Effects of Microencapsulated Probiotics on Performance, Organ Development, Diarrhoea Incidences, Blood Parameters, Intestinal Histomorphology and Microflora in Weaning Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Procedure
2.2. Animals and Housing System
2.3. Probiotics
2.3.1. Strains Isolation, Characterization and Growth Conditions
2.3.2. Bioreactor Batch Fermentation and Spray Drying Process
2.4. Diets
2.5. Growth Performance
2.6. Incidence of Diarrhoea Determination
2.7. Blood Sampling and Analyses
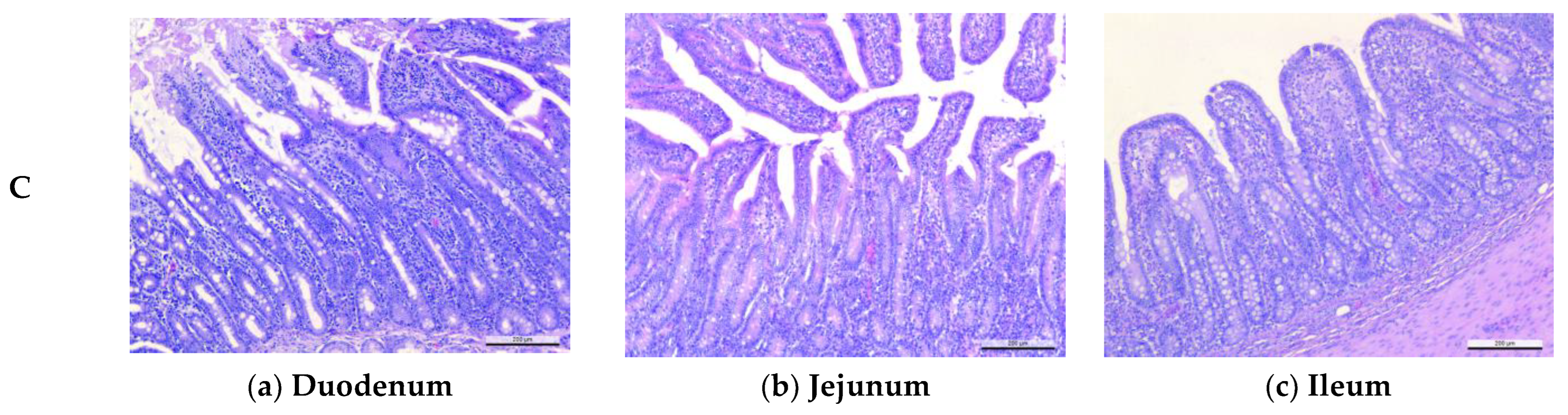
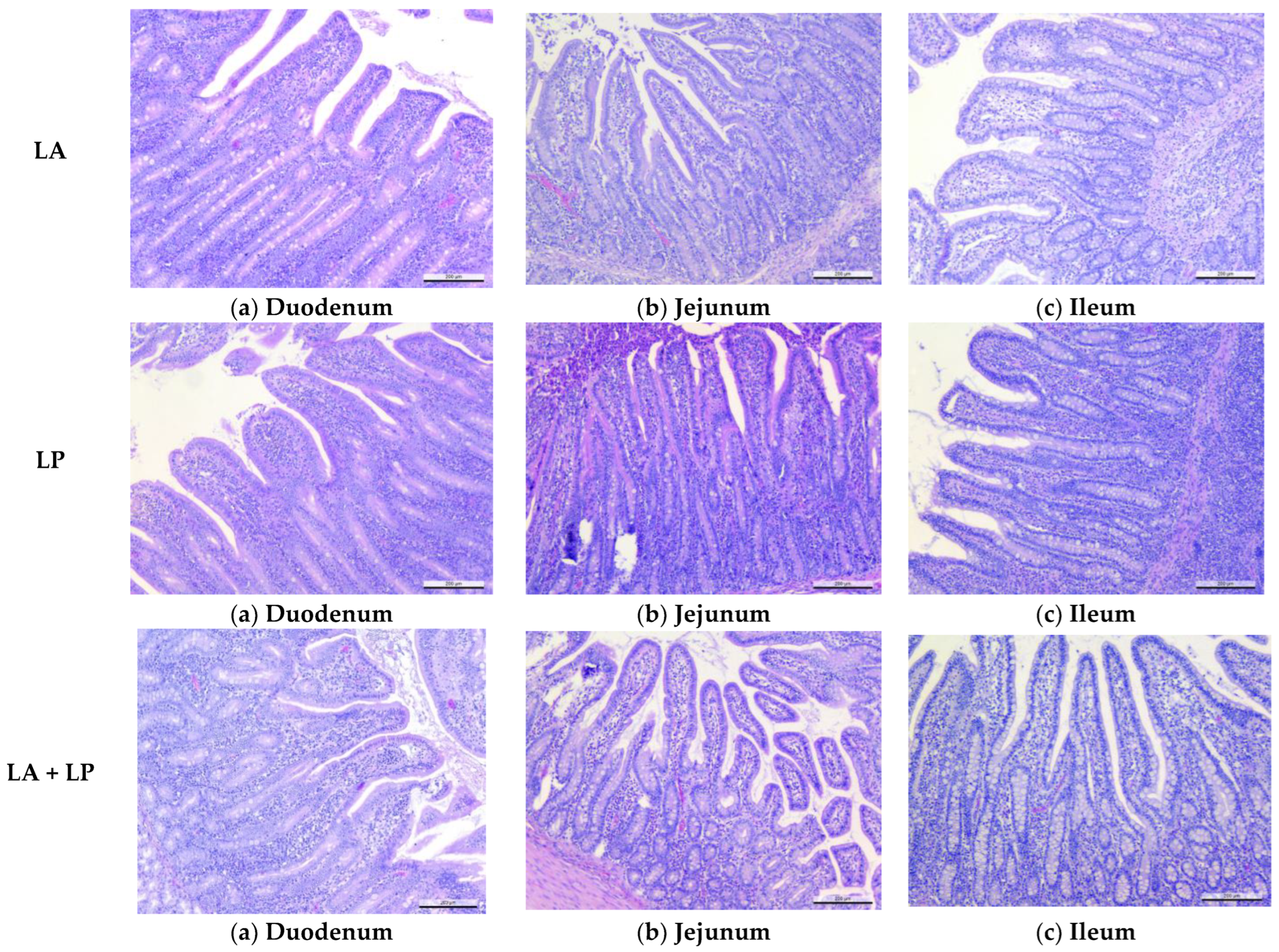
2.8. Intestinal Sampling, Light Microscopy Examination and Intestinal Microflora Analyses
2.8.1. Sampling
2.8.2. Light Microscopy
2.8.3. Microflora Analyses
2.9. Statistical Analysis
3. Results
3.1. Effects of Microencapsulated Probiotics Supplements on Growth Performances of Weaning Piglets
3.2. Effects of Microencapsulated Probiotics Supplements on Organs Development, Intestinal pH and Diarrhoea Incidence of Weaning Piglets
3.3. Effect of Microencapsulated Probiotics Supplements on Biochemical Parameters of Weaning Piglets
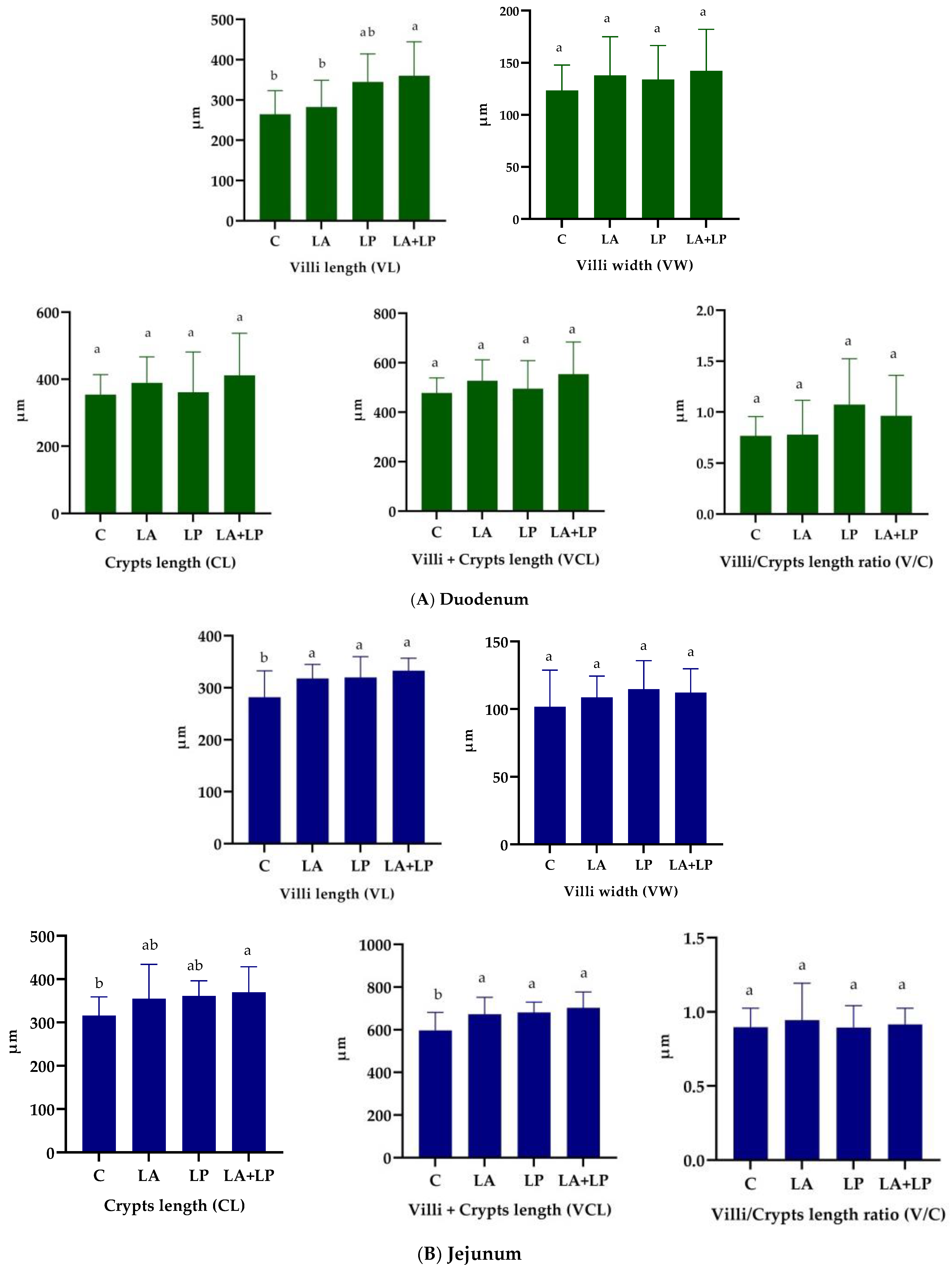
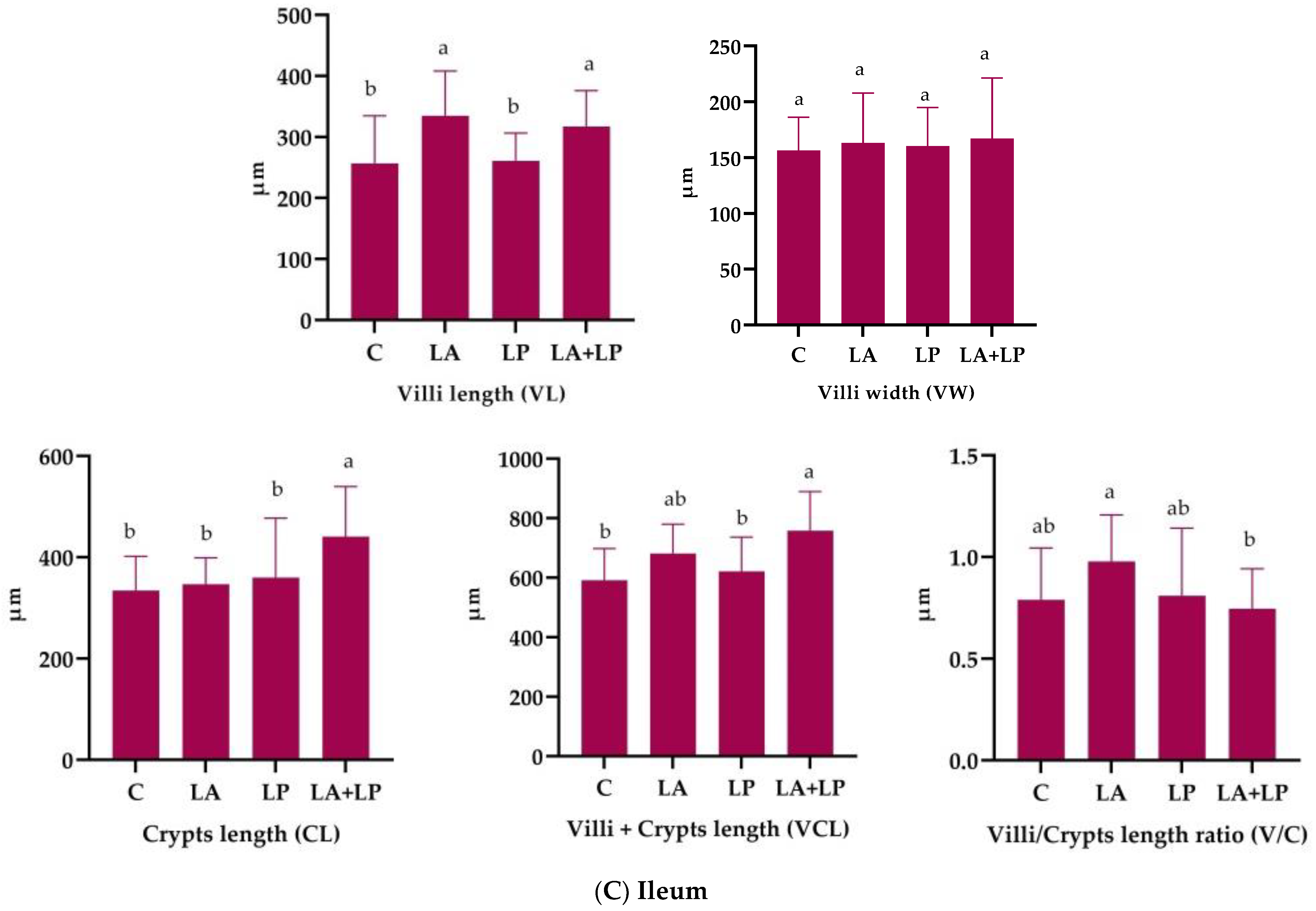
3.4. Effects of Microencapsulated Probiotics Supplements on Intestinal Histomorphology Measurements of Weaning Piglets
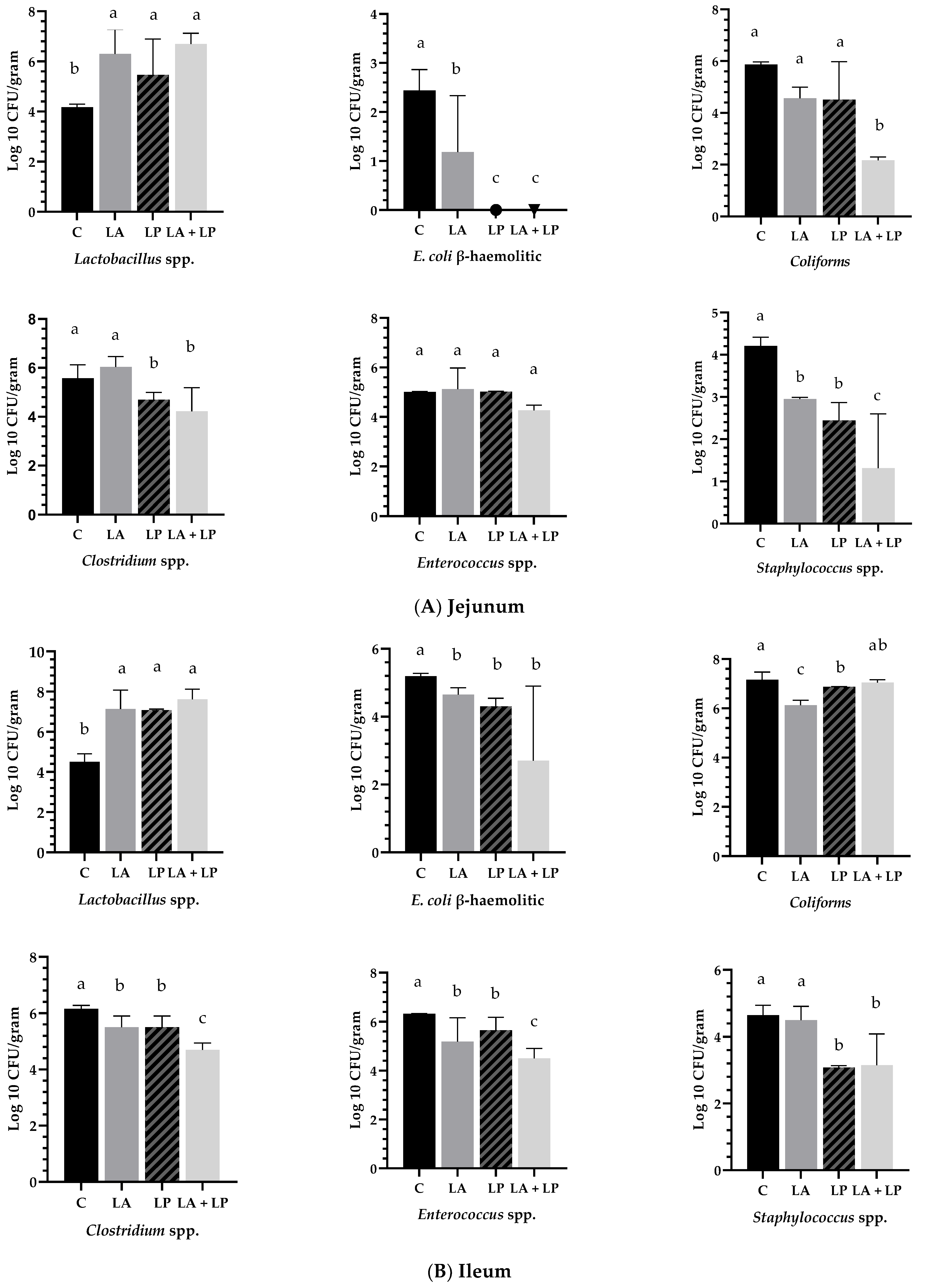
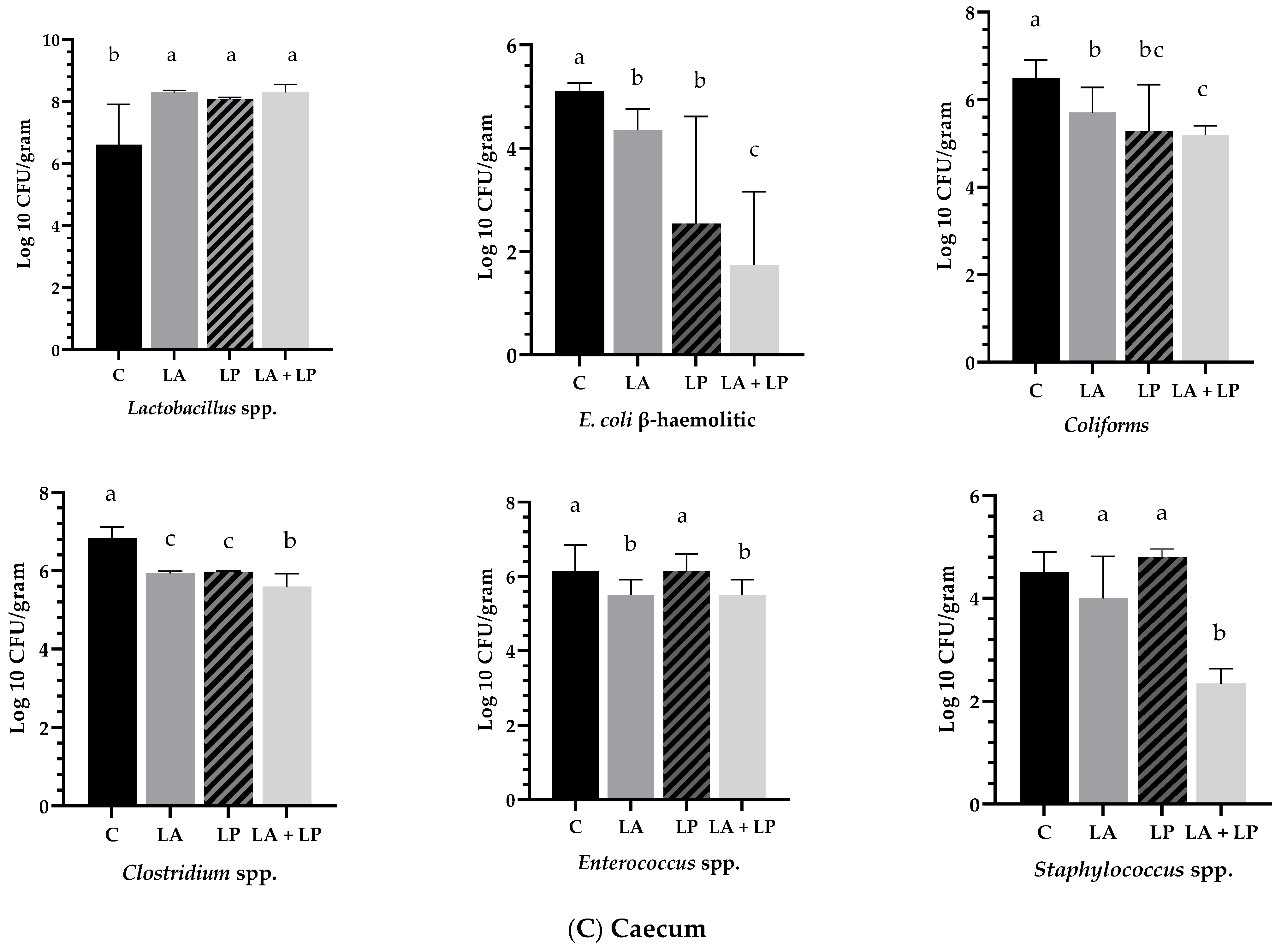
3.5. Effects of Microencapsulated Probiotics Supplements on Intestinal Microbiota of Jejunum, Ileum and Caecum of Weaning Piglets
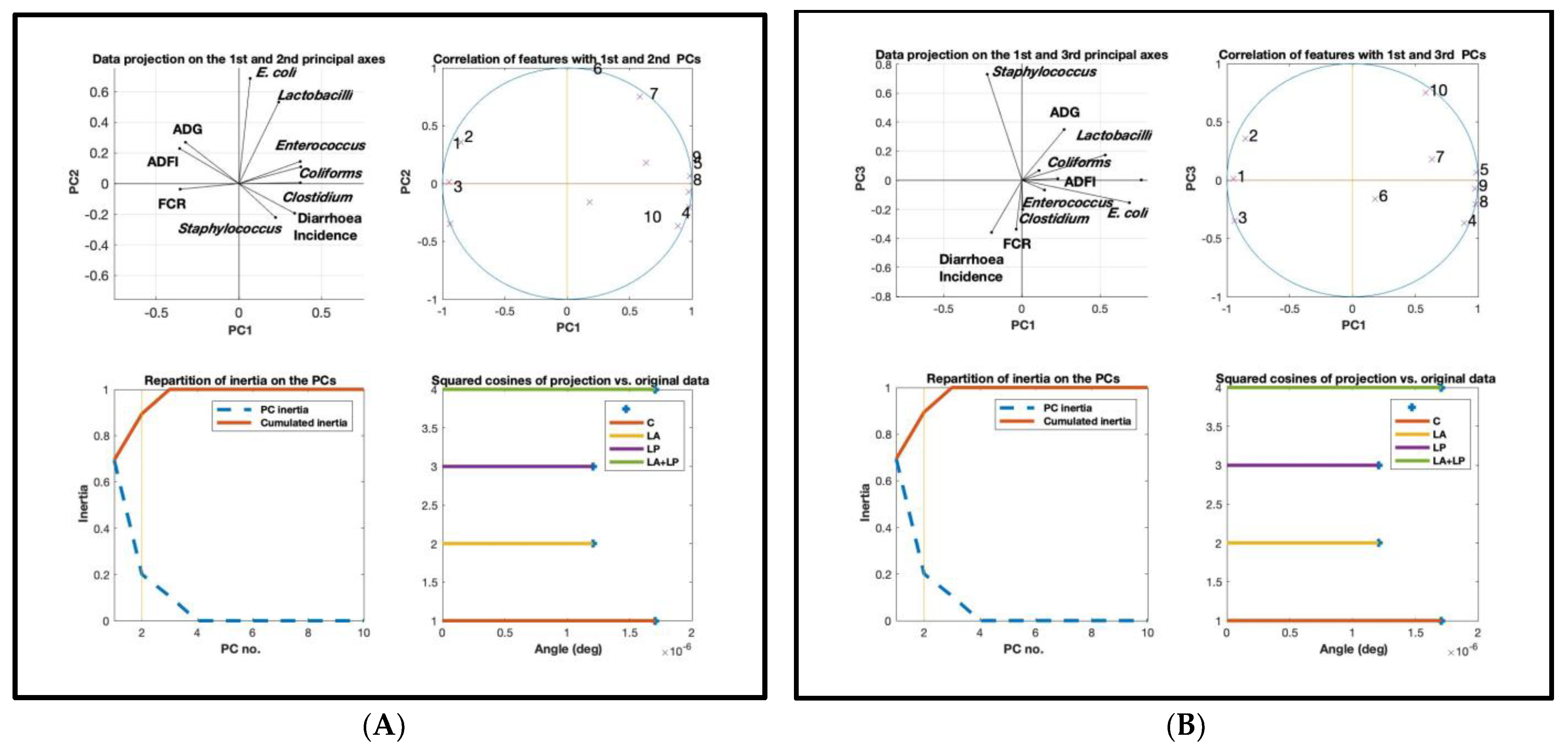
3.6. Principal Component Analysis (PCA)
4. Discussion
4.1. Effects of Microencapsulated Probiotics Supplements on Growth Performances of Weaning Piglets
4.2. Effects of Microencapsulated Probiotics Supplements on Organs Development, Intestinal pH and Diarrhoea Incidence of Weaning Piglets
4.3. Effect of Microencapsulated Probiotics Supplements on Biochemical Parameters of Weaning Piglets
4.4. Effects of Microencapsulated Probiotics Supplements on Intestinal Histomorphology of Weaning Piglets
4.5. Effects of Microencapsulated Probiotics Supplements on Intestinal Microbiota of Jejunum, Ileum and Caecum of Weaning Piglets
