitrogen, phosphorus, and potassium in doses of 20, 80, and 100 kg·ha−1 was applied to all study objects. Ammonium nitrate, 19% superphosphate, i.e., P2O5, 60% potassium salt, i.e., K2O, were used. Nitrogen fertilisation in the form of ammonium nitrate in the years of the full use (2012–2015) was used once in spring, before the start of plant vegetation in the amount adopted in the II study factor.
2.2. Compost Chemical Properties
Table 1 provides the chemical composition of the compost. Compost pH was determined potentiometrically, and EC (electrical conductivity) was measured using the Slandi SC300 conductometer from Slandi Sp. z o. o. (Michałowice, Poland). The total C, N, and S content was analysed with the COSTECH ECS 4010 elemental analyzer from Costech Analytical Technologies Inc. (Valencia, CA, USA). The content of total K, Ca, Mg, Na, Fe, Mn, Cu, Zn, Cr, and Cd was determined after mineralization in a mixture of concentrated acids (HNO3 + HClO4) using the atomic absorption spectrophotometer Unicam Solaar 929 from Pye Unicam Ltd. (Cambridge, UK). Phosphorus (P) was determined colorimetrically using the Merck Vega 400 colorimeter from Merck (Darmstadt, Germany). The analysis revealed that the compost had a neutral pH. Total C, N, and Mg contents were low, while those of P, K, S, Ca, and Na were high. The trace element content (Cu, Fe, Mn, Cr, and Zn) remained within the permissible limits set by the industry standard [40].

Table 1.
Physicochemical properties of the compost used in this study and total doses of minerals brought in with compost fertilisation.
2.3. Study Site and Soil Properties
The experiment was established in the NW region of Poland with a transitional temperate climate, in Lipnik near Stargard (N 53°20′35.8″, E 14°98′10.8″) (Figure 1), in the area of the Agricultural Experimental Station of the West Pomeranian University of Technology in Szczecin. The experiment was conducted on a light soil, specifically a loamy sand, which falls under the category of Haplic Luvisols (Humic) [41]. The soil had the following texture composition: 59% sand, 28% silt, and 13% loam. The soil exhibited an acidic pH (pHKCl: 5.2). Prior to the experiment, the content of total macroelements in the soil was as follows: 8.10 g C per kg of dry matter (DM), 0.92 g N per kg of DM, 0.02 g S per kg of DM, 0.91 g Mg per kg of DM, 0.66 g K per kg of DM, and 0.45 g P per kg of DM. Soil pH was determined using potentiometric methods, while the determination of total C, N, and S content was conducted through the use of an elementary analyzer, COSTECH ECS 4010 from Costech Analytical Technologies Inc. (Valencia, CA, USA). The content of total Mg and K was determined after mineralization in a mixture of concentrated acids (HNO3 + HClO4) using an atomic absorption spectrophotometer, Unicam Solaar 929 from Pye Unicam Ltd. (Cambridge, UK), whereas P content was obtained through a colorimetric analysis using the Merck Vega 400 colorimeter from Merck (Darmstadt, Germany).
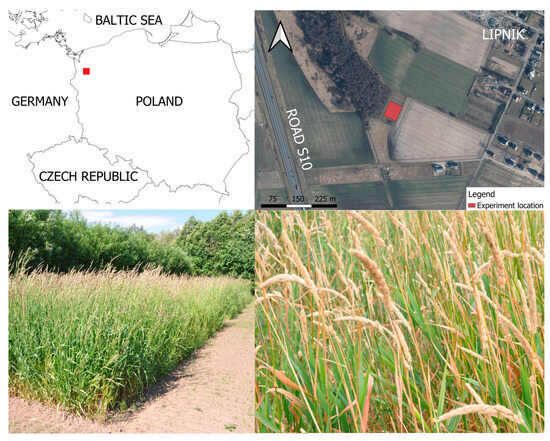
Figure 1.
Experiment location and various development stages of reed canary grass (RCG, Phalaris arundinacea L.).
2.4. Climatic Conditions
Meteorological data from the Agricultural Experimental Station in Lipnik (distance from the field trial of about 500 m) for the years 2012–2015 and 1980–2008 (Figure 2) indicate differences in precipitation and air temperatures. In each year, the average air temperature of the growing season was higher than the long-term average. The warmest years were 2014 and 2013, in which the average air temperature during plant growth was 16.7 and 16.4 °C. The warmest months in 2014 were July, August, and September, and in 2013, were June and July.
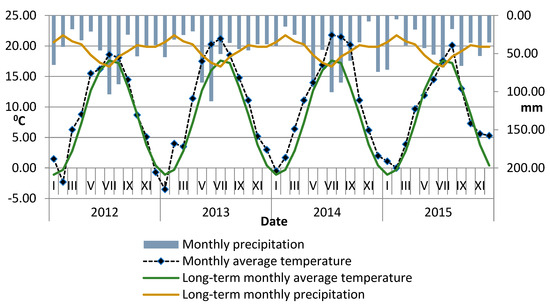
Figure 2.
Average monthly air temperature and monthly precipitation during the experiment. For comparison purposes, the long-term data are also shown.
The wettest year was 2014, which had the highest precipitation of 622.3 mm. This amount was 38 mm more than the long-term annual average, especially during the growing season, which was 64 mm above average. In 2014, the most significant rainfall occurred in July, May, and August, with the respective total precipitation of 100.6 mm, 94.0 mm, and 88.1 mm. On the other hand, the driest year in the study period was 2012, with a total precipitation that was 81 mm lower than the average, and during the growing season, it was 98 mm below the long-term average.
2.5. Harvest and Plant Analysis
Plants were harvested for seeds using a plot harvester, Wintersteiger Classic (Ried, Austria), during the full ripening phase of RCG by cutting the inflorescence parts of plants in the first half of July of each year (2012–2015). The remaining biomass was then sheared with a rotary mower Samba 160 from Samasz Sp z o.o. (Zabłudów, Poland). Autumn RCG growths that were collected in each year of research were not presented. Throughout the plant’s growing season, we evaluated its development and growth, taking biometric measurements from 25 plants or shoots. These measurements included shoot length (cm), shoot thickness (mm), inflorescence length (cm), the number of seeds in a single inflorescence, yield structure (pcs), and seed and plant biomass yield (dt∙ha−1). The above results are presented as the average for the years of the study. The energy value of DM was determined on the KL-10 calorimeter in accordance with the PN-81/G-04513 standard [42].
2.6. Statistical Analysis
The results were statistically processed with the classic analysis of variance at the significance level of p = 0.05 (ANAWAR 5.3 software by Franciszek Rudnicki). ANAWAR 5.3 is based on computational programs for orthogonal data from single, multiple single, double, and tree-factor experiments. To determine significant differences in the results, Tukey’s test at the level of p = 0.05 was used. Principal component analysis (PCA) was also performed for selected results, in addition to the projection of the results on the plane used and was conducted with STATISTICA 12.5 from StatSoft Polska Sp. z o.o. (Kraków, Poland).
