Abstract
Citrullus colocynthis L. is a wild watermelon, commonly named bitter melon or bitter apple, that naturally grows in arid regions of India among other hot arid areas of the world. Its non-edible fruits contain certain phytochemicals of therapeutic and nutraceutical value. The effectiveness of biopesticide formulations that are known to possess insecticidal properties was tested. This is the first botanical pesticide formulation developed from C. colocynthis, named “Thar Jaivik 41 EC”. The phytochemicals of C. colocynthis seed were identified using GC-MS/MS, and a total of 59 constituents were identified, of which seven have significant insecticidal properties: n-hexadecanoic acid; octadecanoic acid; dotriacontance; 9, 12-octadecadienoic acid (Z,Z); 9, 12-octadecadienoic acid (Z,Z)-, methyl ester; 6-octadecenoic acid, methyl ester; and hexatriacontane. Among the different levels of tested concentrations, “Thar Jaivik 41 EC” was found most effective at 3 mL L−1 for managing various insect pests such as pod borer (Helicoverpa armigera) and aphid (Toxoptera citricida) through repellent, deterrent, antifeeding action and by causing respiration abnormalities. Moreover, it caused the least harm to natural enemies such as coccinellids at this concentration. The phytotoxicity response of “Thar Jaivik 41 EC” on tested crops revealed that it is highly safe for plants, showing no toxicity symptoms when applied at higher doses than the recommended one (3 mL L−1). Integration of the “Thar Jaivik 41 EC” formulation in agriculture would help to safeguard farmers’ benefits, such as reduced pest levels, improved food safety and quality of products, which would allow them to fetch higher prices, as well as provide intangible benefits to the consumers and environment.
1. Introduction
Chemical or synthetic pesticides have contributed to disease and pest management concomitantly with improving agriculture production. Still, over the past three decades, their indiscriminate use has raised various concerns, such as concerns relating to the buildup of pesticide resistance in pests, pesticide residues, death of beneficial insects and the outbreak of secondary pests [1]. Such outcomes have instigated the search for eco-friendly, natural, plant-based pest control alternatives [2]. Several plant-based pesticides are available at an affordable cost and are within the reach of members of farming communities, especially small-scale farmers. These alternatives are more selective towards target pests and less toxic to vertebrates, aquatic animals and pollinators and are much safer for terrestrial and marine species and the environment due to their low residual effects [3,4,5,6]. Bioproducts, such as aged cow urine, asafetida, tobacco, aloe vera and chaste tree, are very effective in managing insect pests in many vegetables and field crops [7]. These indigenous biopesticides embody effective and affordable products already adopted in many areas, consequently, reducing pesticide load on the ecosystem [7]. Many researchers have revealed the presence of insecticidal properties in different plant families. In their study, Chandrashekharaiah et al. [8] tested the efficacy of other plant-based indigenous preparations against diamondback moths (DBM). Among different extracts tested, the more detrimental response to DBM larvae was observed for the neem seed kernel extract (NSKE) extracts + Aloe vera + Calotropis + chaste tree + Clerodendron inerme or red chilli + neem fruit + custard apple leaf. In addition to having larval mortality, repellent and antifeedant activities, these extracts also caused morphogenic deformities in DBM. The presence of secondary metabolites, such as flavonoids, phenolics and scented compounds (terpenoids, alkaloids, steroids and organic cyanides), is believed to be the main attribute generating the insecticidal properties of these extracts [9,10]. The phytochemicals present in plants act as defensive chemicals against herbivores, playing a decisive role in host acceptance or rejection by insects by affecting their olfactory and gustatory senses. Plant-origin pesticides or biopesticides have long been used, and they still hold the key as alternatives to synthetic pesticides. Plants are undoubtedly a treasure trove of both chemicals and biological activities. Although there are thousands of secondary metabolites in plants, very few have been investigated for pest control activity [11]. For instance, cow urine has shown some peculiar properties as a repellant to many insect pests and, at the same time, as an attractant to others (e.g., wasps) [12].