4.6. Principal Component Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moturi, J.; Kim, K.Y.; Hosseindoust, A.; Lee, J.H.; Xuan, B.; Park, J.; Kim, E.B.; Kim, J.S.; Chae, B.J. Effects of Lactobacillus salivarius isolated from feces of fast-growing pigs on intestinal microbiota and morphology of suckling piglets. Sci. Rep. 2021, 11, 6757. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhu, W.; Guo, Q.; Luo, W.; Zhang, J.; Xu, W.; Xu, J. Weaning induced hepatic oxidative stress, apoptosis, and aminotransferases through MAPK signaling pathways in piglets. Oxid. Med. Cell. Longev. 2016, 2016, 4768541. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; et al. Effect of sialyllactose on growth performance and intestinal epithelium functions in weaned pigs challenged by enterotoxigenic Escherichia Coli. J. Anim. Sci. Biotechnol. 2022, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Kim, C.; Yoo, J.; Pluske, J.R. A between-experiment analysis of relationships linking dietary protein intake and post-weaning diarrhoea in weanling pigs under conditions of experimental infection with an enterotoxigenic strain of Escherichia coli. JSAS 2015, 86, 286–293. [Google Scholar] [CrossRef]
- Hăbeanu, M.; Lefter, N.A.; Toma, S.M.; Dumitru, M.; Cismileanu, A.; Surdu, I.; Gheorghe, A.; Dragomir, C.; Untea, A. Changes in Ileum and Cecum Volatile Fatty Acids and Their Relationship with Microflora and Enteric Methane in Pigs Fed Different Fiber Levels. Agriculture 2022, 12, 451. [Google Scholar] [CrossRef]
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhoea in Piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef]
- EU Commission. Regulation EC 1831/2003 of the European Parliament and of the Council, of 22 September 2003 on Additives for Use in Animal Nutrition (Text with EEA Relevance); EU Commission: Brussels, Belgium, 2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003R1831 (accessed on 10 November 2022).
- FAO/WHO. Guidelines for the evaluation of probiotics in food Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, ON, Canada, 30 April–1 May 2002; pp. 1–11. Available online: https://www.mhlw.go.jp/file/05-Shingikai-11121000-Iyakushokuhinkyoku-Soumuka/0000197343.pdf (accessed on 10 November 2022).