3. Results and Discussion
3.1. Biometric Parameters
3.1.1. Above-Ground Vegetative Organs
Biometric parameters of RCG depend on the genotype of the plant, habitat, climate conditions, and the methods of cultivation and fertilisation [33]. After the emergence and spreading of plants before winter, the number of shoots per plant was lower than in the following years, and no significant differences were detected. RCG shows higher numbers of shoots compared with Miscanthus species and a similar, large number of shoots as Panicum virgatum L. and Spartina pectinata [43,44,45]. In the years of full use (2012–2015), the number of shoots produced by reed canary grass plants was 19.8–33.6 pieces (Table 2), and the range obtained was slightly higher than in the multi-genotype study conducted in England by Christian [33]. In plants fertilised with compost at doses of 10 and 20 Mg·ha−1, the number of shoots per plant was higher by 24.7% and 30.6%, respectively, compared to the plants not fertilised with compost.

Table 2.
Effects of fertilisation with compost and mineral nitrogen on the number of shoots of RCG during the years of 2012–2015.
Mineral nitrogen fertilisation positively affects the growth of RCG [18,28,46]. The favourable reaction to nitrogen fertilisation was also found in the RCG morphology studies (number, length, and thickness of shoots) (Table 2, Table 3 and Table 4). Nitrogen fertilisation in doses of 40, 80, and 120 kg·ha−1 in both years of use contributed to a significant increase in the number of shoots on one plant. On average, from the years of study, the plants produced more shoots when nitrogen fertilisation was applied and were higher by 9.1%, 20.2%, and 30.6%, respectively, compared to the number of shoots produced by non-fertilised plants. The height of the RCG can be from 0.8 to 2.0 m [20], and in exceptional conditions, even up to 3 m [18,47]. In studies of the effect of fertilisation on the length of RCG shoots, their differentiation from the dose of applied fertilisation was found (Table 3, Figure 3). Plants fertilised with compost in doses of 10 and 20 Mg·ha−1 during the years of use had shorter lengths of shoots by 5.3% and 11.3%, respectively, than plants grown without compost fertilisation. However, plants fertilised with nitrogen at doses of 40, 80, and 120 kg·ha−1 had longer lengths of shoots by 4.3%, 8.2%, and 12.5%, respectively, than plants without nitrogen fertilisation.

Table 3.
Effects of fertilisation with compost and mineral nitrogen on the length of RCG shoots (cm) during the years of 2012–2015.

Table 4.
Effects of fertilisation with compost and mineral nitrogen on the thickness of RCG shoots (mm) during the years of 2012–2015.
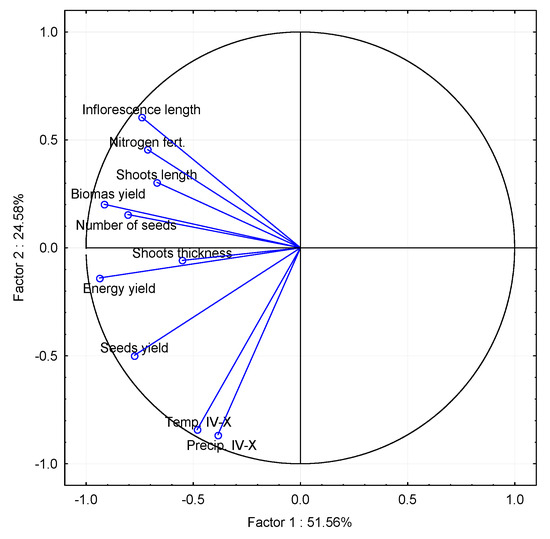
Figure 3.
The principal component analysis (PCA) for RCG’s energy yield, biometric parameters, and climatic conditions.
Considering the years of study, plants in the third year of vegetation had the most considerable length of shoots, which resulted from a greater water availability in the growing season of 2014 (the year with the highest annual rainfall throughout the research period). RCG is a species occurring in humid habitats [14,22]; therefore, when grown on sandy soils with deep groundwater, water availability may be a factor limiting the growth of RCG. The average shoot lengths during studies (Table 3) were slightly longer than in the case of the RCG cultivation (avg. 102–129 cm depending on the diversity of phenotypic features) on Humic Gleysol in Quebec (Canada) [48]. In spite of the presence of ample water due to soil type and higher precipitation levels, the shorter and colder growing season, along with the harsher winters characteristic of Quebec’s continental climate, may have limited plant growth compared to the transitional temperate climate found in the Baltic countries [49]. Nevertheless, when compared to plants in the wet habitats of floodplains in Poland [39] and habitats with heavier and more fertile soils in Poland [50], the studies still resulted in shorter shoot lengths of RCG. Additionally, in England [33], where RCG was cultivated on heavy soils and received nitrogen fertilisation at a rate of 120 kg, the shoot length was slightly longer. However, this increase in shoot length did not correspond to a higher dry matter yield. In an experiment conducted in Finland [49], RCG varieties achieved an average maximum height of 160 cm when grown on clay and organic soils with nitrogen fertilisation ranging from 40 to 70 kg per hectare. The variations in height depended on the specific variety and the year of cultivation, as noted by Sahramaa and Jauhiainen in 2003 [49]. The height of RCG is influenced by the variety itself and phenotypic characteristics arising from the selection of varieties and their natural habitat. For example, native RCG populations in habitats situated north of Finland tend to have shorter heights compared to cultivated varieties.
The assessment of the RCG shoot thickness is presented in Table 4. Organic compost fertilisation (10 and 20 Mg·ha−1) applied to the ground had a significant impact on increasing the thickness of the shoots produced by RCG plant, on average from the years of study, with an increase of 0.20 and 0.42 mm, i.e., of 5.4% and 11.4%. The analysis of the impact of the applied doses of mineral nitrogen fertilisation (40, 80, and 120 kg·ha−1) showed that it had a significant effect on the thickness of shoots of RCG plants, and the increase in shoot thickness was by 5.4%, 7.1%, and 10.9%, respectively, compared to that of non-nitrogen-fertilised plants. As indicated by other studies [39,48,49], habitat conditions and nutrient availability have a positive effect on the morphology of RCG, e.g., the length of leaves, shoots, and panicles increases. Morphological features can be changed by the method of cultivation. The level of fertilisation notably boosts plant height, including the length of leaves, stems, and inflorescences. However, it may also increase the proportion of leaves relative to the overall shoot yield, similar to the impact of frequent mowing. Conversely, delaying the harvest date leads to an increase in the proportion of shoots and is influenced by the frequency of mowing on the development of plant organs [22,28,33].
3.1.2. Inflorescence and Number of Seeds
The inflorescence length of RCG is from 7 to 40 cm [47], most often 8 to 12 cm [39,48,50]. The assessment of the length of RCG inflorescence indicates that they were dependent on the studied factors (Table 5). Organic compost fertilisers (10 and 20 Mg·ha−1) applied to the ground had a significant impact on the length of an inflorescence of an RCG plant; their average length (for the years of study) increased by 4.3% and 9.4%. Analysis of the impact of the applied doses of nitrogen mineral fertilisation (40, 80, and 120 kg·ha−1) showed that it had a significant impact on increasing the length of RCG inflorescence (Table 6, Figure 3) by 9.2%, 16.5%, and 22.9%, respectively.

Table 5.
Effects of fertilisation with compost and mineral nitrogen on the length of RCG inflorescence (cm) during the years of 2012–2015.

Table 6.
Effects of fertilisation with compost and mineral nitrogen on the number of seeds in a single RCG inflorescence during the years of 2012–2015.
The assessment of the number of seeds in one inflorescence of RCG plants indicates their dependency on the studied factors (Table 6). Compost fertilisation levels (10 and 20 Mg·ha−1) applied to the ground had a significant impact on the number of seeds produced in a single inflorescence of RCG, and their number increased by 8.4% and 17.4%, on average, in the years of research.