Citrullus colocynthes L., widely known as bitter melon, is a trailing annual herbaceous plant of the Cucurbitaceae family that resembles the common watermelon (C. lanatus), to which it is closely related [13]. Geographically, it is distributed mainly in hotter parts of the globe, including the Indian Thar desert, where it grows as a weed on the arid dunes and barren lands [13,14]. It is popularly known as ‘Tumba’ or ‘Bitter apple’ in India, ‘Hadla’ in Jordan and ‘Abujahl watermelon’ or ‘Kadu Hanzal’ in Persia [14,15]. Bitter apple plants survive well under the extreme climate conditions of hot arid regions, such as under drought, heat and salt stress, as well as in the poor nutrient conditions of desert soil. From each node, many branches and tendrils grow, which help the vines to spread over dunes of sand, thus, helping to prevent soil erosion. Besides its ecological significance for controlling sand dune erosion, bitter apple has limited use in human consumption. However, recent reports revealed the presence of certain phytochemical constituents, which give it valuable medicinal, nutritional [14] and insecticidal properties. According to Tarraf et al. [16], fruits and other parts of C. colosynthes plants possess several beneficial bioactive compounds characterized by pesticidal effects. These authors mentioned that using bitter apple seed oil considerably decreased nematode (Meloidogyne incognita) density in soil, with a reduced number of eggs and gall formation on treated tomato roots. Furthermore, the properties of different biopesticides were assessed against aphids on coriander by Meena et al. [17] for two years. The reduction in the aphid population on coriander plants by the application of organic salt (bioproduct, at 5 mL L−1), neem seed kernel extract (10 mL L−1) and bitter apple fruit extract (10 mL L−1) corresponded to 67.83 and 70.10%, 67.00 and 68.96% and 64.89 and 68.32% in two consecutive years, respectively, whereas other leaf extract formulations of custard apple, parthenium or caster showed effectiveness in controlling the aphid population at a lower rate. There was no evident toxicity symptom when applying any of the formulations on the plants or on natural enemies. In addition, the antifeedant, deterrent, insect-growth-regulating and fertility-decreasing properties of C. colocynthis on insects were documented by Prabuseenivasan et al. [18]. Cow urine tested at different concentrations, either alone or in combination with recommended insecticides, provided effective control of many insect pests [19]. Miah et al. [20] evaluated the repellency and toxicity effect of fermented cattle urine, neem seed kernel, mahagony seed and allamanda leaf extract at 5, 10 and 15 percent concentrations against mealybugs of sugarcane. The repellency effect of fermented cattle urine at 15% was greater (67.80%) than that of other treatments. Comparing the three probit regression equation lines, the highest probit mortality was found with fermented cattle urine at 24 hours after treatment (HAT) and mahagony seed extract at 48 HAT. It is adequately documented that cow urine, particularly that of indigenous Indian cow breeds, has several beneficial properties, and it is used in treating human and animal diseases as well as crop pests and diseases [21,22]. Moreover, centuries ago, in the ‘Sushruta Samhita’ and ‘Ashtanga Sangraha’ Vedic literature, cow urine was described as the most effective substance with innumerable therapeutic values [21].
Based on those above, and considering the vast plant resource in India’s hot and arid region and the phytochemical characteristics from bitter apple able to manage insect pests, the present investigation was envisaged and executed. We attempted to characterize its insecticidal properties and produced a bitter-apple-fruit-based, novel, eco-friendly bioformulation “Thar Jaivik 41 EC” for commercial application.
2. Materials and Methods
2.1. Plant Material and Experimental Site
Bitter apple fruits were harvested from well-grown plants during the fruiting season of 2018–2019 from the experimental farm of ICAR-CIAH, Bikaner, India (N 28° 06′ E 73° 21′; 224 m ASL). The bitter apple fruits were sliced to remove the seeds and then the seeds were dried at room temperature for 15 days.
2.2. Petroleum Ether Extraction of Bitter Apple Seed
An electric grinder was used to grind the dried bitter apple seeds into powder. The Soxhlet apparatus extracted bioactive compounds from bitter apple seeds in a 1:10 sample:solvent ratio over three hours. Following every extraction, the extract was cleaned using Whatman No. 1 filter paper, and the resulting solution was then exposed to the sun to evaporate the solvent. The sections were kept in a refrigerator in a glass beaker until the performance of the analysis.
2.3. Gas Chromatography and MS/MS Analyses
The phytochemical constituents of bitter apple seed were identified using GC-MS/MS. Bioactive compounds of bitter apple seeds were identified by comparing the outcome with the mass spectra libraries, and the components’ relative percentages were expressed as percentages by peak area normalization. The fatty acid methyl esters (FAMEs) were analyzed using a GC-MS/MS via gas chromatograph with an AOC-20i and interfaced to QP 2010 Plus MS (GC-2010 System, SC, Kyoto, Japan), which was outfitted with a polar fused silica column, COL-ELITE-2560 (extremely polar phase). The oven temperature was set at 100 °C (4 min) and then 240 °C (15 min) with 225 °C injection temperature. The flow speed of helium gas was 1.0 mL min−1 throughout the 65 min program [14].