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.J.; Kim, D.W.; Kang, B.N.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Zhu, C.; Yao, J.; Zhu, M.; Zhu, C.; Yuan, L.; Li, Z.; Cai, D.; Chen, S.; Liu, H.Y.; Hu, P. A meta-analysis of Lactobacillus-based probiotics for growth performance and intestinal morphology in piglets. Front. Vet. Sci. 2022, 9, 1750. [Google Scholar]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Patel, B.H.M.; Singh, P. Effect of swine based probiotic on performance, diarrhoea scores, intestinal microbiota and gut health of grower-finisher crossbred pigs. Livest. Sci. 2017, 195, 74–79. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Yu, Y.H. Evaluation of Bacillus licheniformis-fermented feed additive as an antibiotic substitute: Effect on the growth performance, diarrhoea incidence, and cecal microbiota in weaning piglets. Animals. 2020, 10, 1649. [Google Scholar] [CrossRef] [PubMed]
- Pitino, I.; Randazzo, C.L.; Mandalari, G.; Curto, A.L.; Faulks, R.M.; Le Marc, Y.; Wickham, M.S.J. Survival of Lactobacillus rhamnosus strains in the upper gastrointestinal tract. Food Microbiol. 2010, 27, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Da Rosa, D.P.; Vieira, M.D.M.; Kessler, A.M.; de Moura, T.M.; Frazzon, A.P.G.; McManus, C.M.; Ribeiro, A.M.L. Efficacy of hyperimmunized hen egg yolks in the control of diarrhoea in newly weaned piglets. Food Agric. Immun. 2015, 26, 622–634. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets’: Understanding the keys to health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Jiao, L.F.; Song, Z.H.; Ke, Y.L.; Xiao, K.; Hu, C.H.; Shi, B. Cello-oligosaccharide influences intestinal microflora, mucosal architecture and nutrient transport in weaned pigs. Anim. Feed Sci. Technol. 2014, 195, 85–91. [Google Scholar] [CrossRef]
- Huang, C.H.; Qiao, S.Y.; Li, D.F.; Piao, X.S.; Ren, J.P. Effects of lactobacilli on the performance, diarrhoea incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs. Asian Austral. J. Anim. 2004, 17, 401–409. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation improved probiotics survival during gastric transit. HAYATI J. Biosc. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- García, M.J.; Ruíz, F.; Asurmendi, P.; Pascual, L. Reevaluating a non-conventional procedure to microencapsulate beneficial lactobacilli: Assessments on yield and bacterial viability under simulated technological and physiological conditions. J. Sci. Food Agric. 2022, 102, 2981–2989. [Google Scholar] [CrossRef]