Application of nitrogen mineral doses (40, 80, and 120 kg·ha−1) revealed that they had a significant impact on the number of seeds developed from one inflorescence of RCG, and their number increased by 25.9%, 54.6%, and 68.7%, respectively, compared to the quantity obtained from non-nitrogen-fertilised plants.
3.2. Seed Yield
The seed yield of RCG depended on the research factors that were evaluated (Table 7); PCA analysis (Figure 3) indicates that seed yield in the conducted research may also depend on climatic conditions; higher rainfall and temperature may have a beneficial effect on seed yield. In the case of grasses, temperature and day length are necessary to induce flowering; RCG requires a double induction: primary, with a temperature of 3–12 °C for a day length below 12 h, and secondary, with a higher temperature and a more extended day [38]. For example, in the Nordic countries, autumn with long days and low temperatures causes this primary induction at a low level, which results in low seed yield in the following year [39]. Moreover, RCG, depending on the variety, requires a specific level of temperatures (737 degree days) to achieve the ripeness of its beans. According to Kieloch et al. [40] and Sahramaa and Hömmö [39], insufficient water availability limits seed yield; therefore, a higher water availability in sandy soils with a deep groundwater table will have a positive effect on seed yield. The applied doses of pre-sowing fertilisation with compost positively influenced the increase in RCG seed yields, on average, from 13.8% at a fertilisation of 10 Mg·ha−1 and by 30.6% at 20 Mg·ha−1 compared to the yield from plants not fertilised with the compost. The use of increasing doses of nitrogen fertilisation of plants, compared to plants without fertilisation with this factor, contributed to the increase in RCG seed yield, on average for the years of the study by 8.6% at 40 kg N·ha−1, 20.2% at 80 kg N·ha−1, and 31.7% at 120 kg N·ha−1. The assessment of the study years’ impact on the seed yield of RCG showed that, in the first year of full use (2012), yields were, on average, regardless of research factors, at the level of 386 kg·ha−1. In the second year (2013), in comparison to the first year, seed yields exhibited an average increase of 22.7%. However, in the following years, the yield saw a decline, first by 14.1% and subsequently by 65.2%. The reason for the decrease in seed yield in 2015, apart from the age of cultivation, was also unfavourable weather conditions, i.e., a low precipitation in the growing season, which was 59 mm lower than the long-term average for this period. PCA analysis (Figure 3) suggests that the seed yield of RCG in this research may be influenced by climatic conditions, with higher rainfall and temperature potentially exerting a positive impact on seed yield.

Table 7.
Effects of fertilisation with compost and mineral nitrogen on the seed yield of RCG (kg·ha−1) during the years of 2012–2015.
The yield of PG seeds depends on the species, species variety, applied agrotechnical operations, or weather conditions that affect their growth and development, as evidenced by the high correlation coefficients of hydrothermal indices with the number of generative shoots [39,48]. The increase in the production of vegetative parts of RCG is associated with a reduction in the yield of generative parts and seeds [39]. An additional essential element is the ability of individual varieties to retain seeds in ears, which depends on both the RCG variety and the harvest date [48]. According to Sahramaa and Hömmö [39], RCG’s seed yield stability, in subsequent years of use, is a more species-specific rather than a variety-specific feature. The initial year of harvest typically delivers the highest yields for most species. In the subsequent years, yields consistently diminish, mainly due to the influence of external environmental factors. In our own research, we observed stable seed yields in the first two years of utilization, followed by a decline in the quantity of harvested seeds, particularly in the third and fourth years of harvest. Sahramaa and Hömmö [39] obtained a lower RCG seed yield in less favourable years, especially in dry vegetation periods. The amount of harvested yield depends on many factors, e.g., the year of use, and ranges from 100 to even over 500 kg·ha−1 [39,48], which entirely coincides with the number of seeds collected in the production of energy biomass. Leaving plants until late autumn and, consequently, having one harvest during the growing season are not economical because the delay in harvest results in an increase in biomass yield, but simultaneously, it reduces the efficiency of biogas and energy concentration in 1 kg of dry matter due to a decline in the share of highly digestible organic matter and an increase in the content of insoluble crude fibre and an increase in the amount of lignin and thus of the lignification of cell walls with age [22,46,47]; thus, some authors [22,47,48,51] recommend harvesting the plant every year in spring for energy purposes, i.e., for direct combustion. One of the explanations for the reduced yield when harvesting RCG only once is its tendency to undergo lodging, particularly after reaching full maturity. This lodging can lead to plant decay and even plant loss. Our own observations of this study align with this perspective. Consequently, it is advisable to harvest RCG plants for biomass when they have fully matured. This approach also allows for the collection of seeds, which can serve as an additional source of income. As Kieloch et al. [40] point out, seeds obtained from plants growing in better conditions are characterised by a higher germination capacity.
3.3. Dry Matter Yields and Morphological Structure of Yield
The applied study factors (organic fertilisation with compost and mineral nitrogen fertilisation) affected the amount of dry matter yields of RCG plants harvested for biomass that is intended for combustion (Table 8). The applied levels of compost fertilisation (10 and 20 Mg·ha−1) provided yields of 95 and 190 kg N·ha−1, 20 and 40 kg P·ha−1, and 35 and 70 kg K·ha−1, respectively, and had positive effects on the obtained dry matter yields. Organic fertilisation provides essential nutrients (NPK) and other minerals necessary for plant nutrition. Compost is a source of slow-release nutrients that depends on the rate of mineralisation, which can last up to several years [28,52]. Research by Bélanger et al. [51] indicates that, with organic fertilisation (pig slurry and municipal biosolid) containing 40, 80, and 120 kg N·ha−1, RCG’s DM yield is comparable to that with the same doses of mineral fertilisation. Therefore, organic fertilisation can be a suitable replacement for mineral fertilisers in the cultivation of bioenergy crops. However, according to Lord [28], in the first year, approximately 6% of total N, 15% of total P, and up to 80% of total K are available. Therefore, the availability of fertilisers from compost should be taken into account and possibly supplemented with mineral fertilisation. Organic fertilisation is vital in the case of sandy soils; it improves the physical and chemical properties of the soil, among others, enables the development of a better soil structure, increases water retention and the availability of fertiliser ingredients, and improves the microbiological activity of soils [53,54]. In all the years of study, the increase in yield of fertilised plants was significant compared to the yield from plants not fertilised with compost, which averaged 14.8% and 71.6% in the first, 9.9% and 17.4% in the second, and 14.9% and 55.2% in the fourth year of study, which is related to the slow release of fertiliser ingredients [28]. Despite the lowest rainfall in the first year of cultivation, the highest increase in yield of plants fertilised with compost at a dose of 20 Mg·ha−1 was obtained, which indicates that organic fertilisation on sandy soils can lead to the increase and stabilisation of RCG yield even with a lower water availability. The average of four years of the experiment showed that the applied compost fertilisation caused a significant increase in dry matter yields by 8.4% and 26.1%, respectively, compared to the yields obtained from plants not fertilised with compost.

Table 8.
Effects of fertilisation with compost and mineral nitrogen on the dry matter yield of RCG (Mg ha−1) during the years of 2012–2015.
The average annual dry matter yield over the entire research period with single fertilisation with compost at doses of 10 and 20 Mg·ha−1 was 8.2 and 10.6 DM Mg·ha−1, respectively. The obtained results of dry matter yield were higher than those obtained in studies conducted in Canada on silty clay soils [51], where, with organic and mineral fertilisation, calculated N doses were from 40 to 120 kg N·ha−1 dry matter yield ranged from 3.8 to 8.0 DM Mg·ha−1, respectively. In studies conducted in England on degraded sandy clay loam, clay, and clay loam soils, compost was used at doses of up to 500 Mg·ha−1, which, after taking the N content and its availability in the first year of cultivation into account, resulted in a yield of 190 kg N·ha−1, and in the next year, of 50–100 kg N·ha−1. The applied fertilisation allowed to obtain an average dry matter yield (oven dried at 105 °C) of 3.5–6.7 DM Mg·ha−1. Research conducted on sandy loam soils in Latvia [54] confirms the beneficial effect of organic fertilisation (fermentation residues every year in doses of 30–150 kg N ha−1) on the dry matter yield of RCG. In these studies, dry matter yields in the range of 5.8–13.9 DM Mg·ha−1 were obtained, depending on the N dose and the year of cultivation. Fertilisation, in the form of fermentation residues, provides readily available nutrients for RCG, and ammonium is produced during fermentation, which plants easily absorb. The beneficial effect of organic fertilisation was also demonstrated by studies carried out with sewage sludge on clay loam soil in Poland by Antonkiewicz [54]. In this experiment, RCG with sewage sludge content was fertilised once before sowing to obtain 74.5 g N·kg−1 DM with doses 10, 20, 40, and 60 Mg·ha−1; the obtained biomass yield ranged from 5.3 (control) to 13.4 Mg·ha−1 (sewage sludge in a dose of 40 Mg·ha−1). Research conducted on sandy loam with digestate in the Czech Republic [55] allowed to obtain a dry matter yield of RCG in the range of 3.9–6.1 Mg·ha−1 at an annual dose of digestate corresponding to 100 kg N·ha−1. The yield of RCG biomass obtained with the same dose of nitrogen from mineral fertilisers was similar. However, Kopecký et al. indicate a better effect of organic fertilisation with digestate compared to mineral fertilisation on light soils. After analysing the impact of the applied doses of nitrogen mineral fertilisation (40, 80, and 120 kg·ha−1) on the dry matter yield of RCG in light soil conditions, it is noteworthy that this factor significantly increases the yield of plants in all years of the study; however, the plants’ reaction towards this study factor varied over the years. The lowest yield (6.4 Mg·ha−1) with mineral fertilisation at a dose of 40 kg N·ha−1 was obtained in the year with the lowest precipitation (2012). In comparison, with the same level of fertilisation, the biomass yield was 11.3 DM Mg·ha−1 in 2013. Higher doses of N from mineral fertilisers resulted in reduced differences in yields between the first year of cultivation and subsequent years. However, fertilisation with compost, in 2012, at a dose of 20 Mg·ha−1 made it possible to obtain one of the highest biomass yields (11.0 DM Mg·ha−1) in the entire research period. The reduction in yield variability with increasing N fertilisation is also confirmed by Strašil et al. [46], who indicate significant fluctuations (4.0–14.6 DM Mg·ha−1) in the dry matter yield of RCG with mineral fertilisation at doses of 30 and 60 kg N·ha−1 on clay loam and sandy loam soils. The greatest fluctuation in yields in individual years occurred on sandy soils. The highest yields were obtained on clay loam soil, while in the control, the difference in yields depending on the location and soil was as much as 45%, and in the variants with fertilisation (60 kg N·ha−1), it was 33%. The authors indicate that this variability was related to the distribution of precipitation and temperature during the growing season. Also, Bélanger et al. [51] obtained lower yields and a greater variability on poor-quality soil with mineral fertilisation at doses ranging from 40 to 120 kg N·ha−1. The obtained results may indicate that, in the case of sandy, poor, and marginal soils, it is necessary to use organic fertilisation to obtain a satisfactory and stable yield because mineral fertilisation alone may turn out to be less effective in dry years. The average annual yield of RCG without fertilisation was 7.0 Mg·ha−1, while mineral fertilisation allowed obtaining a yield in the range of 8.8–12.8 DM Mg·ha−1, with the increasing dose of N clearly influencing the increase in the obtained yield, which is also confirmed by other studies [33,49,51,56,57]. Compared to the obtained dry matter yields from plants not fertilised with nitrogen, the increases in dry matter yields in plants fertilised with nitrogen were 19.2%, 44.9%, and 69.2% in the first, 35.5%, 49.5%, and 81.7% in the second, and 35.4%, 41.8%, and 65.8% in the fourth year of nitrogen fertilisation. The results of average yields of RCG dry matter from the research years showed that the plants exhibited significantly higher yields with nitrogen fertiliser, and the increase in yield compared to the control (without mineral nitrogen fertilisation) was 26.7% (with fertilisation of 40 kg N·ha−1), 41.9% (at 80 kg N·ha−1 fertilisation), and 65.1% (at 120 kg N·ha−1 fertilisation). Similar fluctuations in RCG dry matter yield (up to 72%) with mineral fertilisation at a dose of 120 kg N·ha−1 compared to the control were obtained in studies conducted in Canada [51], with the average biomass yield (from 4.2 to 8.0 DM Mg·ha−1) being significantly lower and fluctuating for the variants fertilisation (40, 80, and 120 kg N·ha−1). RCG in this experiment obtained significantly lower yields in the site with lower average temperatures and precipitation during the growing season. The notably lower yield of dry matter in the RCG experiment, despite similar fertilisation practices, can be attributed to the less favourable continental climatic conditions as opposed to the more conducive temperate climates [48,49]. Nevertheless, research conducted in Lithuania [58] revealed a significantly more substantial impact of mineral nitrogen fertilisation on RCG yields. In the case of acidic moraine soils with a clay content of less than 15%, the application of 120 kg N·ha−1 in mineral fertilisation led to a remarkable yield increase, up to 146% more than the control group. Average annual yields over the entire research period amounted to 6.1–9.9 DM Mg·ha−1, but the authors indicate that rainfall conditions also influenced the yield, and, for example, with high rainfall, it was possible to obtain 11.1 DM Mg·ha−1. When assessing the amount of RCG dry matter yields obtained in the years of study, it should be stated that in light soil conditions and applied experimental factors, in the first year of plantation use, a dry matter yield of 5.3–15.9 Mg·ha−1 was obtained, and in subsequent years, a 26.6% higher yield was obtained in the second year of research (2013), and a 11.7% higher yield in the third year. Contrastingly, the average yield of plants was 3.2% higher in the fourth year than in the first year of study.
The amount of harvested dry matter yield of native grass species intended for biomass varies greatly and is predicated on the tested species, weather conditions, habitat, harvest time, and fertilisation level. However, when it comes to RCG, numerous studies confirm a significant favourable response to nitrogen fertilisation [22,28,33,39,45,46,47,49,53,54,55,56,57,58].