- Sorescu, I.; Dumitru, M.; Hăbeanu, M.; Costin, S. Lactobacillus Spp. strains isolation, identification, preservation and quantitative determinations from the intestinal content and faeces of weaned piglets. Arch. Zootech. 2020, 23, 84–100. [Google Scholar]
- Dumitru, M.; Vodnar, D.C.; Elemer, S.; Ciurescu, G.; Hăbeanu, M.; Sorescu, I.; Georgescu, S.-E.; Dudu, A. evaluation of non-encapsulated and microencapsulated lactic acid bacteria. Appl. Sci. 2021, 11, 9867. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Q.; Chang, J.; Yin, Q.; Song, A.; Li, Z.; Wang, E.; Lu, F. Effects of Lactobacillus casei and Enterococcus faecalis on growth performance, immune function and gut microbiota of suckling piglets. Arch. Anim. Nutr. 2017, 71, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Sorescu, I.; Dumitru, M.; Ciurescu, G. Lactobacillus spp. and Enterococcus faecium strains isolation, identification, preservation and quantitative determinations from Turkey gut content. Rom. Biotechnol. Lett. 2019, 24, 41–49. [Google Scholar] [CrossRef]
- Dumitru, M.; Sorescu, I.; Habeanu, M.; Tabuc, C.; Idriceanu, L.; Jurcoane, S. Preliminary characterisation of Bacillus subtilis strain use as a dietary probiotic bio-additive in weaning piglet. Food Feed. Res. 2018, 45, 203–211. [Google Scholar] [CrossRef]
- SPSS. Statistics Version 20.0; IBM SPSS: Chicago, IL, USA, 2011. [Google Scholar]
- Estrada, A.; Drew, M.D.; Van Kessel, A. Effect of the dietary supplementation of fructooligosaccharides and Bifidobacterium longum to early-weaned pigs on performance and fecal bacterial populations. Can. J. Anim. Sci. 2001, 81, 141–148. [Google Scholar] [CrossRef]
- Guerra-Ordaz, A.A.; Molist, F.; Hermes, R.G.; de Segura, A.G.; La Ragione, R.M.; Woodward, M.J.; Tchorzewska, M.A.; Collins, J.W.; Pérez, J.F.; Martín-Orúe, S.M.; et al. Effect of inclusion of lactulose and Lactobacillus plantarum on the intestinal environment and performance of piglets at weaning. Anim. Feed Sci. Technol. 2013, 185, 160–168. [Google Scholar] [CrossRef]
- Fan, G.; Chang, J.; Yin, Q.; Wang, X.; Dang, X. Effects of probiotics, oligosaccharides, and berberine combinations on growth performance of pigs. Turkish J. Vet. Anim. Sci. 2015, 39, 637–642. [Google Scholar] [CrossRef]
- Liu, G.; Chen, S.; Guan, G.; Tan, J.; Al-Dhabi, N.A.; Wang, H.; Duraipandiyan, V.; Fang, J. Chitosan modulates inflammatory responses in rats infected with enterotoxigenic Escherichia coli. Mediat. Inflamm. 2016, 2016, 7432845. [Google Scholar] [CrossRef]
- Almeida, J.A.S.; Liu, Y.; Song, M.; Lee, J.J.; Gaskins, H.R.; Maddox, C.W.; Osuna, O.; Pettigrew, J.E. Escherichia coli challenge and one type of smectite alter intestinal barrier of pigs. J. Anim. Sci. Biotechnol. 2013, 4, 52. [Google Scholar] [CrossRef]
- Cao, S.; Wang, L.; Jiao, L.; Lin, F.; Xiao, K.; Hu, C. Effects of diosmectite-Lactobacillus acidophilus on growth performance, intestine microbiota, mucosal architecture of weaned pigs. Anim. Feed Sci. Technol. 2016, 220, 180–186. [Google Scholar] [CrossRef]
- Qiao, J.; Li, H.; Wang, Z.; Wang, W. Effects of Lactobacillus acidophilus dietary supplementation on the performance, intestinal barrier function, rectal microflora and serum immune function in weaned piglets challenged with Escherichia coli lipopolysaccharide. Antonie Van Leeuwenhoek 2015, 107, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, M.; Callegari, M.L.; Reggi, S.; Caprarulo, V.; Giromini, C.; Spalletta, A.; Coranelli, S.; Sgoifo Rossi, C.A.; Rossi, L. Lactobacillus plantarum and Lactobacillus reuteri as Functional Feed Additives to Prevent Diarrhoea in Weaned Piglets. Animals 2021, 11, 1766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.Y.; Kim, I.H. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhoea score in weanling pigs. Anim. Feed Sci. Technol. 2015, 200, 86–92. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Hu, Y.; Huang, J.; Yang, H.; Wang, L.; Chen, S.; Chen, C.; He, S. Effects of dietary microencapsulated tannic acid supplementation on the growth performance, intestinal morphology, and intestinal microbiota in weaning piglets. J. Anim. Sci. 2020, 98, skaa112. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Omogbenigun, F.O.; Rademacher, M.; Blank, G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 2006, 84, 125–134. [Google Scholar] [CrossRef]