The average dry matter yield of RCG obtained in our study, utilizing only mineral fertilisation with similar N and K doses, exceeded that reported in a Swedish study with a slightly lower P fertilisation. This is observed across light, medium, and heavy mineral soils, as well as for varying biomass harvest timings [52]. For instance, some studies observed a decrease in yield during the growing season as the crop aged, while others found no such correlation [56]. In the Czech Republic, lower RCG yields were recorded on mineral soils with a lower mineral nitrogen fertilisation, with the highest values achieved on heavy soils [46,57]. Estonia reported lower RCG dry matter yields, particularly on organic soils, in contrast to mineral soils with fertilisation [56]. Similarly, in Lithuania, lower yields, even with two harvests per season, were observed on clay soils with similar N and P fertilisation, along with slightly lower K levels [58]. This research also illustrated the positive impact of N fertilisation on increasing RCG dry matter yield. In England, studies indicated lower yields of RCG dry matter, especially with similar N and K fertilisation on clay soil [33]. The genotype of the plant and the timing of harvest had a significant influence on the obtained dry matter yield, with harvest delay having an adverse effect. Similar yields with equivalent mineral fertilisation were reported in Poland [59]. Research conducted by Lewandowski and Schmidt [60] in Germany, which analysed RCG yield with N mineral fertilisation, also highlighted the dependence of RCG yield on soil type. Significantly higher yields were achieved on heavy soils compared to light soils, even in cases of lower precipitation. Multiple studies [18,28,39,46,47,48,55] have suggested that water scarcity in the environment can limit RCG yield. However, Krzyżaniak and Stolarski [20] indicated that RCG can thrive in dry soils, provided these soils are sufficiently fertile. Based on the cited studies and the obtained results, it can be concluded that it is possible to obtain high RCG yields on light mineral soils in temperate climate conditions with compost fertilisation, and outstanding results are obtained with a combination of organic and mineral fertilisation, even with periodic moisture deficits.
An essential element of biomass yield quality is its structure, when RCG is used as a fodder or for the production of biogas or combustion [23,33,48,52,58]. For biogas and fodder production, 3–4 cutting per year with a large proportion of leaves and young shoots are recommended, while burning is usually recommended only once a year, and is often delayed [57]. The morphological structure of RCG crop yield in the years of use was similar; hence, its average over the years of the study is presented in Figure 4 In the years of the study, the share of stems in the dry matter yield ranged from 63.1% to 70.9%. The share of leaves ranged from 19.1% to 23.2%, and inflorescence from 10.0% to 13.7%. In the years of study, the used compost fertilisation levels (10 and 20 Mg∙ha−1) only slightly affected the reduction in the share of stems in the annual yield of RCG dry matter by 1.4% and 2.1%, respectively, and the effect of nitrogen fertilisation on reducing the share of shoots in the yield structure was also confirmed by other studies [48,52]. There was also no apparent impact of the applied nitrogen doses on this characteristic. Instead, there was merely a tendency for a slight decrease in the proportion of stems, favouring a higher proportion of leaves and inflorescences in the yield. Over the course of the research years, this difference amounted to 2.7–5.9%. The share of inflorescence in the dry matter yield was, on average, 12.2%. According to Landström et al. [52], a large number of RCG stems in yield structure is desirable because they contain less undesirable elements like Cl, S, K, Ca, and Mg for combustion [47,48,52] and better-quality fibres for pulp production. Reducing the levels of these elements can also be achieved by postponing the harvesting of biomass until winter or even early spring [18,47,48,52]. However, Christian et al. [33] and Ustak et al. [47] suggest that a higher number of shoots, which may be influenced by the abundant supply of nitrogen and rising average temperatures, can lead to a decline in the biomass quality required for combustion. This is because the shoots may contain a significant quantity of minerals and water.
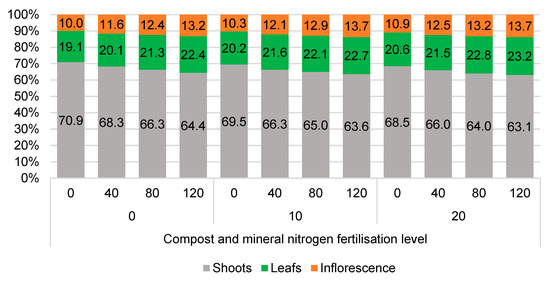
Figure 4.
Effect of the level of compost (Mg·ha−1) and nitrogen (kg·ha−1) fertilisation on the structure (%) of RCG plant yield grown in light soils, an average over the years of research.
3.4. Energy Properties
The energy value of the harvested biomass in the years of research ranged from 16.9 to 17.4 MJ·kg−1 DM. The analysis revealed no substantial impact of the examined factors on its concentration in plants (as shown in Table 9). It is evident that the energy efficiency of RCG plants exhibited a slight decrease when grown on sites fertilised with compost, while nitrogen fertilisation resulted in an average increase of 1.8% in its concentration.

Table 9.
Effects of fertilisation with compost and mineral nitrogen on the calorific value of RCG (MJ·kg−1 DM) and the per cent share of dry matter (%) during the years of 2012–2015.
The suitability of grass biomass for energy applications depends on more factors than just its yield. Factors such as the heat of combustion, calorific value, and chemical composition also play a crucial role as they significantly impact the technological processing conditions and the quality of the end product [20,58,60]. The calorific value of the tested plant ranged from 16.9 to 17.4 MJ·kg−1DM. Similar results were also obtained in other studies [25,28,45,46,55,58]. Comparing the obtained results to other species, it can be stated that they were similar to the calorific value (16.8–17.4 MJ·kg−1 DM) of Virginia fanpetals (Sida hermaphrodita) cultivated on light soils (under the same climatic conditions) [61], and a similar caloric value was also obtained in the studies on Miscanthus sacchariflorus, Miscanthus x giganteus [62,63], Dactylis glomerata, Bromus inermis, and Arrhenatherum elatius as well as on Phragmites australis, RCG, and Spartina pectinata [25,64,65,66,67]. The obtained calorific value for RCG was slightly lower than Salix ssp. [68]. The examined grasses, under the influence of a dose of 40 kg N·ha−1, did not show any differentiation in terms of calorific value. Only an increase in the dose to 80 and 120 kg N·ha−1 caused an increase in the calorific value of RCG. Similar results showing favourable reactions to nitrogen fertilisation were also obtained in other studies on RCG [57,59] as opposed to Strašil’s [46] studies. According to Kacprzak et al. [59] and Allison et al. [67], nitrogen fertilisation can increase lignin content and RCG calorific value and improve crop yields, but it may have a negative effect on energy production in anaerobic digestion.
A crucial parameter of biomass is also the share of dry matter, which is vital in biomass storage and energy production processes [18,22,33,47,58]. Elevated moisture levels in biomass can result in self-ignition when stored, reduced combustion efficiency, and the degradation of carbohydrates due to microbial activity [22,47]. For instance, it is recommended to maintain a moisture content of less than 23% when storing RCG biomass for direct combustion [47]. The dry matter content of RCG plants harvested every year showed that they were similar in the years of the study (Table 9). The dry matter content in the examined years and under the influence of the applied research factors (organic fertilisation with compost and mineral nitrogen) ranged from 69.8% to 72.4%, and the applied study factors only indicate a slight increase in this content from 0.8% (when fertilised with compost at a dose of 20 Mg∙ha−1) to 2.9% (when fertilised with nitrogen at a dose of 120 kg N∙ha−1).