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Scie. 2019, 6, 48. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Panaite, T.D.; Turcu, R.P. Effect of dietary orange and grapefruit peel on growth performance, health status, meat quality and intestinal microflora of broiler chickens. Ital. J. Anim. Sci. 2020, 19, 1394–1405. [Google Scholar] [CrossRef]
- Owusu-Asiedu, A.N.; Baidoo, C.M.S.K.; Marquardt, R.R.; Yang, X. Response of early-weaned pigs to an enterotoxigenic Escherichia coli (K88) challenge when fed diets containing spray-dried porcine plasma or pea protein isolate plus egg yolk antibody. J. Anim. Sci. 2003, 81, 1781–1789. [Google Scholar] [CrossRef]
- Sakata, T.; Kojima, T.; Fujieda, M.; Takahashi, M.; Michibata, T. Influences of probiotic bacteria on organic acid production by pig caecal bacteria in vitro. Proc. Nutr. Soc. 2003, 62, 73–80. [Google Scholar] [CrossRef]
- Chen, H.S.; Velayudhan, D.E.; Li, A.; Feng, Z.; Liu, D.; Yin, Y.L.; Nyachoti, C.M. Growth performance, gastrointestinal microbial activity, and immunological response of piglets’ receiving microencapsulated Enterococcus faecalis CG1. 0007 and enzyme complex after an oral challenge with Escherichia coli (K88). Can. J. Anim. Sci. 2016, 96, 609–618. [Google Scholar] [CrossRef]
- Peng, X.; Wang, R.; Hu, L.; Zhou, Q.; Liu, Y.; Yang, M.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; et al. Enterococcus faecium NCIMB 10415 administration improves the intestinal health and immunity in neonatal piglets’ infected by enterotoxigenic Escherichia coli K88. J. Anim. Sci. Biotechnol. 2019, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Nedumpun, T.; Hampson, D.J.; Muangsin, N.; Prapasarakul, N. Microencapsulated probiotic Lactiplantibacillus plantarum and/or Pediococcus acidilactici strains ameliorate diarrhoea in piglets’ challenged with enterotoxigenic Escherichia coli. Sci. Rep. 2022, 12, 7210. [Google Scholar] [CrossRef] [PubMed]
- Aiyegoro, O.; Dlamini, Z.; Okoh, A.; Langa, R. Effects of Probiotics on Growth Performance, Blood Parameters, and Antibody Stimulation in Piglets. S. Afr. J. Anim. Sci. 2017, 47, 766–775. Available online: https://hdl.handle.net/10520/EJC-adf1a0eb5 (accessed on 10 September 2022).