The energy yield of the harvested biomass from RCG plants increased in all years of the study under the influence of the applied levels of compost fertilisation. This increase was primarily attributed to the higher biomass yield that was obtained, with the concentration of energy in the harvested crop contributing only to a limited extent (as indicated in Table 10). The average energy yield of plants fertilised with compost in doses of 10 and 20 Mg∙ha−1 was higher by 6.7% and 24.0% than the energy yield of plants not fertilised with compost. The obtained energy yield results under the influence of the applied doses of nitrogen mineral fertilisation (40, 80, and 120 kg N·ha−1) prove that the energy yield was significantly higher in fertilised plants than in non-fertilised plants, which is also confirmed by other studies [28,39,46,48,52,55]. The average increase in energy efficiency over the course of the experiment was 20.6%, 33.3%, and 49.9%, respectively. The energy yield achieved was noteworthy, surpassing the results obtained in the studies on RCG by Lord [28] and Strašil [46]. Moreover, it exceeded the energy yield in similar climatic conditions, particularly light soils, when compared to Virginia fanpetals (Sida hermaphrodita) [61]. According to Krzyżaniak and Stolarski [20], the energy consumption of RCG cultivation is approximately 8.8 GJ·ha−1 (seed material, fuel, fertilisers, and machine production), and NPK mineral fertilisation accounts for as much as 50% of the demand. Therefore, the complete or partial replacement of mineral fertilisation with cheaper compost will significantly reduce production costs and improve the profitability of RCG cultivation.

Table 10.
Effects of fertilisation with compost and mineral nitrogen on the energy yield of RCG (GJ·ha) during the years of 2012–2015.
4. Conclusions
Organic compost and mineral nitrogen fertilisation increased the height and thickness of shoots of RCG. The applied levels of compost fertilisation (10 and 20 Mg∙ha−1) had a positive effect on the obtained dry matter yields in all years of the study compared to plants not fertilised with compost, and their yield was higher, respectively, by 12.1% and 41.0%, on average, for the years of research. Nitrogen fertilisation in light soil conditions (in doses of 40, 80, and 120 kg·ha−1) significantly increased RCG yield in all years of research. Compared to the obtained dry matter yields of non-nitrogen fertilised plants, the increase in this yields of fertilised plants was higher by 26.8%, 41.6%, and 65.0%, respectively. The highest energy value of RCG dry matter yield per unit area was determined for plants subjected to the most extensive fertilisation with organic compost and mineral nitrogen. When harvesting RCG plants for energy biomass at the stage of full maturity, a significant seed yield of 242 to 600 (kg·ha−1) can also be obtained in the first three years, while in the fourth year of use, the seed yield was almost three times lower (90–158 kg·ha−1). According to these investigations, RCG can thrive in moderate climate conditions on light soils, and the application of both organic and mineral fertilisation strategies enables the attainment of substantial biomass yields in such environments. In the initial years of cultivating RCG for biomass, an added advantage that was noted is the potential for achieving a significant seed yield, which can be utilised when establishing subsequent crops. When grown on light mineral soils in the context of a Central European climate, the combination of organic fertilisers (compost derived from urban greenery) and mineral nitrogen fertilisation is employed to secure high yields of both biomass and seeds.
Implementation of RCG-based biomass-to-bioproduct-pathways requires SWAT and stakeholders analyses, an economic assessment (the average costs of fertiliser, working hours, and application technique) and life cycle analyses. In plant life cycle research, it will be important to determine the impact of RCG cultivation on the environment with the analyses of GHG emissions and the CO2 equivalent and the migration of fertiliser ingredients into the soil and water environment, among others.
Author Contributions
Conceptualization, T.K.; methodology, T.K.; software, T.K. and G.J.; validation, G.J., T.K. and R.M.; formal analysis, G.J.; investigation, T.K., G.J. and R.M.; resources, T.K. and G.J.; data curation, T.K.; writing—original draft preparation, T.K. and G.J.; writing—review and editing, G.J. and R.M.; visualisation, T.K. and G.J.; supervision, G.J.; project administration, T.K.; funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
West Pomeranian University of Technology in Szczecin, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gabryszuk, M.; Barszczewski, J.; Wróbel, B. Characteristics of grasslands and their use in Poland. J. Water Land Dev. 2021, 51, 243–249. [Google Scholar] [CrossRef]
- Kitczak, T.; Malinowski, R.; Jarnuszewski, G.; Podlasiński, M. Changes within permanent grasslands used for agriculture in the West Pomeranian Voivodship. J. Water Land Dev. 2023, 59. in press. [Google Scholar]
- Schils, R.L.M.; Bufe, C.; Rhymer, C.M.; Francksen, R.M.; Klaus, V.H.; Abdalla, M.; Milazzo, F.; Lellei-Kovács, E.; Berge, H.T.; Bertora, C.; et al. Permanent grasslands in Europe: Land use change and intensification decrease their multifunctionality. Agric. Ecosyst. Environ. 2022, 330, 107891. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More Important for Ecosystem Services than You Might Think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Linke, B.; Idler, C.; Amon, T.; Hobbs, P.J. Bioenergy from permanent grassland—A review: 1. Biogas. Bioresour. Technol. 2009, 100, 4931–4944. [Google Scholar] [CrossRef]
- EC. Communication (2014) 0015 from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions. A Policy Framework for Climate and Energy in the Period from 2020 to 2030, COM/2014/015 Final. Available online: https://www.eea.europa.eu/policy-documents/com-2014-15-final (accessed on 7 September 2023).
- Gugele, B.; Pinterits, M.; EEA; EU; EC; DG Climate Action; EEA. Annual European Union Greenhouse Gas Inventory 1990–2021 and Inventory Report. 2023, p. 752. Available online: https://www.eea.europa.eu/publications/annual-european-union-greenhouse-gas-2 (accessed on 7 September 2023).
- Wiśniewski, T.P. Investigating Divergent Energy Policy Fundamentals: Warfare Assessment of Past Dependence on Russian Energy Raw Materials in Europe. Energies 2023, 16, 2019. [Google Scholar] [CrossRef]
- Directive 2018/2001/EU of the European Parliament and of the Council of Dec 11 2018 on the Promotion of the Use of Energy from Renewable Sources (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/HTML/?uri=CELEX:32018L2001 (accessed on 9 September 2023).
- Share of Energy Consumption from Renewable Sources in Europe (8th EAP). Available online: https://www.eea.europa.eu/ims/share-of-energy-consumption-from (accessed on 7 September 2023).