- Busanello, M.; dos Santos, M.S.; Pozza, P.C.; Nunes, R.V.; Chambo, A.P.S.; Eckstein, I.I. Probiotics: Viable and inactivated cells on the performance, microflora and blood parameters of piglets. Rev. Bras. Saúde Prod. Anim. 2015, 16, 387–396. [Google Scholar] [CrossRef]
- Kim, D.; Min, Y.; Yang, J.; Heo, Y.; Kim, M.; Hur, C.G.; Lee, S.C.; Lee, H.K.; Song, K.D.; Heo, J.; et al. multiprobiotic Lactobacillus supplementation improves liver function and reduces cholesterol levels in Jeju native pigs. Animals 2021, 11, 2309. [Google Scholar] [CrossRef]
- Zheng, L.; Duarte, E.M.; Loftus, S.A.; Kim, W.S. Intestinal health of pigs upon weaning: Challenges and nutritional intervention. Front. Vet. Sci. 2021, 8, 628258. [Google Scholar] [CrossRef]
- Jang, Y.D.; Kang, K.W.; Piao, L.G.; Jeong, T.S.; Auclair, E.; D’Inca, J.R.; Kima, Y.Y. Effects of live yeast supplementation to gestation and lactation diets on reproductive performance, immunological parameters and milk composition in sows. Live. Sci. 2013, 152, 167–173. [Google Scholar] [CrossRef]
- Nyachoti, C.M.; Kiarie, E.; Bhandari, S.K.; Zhang, G.; Krause, D.O. Weaned pig responses to Escherichia coli K88 oral challenge when receiving a lysozyme supplement. J. Anim. Sci. 2012, 90, 252–260. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, H.S.; Wang, X.C.; Hu, Q.; Liu, C.X.; Wu, X.; Deng, D.; Hou, Y.Q.; Nyachoti, C.M.; Xiao, D.F.; et al. Effect of low dosage of chitooligosaccharides supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J. Anim. Sci. 2015, 93, 1089–1097. [Google Scholar] [CrossRef]
- Cao, G.T.; Zeng, X.F.; Chen, A.G.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Yang, C.M. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013, 92, 2949–2955. [Google Scholar] [CrossRef]
- Kamboh, A.A.; Arain, M.A.; Mughal, M.J.; Zaman, A.; Arain, Z.M.; Soomro, A.H. Flavonoids: Health-promoting phytochemicals for animal production-a review. J. Anim. Health Prod. 2015, 3, 6–13. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Zhou, H.; Wang, L.; Ding, S.; Wang, Y.; Li, A. Effects of microencapsulated Lactobacillus plantarum and fructooligosaccharide on growth performance, blood immune parameters, and intestinal morphology in weaned piglets. Food Agric. Imunol. 2018, 29, 84–94. [Google Scholar] [CrossRef]
- Also, K.; Velayudhan, D.E.; Khafipour, E.; Li, A.; Yin, Y.; Nyachoti, M. Combined effects of chitosan and microencapsulated Enterococcus faecalis CG1. 0007 probiotic supplementation on performance and diarrhoea incidences in enterotoxigenic Escherichia coli K88+ challenged piglets. Anim. Nutr. 2017, 3, 366–371. [Google Scholar]
- Isaacson, R.; Kim, H.B. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Slifierz, M.; Jalali, M.; Friendship, R. Evaluation of the nasal microbiota in slaughter-age pigs and the impact on nasal methicillin-resistant Staphylococcus aureus (MRSA) carriage. BMC Vet. Res. 2014, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ji, H.F.; Zhang, D.Y.; Wang, S.X.; Wang, J.; Shan, D.C.; Wang, Y.M. Effects of Lactobacillus brevis preparation on growth performance, fecal microflora and serum profile in weaned pigs. Livest. Sci. 2015, 178, 251–254. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Awati, A.A.; Williams, B.A.; Miller, B.G.; Jones, P.; Stokes, C.R.; Akkermans, A.D.; Smidt, H.; De Vos, W.M. Post-natal development of the porcine microbiota composition and activities. Environm. Microbiol. 2006, 8, 1191–1199. [Google Scholar] [CrossRef]
- Wei, H.K.; Xue, H.X.; Zhou, Z.X.; Peng, J. A carvacrol–thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in the jejunal mucosa of weaning piglets. Animal 2017, 11, 193–201. [Google Scholar] [CrossRef]
- Bian, G.; Ma, S.; Zhu, Z.; Su, Y.; Zoetendal, E.G.; Mackie, R.; Liu, J.; Mu, C.; Huang, R.; Smidt, H.; et al. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets’ than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environm. Microbiol. 2016, 18, 1566–1577. [Google Scholar] [CrossRef]
- Mach, N.; Berri, M.; Estellé, J.; Levenez, F.; Lemonnier, G.; Denis, C.; Leplat, J.; Chevaleyre, C.; Billon, Y.; Doré, J.; et al. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environm. Microbiol. Rep. 2015, 7, 554–569. [Google Scholar] [CrossRef]
- Tao, X.; Xu, Z.; Wan, J. Intestinal microbiota diversity and expression of pattern recognition receptors in newly weaned piglets. Anaerobe 2015, 32, 51–56. [Google Scholar] [CrossRef] [PubMed]
- McLamb, B.L.; Gibson, A.J.; Overman, E.L.; Stahl, C.; Moeser, A.J. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS ONE 2013, 8, e59838. [Google Scholar] [CrossRef] [PubMed]
- Suo, C.; Yin, Y.; Wang, X.; Lou, X.; Song, D.; Wang, X.; Gu, Q. Effects of lactobacillus plantarumZJ316 on pig growth and pork quality. BMC Vet. Res. 2012, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Bomba, A.; Nemcová, R.; Gancarčíková, S.; Herich, R.; Kaštel, R. Potentiation of the effectiveness of Lactobacillus casei in the prevention of E. coli induced diarrhoea in conventional and gnotobiotic pigs. In Mechanisms in the Pathogenesis of Enteric Diseases 2; Springer: Boston, MA, USA, 1999; pp. 185–190. [Google Scholar]