- Statistics Poland. Energy from Renewable Sources in 2021; Statistics Poland: Warsaw, Poland, 2022; pp. 1–96. [Google Scholar]
- Marks-Bielska, R.; Bielski, S.; Pik, K.; Kurowska, K. The Importance of Renewable Energy Sources in Poland’s Energy Mix. Energies 2020, 13, 4624. [Google Scholar] [CrossRef]
- Pietrzak, M.B.; Igliński, B.; Kujawski, W.; Iwański, P. Energy Transition in Poland—Assessment of the Renewable Energy Sector. Energies 2021, 14, 2046. [Google Scholar] [CrossRef]
- Maciaszczyk, M.; Czechowska-Kosacka, A.; Rzepka, A.; Lipecki, T.; Łazuka, E.; Wlaź, P. Consumer Awareness of Renewable Energy Sources: The Case of Poland. Energies 2022, 15, 8395. [Google Scholar] [CrossRef]
- Bełdycka-Bórawska, A.; Bórawski, P.; Borychowski, M.; Wyszomierski, R.; Bórawski, M.B.; Rokicki, T.; Ochnio, L.; Jankowski, K.; Mickiewicz, B.; Dunn, J.W. Development of Solid Biomass Production in Poland, Especially Pellet, in the Context of the World’s and the European Union’s Climate and Energy Policies. Energies 2021, 14, 3587. [Google Scholar] [CrossRef]
- Jensen, E.F.; Casler, M.D.; Farrar, K.; Finnan, J.M.; Lord, R.; Palmborg, C.; Valentine, J.; Donnison, I.S. Reed Canary Grass: From Production to End Use. In Perennial Grasses for Bioenergy and Bioproducts; Alexopoulou, E., Ed.; Academic Press: London, UK, 2018; pp. 153–173. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L. Perennial Energy Grasses: Resilient Crops in a Changing European Agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal Agricultural Land Low-Input Systems for Biomass Production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef]
- Duchene, O.; Celette, F.; Ryan, M.; Dehaan, L.R.; Crews, T.E.; David, C. Integrating multipurpose perennial grains crops in Western European farming systems. Agric. Ecosyst. Environ. 2019, 284, 106591. [Google Scholar] [CrossRef]
- Krzyżaniak, M.; Stolarski, M.J. Perennial Grasses for Energy. In Encyclopedia of Sustainable Technologies; Abraham, M.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–140. [Google Scholar]
- Zahorec, A.; Reid, M.L.; Tiemann, L.K.; Landis, D.A. Perennial grass bioenergy cropping systems: Impacts on soil fauna and implications for soil carbon accrual. GCB Bioenergy 2022, 14, 4–23. [Google Scholar] [CrossRef]
- Wrobel, C.; Coulman, B.E.; Smith, D.L. The potential use of reed canarygrass (Phalaris arundinacea L.) as a biofuel crop. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2009, 59, 1–18. [Google Scholar] [CrossRef]
- Valentine, J.; Clifton-Brown, J.; Hastings, A.; Robson, P.; Allison, G.; Smith, P. Food vs. fuel: The use of land for lignocellulosic ‘next generation’ energy crops that minimise competition with primary food production. Glob. Change Biol. Bioenergy 2012, 4, 1–19. [Google Scholar] [CrossRef]
- Nanda, S.; Azargohar, R.; Dalai, A.K.; Kozinski, J.A. An assessment on the sustainability of lignocellulosic biomass for biorefining. Renew. Sustain. Energy Rev. 2015, 50, 925–941. [Google Scholar] [CrossRef]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Karsznicka, A.M.; Grzesik, M.; Mika, B. Cultivation of grasses for biomass-possibilities and restrictions. Zesz. Probl. Postep. Nauk. Rol. 2005, 504, 631–637. (In Polish) [Google Scholar]
- Malinowska, E.; Wiśniewska-Kadżajan, B.; Jankowski, K.; Sosnowski, J.; Wyrębek, H. Evaluation of the usefulness of biomass of different crops for energy. Zesz. Nauk. Uniw. Przyr.-Humanist. Siedlcach Ser. Adm. Zarządzanie 2014, 102, 49–61. (In Polish) [Google Scholar]
- Lord, R.A. Reed canarygrass (Phalaris arundinacea) outperforms Miscanthus or willow on marginal soils, brownfield and non-agricultural sites for local, sustainable energy crop production. Biomass Bioenergy 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Čížková, H.; Rychterová, J.; Hamadejová, L.; Suchý, K.; Filipová, M.; Květ, J.; Anderson, N.O. Biomass production in permanent wet grasslands dominated with Phalaris arundinacea: Case study of the Třeboň basin biosphere reserve, Czech Republic. In The Role of Natural and Constructed Wetlands in Nutrient Cycling and Retention on the Landscape; Vymazal, V., Ed.; Springer International: Cham, Switzerland, 2015; pp. 1–16. [Google Scholar] [CrossRef]
- Piskier, T. A method of estimation of the caloric value of the biomass. Part I–Biomass energy potential. J. Mech. Energy Eng. 2017, 1, 189–194. [Google Scholar]
- Reinhardt, J.; Hilgert, P.; Von Cossel, M. Yield performance of dedicated industrial crops on low-temperature characterised marginal agricultural land in Europe—A rewiev. Biofuels Bioprod. Biorefining 2021, 16, 609–622. [Google Scholar] [CrossRef]
- Hadders, G.; Olsson, R. Harvest of grass for combustion in late summer and in spring. Biomass Bioenergy 1997, 12, 171–175. [Google Scholar] [CrossRef]
- Christian, D.G.; Yates, N.E.; Riche, A.B. The effect of harvest date on the yield and mineral content of Phalaris arundinacea L. (reed canary grass) genotypes screened for their potential as energy crops in southern England. J. Sci. Food Agric. 2006, 86, 1181–1188. [Google Scholar] [CrossRef]
- Nabel, M.; Tenperton, V.M.; Poorter, H.; Lücke, A.; Jablonowski, N.D. Energising marginal soils—The establishment of the energy crop Sida hermaphrodita as dependent on digestate fertilisation, NPK, and legume intercropping. Biomass Bioenergy 2016, 87, 9–16. [Google Scholar] [CrossRef]
- Pszczólkowska, A.; Romanowska-Duda, Z.; Pszczólkowski, W.; Grzesik, M.; Wysokińska, Z. Sustainable energy crop production in Poland: Perspectives. Comp. Econ. Res. Cent. East. Eur. 2012, 15, 57–75. [Google Scholar] [CrossRef][Green Version]
- FAO. IUSS Working Group WRB World Reference Base for Soil Resources 2014; Update 2015; FAO: Rome, Italy, 2014. [Google Scholar]
- Tröster, M.F. Assessing the Value of Organic Fertilizers from the Perspective of EU Farmers. Agriculture 2023, 13, 1057. [Google Scholar] [CrossRef]
- Ferdini, S.; von Cossel, M.; Wulfmeyer, V.; Warrach-Sagi, K. Climate-based identification of suitable cropping areas for giant reed and reed canary grass on marginal land in Central and Southern Europe under climate change. GCB Bioenergy 2023, 15, 424–443. [Google Scholar] [CrossRef]
- Sahraama, M.K.; Hömmö, L. Seed production characters and germination performance of reed canary grass in Finland. Agric. Food Sci. Finl. 2000, 9, 239–251. [Google Scholar] [CrossRef]
- Kieloch, R.; Gołębowska, H.; Sienkiewicz-Cholewa, U. Impact of habitat conditions on the biological traits of the reed canary grass (Phalaris arundinacea L.). Acta Agrobot. 2015, 68, 205–210. [Google Scholar] [CrossRef][Green Version]