- Zhang, D.; Ji, H.; Liu, H.; Wang, S.; Wang, J.; Wang, Y. Changes in the diversity and composition of gut microbiota of weaned piglets’ after oral administration of Lactobacillus or an antibiotic. Appl. Microbiol. Biotechnol. 2016, 100, 10081–10093. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Xie, Z.; Li, M.; Qian, M.; Yang, Z.; Han, X. Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids. Nutrients 2022, 14, 4475. [Google Scholar] [CrossRef]
| Ingredients (g/kg as-Fed Basis) | Basal Diet |
|---|---|
| Ground corn | 667.4 |
| Mustard meal | 20.0 |
| Hempseed meal | 10.0 |
| Soybean meal | 150.0 |
| Corn gluten | 30.0 |
| Milk powder | 50.0 |
| Hempseed oil | 25.0 |
| DL-Methionine | 2.2 |
| L-Lysine HCl | 4.4 |
| Carbonate calcium | 14.6 |
| Monocalcium phosphate | 14.3 |
| Salt | 1.0 |
| Premix Choline | 1.0 |
| Vitamin-mineral premix *& | 10.0 |
| Phytase | 0.1 |
| Total | 1000.0 |
| Nutritional value | |
| Metabolizable energy (EM, MJ/kg) ** | 14.0 |
| Crude protein (%) | 172.5 |
| Lysine (%) | 10.5 |
| Methionine + Cysteine (%) | 7.3 |
| Calcium (%) | 10.1 |
| Total Phosphorus (%) | 7.8 |
| Items | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | LA | LP | LA + LP | |||
| BW (at weaning, kg) | 8.53 | 8.52 | 8.53 | 8.52 | 0.011 | 0.995 |
| 1 to 14 days | ||||||
| ADFI (g/day) | 202 b | 210 a | 215 a | 217 a | 0.013 | 0.041 |
| ADG (g/day) | 92.9 | 95.7 | 96.4 | 98.9 | 0.083 | 0.063 |
| FCR (g feed/g gain) | 2.15 b | 2.33 a | 2.23 a | 1.99 c | 0.026 | 0.003 |
| 15 to 21 days | ||||||
| ADFI (g/day) | 475 | 476 | 476 | 482 | 0.021 | 0.317 |
| ADG (g/day) | 341 | 365 | 358 | 359 | 0.071 | 0.353 |
| FCR (g feed/g gain) | 1.40 | 1.31 | 1.33 | 1.36 | 0.020 | 0.255 |
| 1 to 21 days | ||||||
| ADFI (g/day) | 263 | 288 | 286 | 289 | 0.014 | 0.351 |
| ADG (g/day) | 180 | 186 | 188 | 190 | 0.091 | 0.694 |
| FCR (g feed/g gain) | 1.55 | 1.64 | 1.59 | 1.61 | 0.018 | 0.745 |
| Items | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| C | LA | LP | LA + LP | |||
| Carcass yield, % | 82.3 | 81.4 | 79.6 | 79.9 | 0.640 | 0.098 |
| Liver, g | 205.31 | 226.13 | 214.63 | 233.75 | 4.009 | 0.593 |
| Spleen, g | 21.63 | 19.58 | 21.04 | 19.70 | 1.249 | 0.937 |
| Kidneys, g | 45.0 | 45.65 | 46.33 | 47.00 | 1.212 | 0.960 |
| Heart, g | 47.75 | 50.00 | 48.00 | 50.75 | 1.234 | 0.818 |
| SI length, cm | 1025 | 1070.75 | 1086.50 | 1147.38 | 17.85 | 0.094 |
| Intestinal pH | ||||||
| Duodenum | 6.35 | 6.16 | 6.10 | 6.21 | 0.264 | 0.337 |
| Jejunum | 6.51 | 6.24 | 6.30 | 6.47 | 0.195 | 0.061 |
| Ileum | 6.82 a | 6.32 b | 6.41 ab | 6.28 b | 0.014 | 0.023 |
| Days with diarrhoea | 10 | 10 | 10 | 10 | - | - |
| Diarrhoea incidence | 17.86 a | 10.71 ba | 9.52 bc | 5.95 c | 0.049 | 0.001 |
| Items | Parameters | C | LA | LP | LA + LP | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Lipid profile | GLU, mg dL−1 | 111.13 | 104.25 | 108.88 | 104.75 | 3.041 | 1.035 |
| TG, mg dL−1 | 28.15 | 28.50 | 29.46 | 28.89 | 0.953 | 0.971 | |
| TCH, mg dL−1 | 93.00 | 92.88 | 92.38 | 92.01 | 1.790 | 0.998 | |
| HDL, mg dL−1 | 28.63 | 29.75 | 29.13 | 31.63 | 1.482 | 0.909 | |
| LDL, mg dL−1 | 58.87 | 58.57 | 58.54 | 56.63 | 1.030 | 1.000 | |
| Protein profile | TP, g dL−1 | 4.76 | 4.88 | 4.70 | 4.80 | 0.101 | 0.951 |
| ALB, g dL−1 | 2.81 | 2.88 | 2.76 | 2.81 | 0.063 | 0.811 | |
| BIL, mg dL−1 | 0.28 | 0.24 | 0.26 | 0.25 | 0.010 | 0.746 | |
| BUN, mg dL−1 | 16.69 | 17.75 | 17.72 | 16.96 | 0.622 | 0.856 | |
| UA, mg dL−1 | 0.53 | 0.53 | 0.54 | 0.51 | 0.011 | 0.941 | |
| CRE, mg dL−1 | 1.56 | 1.55 | 1.53 | 1.53 | 0.047 | 0.981 | |
| Enzymatic profile | ALT, U/L | 27.75 | 28.19 | 28.25 | 27.50 | 1.090 | 0.818 |
| AST, U/L | 25.75 | 25.25 | 26.00 | 24.13 | 1.168 | 0.949 | |
| AP, U/L | 1200 | 1120 | 1122 | 1222 | 52.33 | 0.314 | |
| CK, UI/L | 390.00 | 386.63 | 392.38 | 388.50 | 6.202 | 0.981 | |
| LD, UI/L | 1107.13 | 1110.63 | 1115.00 | 1105.8 | 34.18 | 1.000 | |
| GGT, UI/L | 45.38 | 45.63 | 46.50 | 46.13 | 1.921 | 0.997 | |
| Mineral profile | Ca, mg dL−1 | 11.38 | 11.36 | 11.31 | 11.35 | 0.261 | 1.000 |
| Mg, mg dL−1 | 2.28 | 2.29 | 2.23 | 2.30 | 0.053 | 0.948 | |
| IP, mg dL−1 | 6.13 | 6.08 | 6.04 | 6.09 | 0.164 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefter, N.A.; Hăbeanu, M.; Gheorghe, A.; Dumitru, M.; Gal, C.; Vlaicu, P.A. Effects of Microencapsulated Probiotics on Performance, Organ Development, Diarrhoea Incidences, Blood Parameters, Intestinal Histomorphology and Microflora in Weaning Piglets. Agriculture 2023, 13, 39. https://doi.org/10.3390/agriculture13010039
Lefter NA, Hăbeanu M, Gheorghe A, Dumitru M, Gal C, Vlaicu PA. Effects of Microencapsulated Probiotics on Performance, Organ Development, Diarrhoea Incidences, Blood Parameters, Intestinal Histomorphology and Microflora in Weaning Piglets. Agriculture. 2023; 13(1):39. https://doi.org/10.3390/agriculture13010039
Chicago/Turabian StyleLefter, Nicoleta Aurelia, Mihaela Hăbeanu, Anca Gheorghe, Mihaela Dumitru, Claudiu Gal, and Petru Alexandru Vlaicu. 2023. "Effects of Microencapsulated Probiotics on Performance, Organ Development, Diarrhoea Incidences, Blood Parameters, Intestinal Histomorphology and Microflora in Weaning Piglets" Agriculture 13, no. 1: 39. https://doi.org/10.3390/agriculture13010039
APA StyleLefter, N. A., Hăbeanu, M., Gheorghe, A., Dumitru, M., Gal, C., & Vlaicu, P. A. (2023). Effects of Microencapsulated Probiotics on Performance, Organ Development, Diarrhoea Incidences, Blood Parameters, Intestinal Histomorphology and Microflora in Weaning Piglets. Agriculture, 13(1), 39. https://doi.org/10.3390/agriculture13010039