- BN-89/9103-09; Disposal of Municipal Waste Compost from Waste. Polish Committee for Standardization: Warsaw, Poland, 1989. (In Polish)
- PN-81/G-04513; Solid Fuels–Determination of Heat of Combustion and Calculation of Calorific Value. Polish Committee for Standardization: Warsaw, Poland, 1981. (In Polish)
- Steinhoff-Wrześniewska, A.; Dąbrowski, P.; Paszkiewicz-Jasińska, A.; Wróbel, B.; Strzelczyk, M.; Helis, M.; Kalaji, M.H. Studying the Physiological Reactions of C4 Grasses in Order to Select Them for Cultivation on Marginal Lands. Sustainability 2022, 14, 4512. [Google Scholar] [CrossRef]
- Rakhmetova, S.O.; Vergun, O.M.; Kulyk, M.I.; Blume, R.Y.; Bondarchuk, O.P.; Blume, Y.B.; Rakhmetov, D.B. Efficiency of Switchgrass (Panicum virgatum L.) Cultivation in the Ukrainian Forest-Steppe Zone and Development of Its New Lines. Open Agric. J. 2020, 14, 273–289. [Google Scholar] [CrossRef]
- Dradrach, A.; Gąbka, D.; Szlachta, J.; Wolski, K. Wartość energetyczna kilku gatunków traw uprawianych na glebie lekkiej (Energy value of several grass species cultivated on light soil). Grassl. Sci. Pol. 2007, 10, 29–35. (In Polish) [Google Scholar]
- Strašil, Z. Evaluation of reed canary grass (Phalaris arundinacea L.) grown for energy use. Res. Agric. Eng. 2012, 58, 119–130. [Google Scholar] [CrossRef]
- Ustak, S.; Šinko, J.; Muňoz, J. Reed canary grass (Phalaris arundinacea L.) as a promising energy crop. J. Cent. Eur. Agric. 2019, 20, 1143–1168. [Google Scholar] [CrossRef]
- Wrobel, C.; Coulman, B.E.; Smith, D.L. Relationship between seed retention and a folded-leaf trait in reed canarygrass (Phalaris arundinacea L.). Acta Agric. Scand. Sect. B—Soil Plant Sci. 2009, 59, 279–285. [Google Scholar] [CrossRef]
- Sahraama, M.; Jauhiainen, L. Characterization of development and stem elongation of reed canary grass under northern conditions. Ind. Crops Prod. 2003, 18, 155–169. [Google Scholar] [CrossRef]
- Śpiewakowski, E.R.; Wielicka, M.; Piasecki, J. Anatomical-morphological changes in Glyceria aquatica (L.) Wahlb. and Phalaris arundinacea L. growing in the zone inundated by the Kwiecko lake. Acta Soc. Bot. Pol. 1987, 56, 147–154. [Google Scholar] [CrossRef][Green Version]
- Bélanger, G.; Cambouris, A.N.; Ziadi, N.; Parent, G.; Mongrain, D.; Lajeunesse, J.; Martel, H.; Seguin, P. Biomass Production and Environmental Considerations from Reed Canarygrass Fertilized with Organic Residues in Northern Environments. Agron. J. 2018, 110, 664–674. [Google Scholar] [CrossRef]
- Landström, S.; Lomakka, L.; Andersson, S. Harvest in spring improves yield and quality of reed canary grass as a bioenergy crop. Biomass Bioenergy 1996, 11, 333–341. [Google Scholar] [CrossRef]
- Rancane, S.; Karklins, A.; Lazdina, D.; Berzins, P.; Bardule, A.; Butlers, A.; Lazdis, A. Biomass yield and chemical composition of Phalaris aruninacea L. using different rates of fermentation residues as fertiliser. Agron. Res. 2017, 15, 521–529. [Google Scholar]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J.; Popławska, A. The possibility of using sewage sludge for energy crop cultivation exemplified by reed canary grass and giant miscanthus. Soil Sci. Annu. 2019, 40, 21–33. [Google Scholar] [CrossRef]
- Kopecký, M.; Mráz, P.; Kolář, L.; Váchalová, R.; Bernas, J.; Konvalina, P.; Perná, K.; Murindangabo, Y.; Menšík, L. Effect of Fertilization on the Energy Profit of Tall Wheatgrass and Reed Canary Grass. Agronomy 2021, 11, 445. [Google Scholar] [CrossRef]
- Heinsoo, K.; Hein, K.; Melts, I.; Holm, B.; Ivask, M. Reed canary grass yield and fuel quality in Estonian farmers’ fields. Biomass Bioenergy 2011, 35, 617–625. [Google Scholar] [CrossRef]
- Strašil, Z.; Váňa, V.; Káš, M. The reed canary grass (Phalaris arundinacea L.) cultivated for energy utilisation. Res. Agric. Eng. 2005, 51, 7–12. [Google Scholar] [CrossRef]
- Šiaudinis, G.; Jasinskas, A.; Šarauskis, E.; Skuodienė, R.; Repšienė, R.; Karčauskienė, D. The Influence of Lime Material and Nitrogen Fertilization on Reed Canary Grass Productivity, Plant Quality and Environmental Impact of Using Biomass for Energy Purposes. Agronomy 2021, 11, 895. [Google Scholar] [CrossRef]
- Kacprzak, A.; Matyka, M.; Krzystek, L.; Ledakowicz, S. Evaluation of biogas collection from reed canary grass, depending on nitrogen fertilisation levels. Chem. Process Eng. 2012, 33, 697–701. [Google Scholar] [CrossRef]
- Lewandowski, I.; Schmidt, U. Nitrogen, energy and land use efficiencies of miscanthus, reed canary grass and triticale as determined by the boundary line approach. Agric. Ecosyst. Environ. 2005, 112, 335–346. [Google Scholar] [CrossRef]
- Kitczak, T.; Jarnuszewski, G.; Łazar, E.; Malinowski, R. Sida hermaphrodita Cultivation on Light Soil—A Closer Look at Fertilization and Sowing Density. Agronomy 2022, 12, 2715. [Google Scholar] [CrossRef]
- Dubis, B.; Jankowski, K.J.; Załuski, D.; Bórawski, P.; Szempliński, W. Biomass production and energy balance of Miscanthus over period of 11 years: A case study in large-scale farm in Poland. GCB Bioenergy 2019, 11, 1187–1201. [Google Scholar] [CrossRef]
- Voća, N.; Leto, J.; Karažija, T.; Bilandžija, N.; Peter, A.; Kutnjak, H.; Šurić, J.; Poljak, M. Energy Properties and Biomass Yield of Miscanthus x Giganteus Fertilised by Municipal Sewage Sludge. Molecules 2021, 26, 4371. [Google Scholar] [CrossRef]
- Harkot, W.; Warda, M.; Sawicki, J.; Lipińska, H.; Wyłupek, T.; Czarnecki, Z.; Kulik, M. Możliwości wykorzystania runi łąkowej do celów energetycznych (The possibility of meadow sward use for energy purposes). Grassl. Sci. Pol. 2007, 10, 59–67. (In Polish) [Google Scholar]
- Grzelak, M.; Gaweł, E.; Gajewski, P.; Kaczmarek, Z.; Majchrzak, L. Floristic diversity, yielding and calorific value of plant communities with dominant native grass species. J. Res. Appl. Agric. Eng. 2019, 64, 25–28. [Google Scholar]
- Malinowska, E.; Wiśniewska-Kadżajan, B. The Effects of Different Doses of Organic Waste on Prairie Cordgrass (Spartina Pectinata L.) Yield and Selected Energy Parameters. Energies 2023, 16, 5599. [Google Scholar] [CrossRef]
- Allison, G.; Morris, C.; Lister, S.; Barraclough, T.; Yates, N.; Shield, I.; Donnison, I. Effect of nitrogen fertiliser application on cell wall composition in switchgrass and reed canary grass. Biomass Bioenergy 2012, 40, 19–26. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Warmiński, K.; Załuski, D.; Olba-Zięty, E. Willow Biomass as Energy Feedstock: The Effect of Habitat, Genotype and Harvest Rotation on Thermophysical Properties and Elemental Composition. Energies 2020, 13, 4130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).