2.4. Formulation of Biopesticide “Thar Jaivik 41 EC”
The petroleum-ether-extracted bioactive compounds were combined with urine of indigenous cow and a surfactant to create the biopesticide formulation “Thar Jaivik 41 EC”. The urine used in making the formulation was from an indigenous breed named Raathi (Bos indicus) from a local dairy farm. The diagrammatic depiction of the simplified flow chart for isolation and formulation of biopesticide “Thar Jaivik 41 EC” is illustrated in Figure 1. The bioactivity of “Thar Jaivik 41 EC” was reported 18 months after manufacturing the biopesticide.
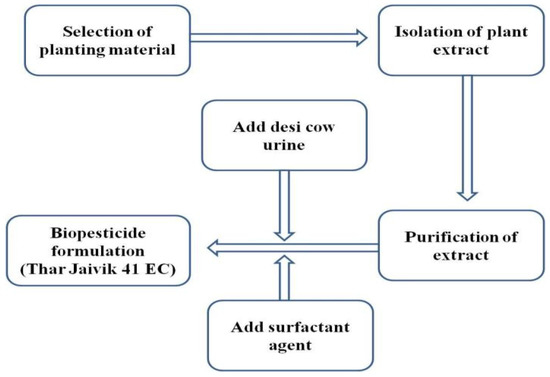
Figure 1.
Diagrammatic depiction of the simplified flow chart for isolation and formulation of biopesticide “Thar Jaivik 41 EC”.
2.5. Rearing of Insects
The larvae of gram pod borer (Helicoverpa armigera) were collected from the naturally grown unsprayed vegetable block at the institute. The furnished cultures were maintained on leaves and fruits of cucurbits plants under laboratory conditions (28 °C ± 2 °C, 55% ± 5% RH). The I and II larval instars of borer were reared in a plastic pot in a batch of twenty larvae, and the remaining instar from III to V larvae, were reared individually in a plastic jar with the cucurbit fruits for one generation under laboratory conditions following the previously reported procedures [23]. The rearing jars and vials were cleaned, and new plant parts were changed daily in the morning throughout the rearing period. Aphids (Toxoptera citricida) were collected from the institute’s pesticide-free citrus research block. The aphids were kept on their host plant leaves in the laboratory for 24 h before the trials were conducted.
2.6. Bioassays Used as Treatments
Seven treatments were adopted: “Thar Jaivik 41 EC” at 2.0 mL, 3.0 mL and 4.0 mL L−1 water, NSKE at 5%, neem oil 4.0 mL L−1 water and spinosad 0.5 mL L−1 water and water spray as a control. The experiments were conducted separately for H. armigera and T. citricida in the laboratory and H. armigera, T. citricida and coccinellids (Coccinella septempunctata) in field conditions on plants. To prevent cannibalism, different gram pod borer larvae instars were reared in a separate jar, as mentioned above. The treatments were designed for the laboratory and field investigation using a completely randomized and randomized block design, respectively, with four replicates. The different treatments were applied twice in the afternoon (at 5.00 p.m.) at 15-day intervals when the insect population in the field was at its peak, whereas, in the laboratory, it was based on the count of the insects. The mortality of the insects upon each treatment was recorded after one day and three days of treatment exposure and is illustrated in Figure 2. Abbott’s formula [24]: Corrected mortality = (mortality in treatment (%) − mortality in control (%)) × 100/(100% − mortality in control (%)).
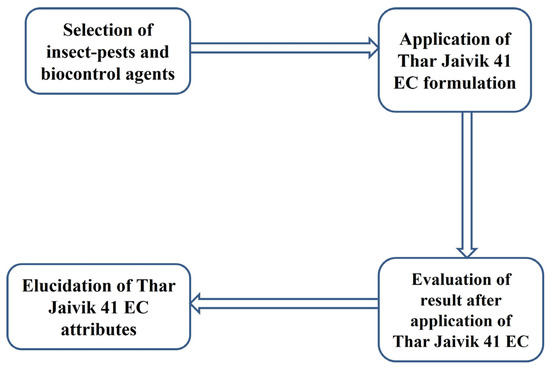
Figure 2.
Diagrammatic depiction of the effect of biopesticide formulation “Thar Jaivik 41 EC” against insect pests and biocontrol agent.
2.7. Phytotoxicity Effect of “Thar Jaivik 41 EC”
Field experiments were conducted to assess the phytotoxic effect of “Thar Jaivik 41 EC” on cucurbit plants (snap melon, muskmelon and kachri) in two seasons. In all the crops, the “Thar Jaivik 41 EC” (3 mL, 10 mL, 20 mL, 30 mL L−1 water) was treated. There were four replicates with randomized block design and three applications at 15-day intervals. “Thar JaivikJ 41 EC” was sprayed to the run-off point using a high-volume, hand-operated knapsack sprayer. Observations of phytotoxic symptoms such as leaf injury, wilting, vein clearing and necrosis were assessed on pre-treatment and 1, 3, 7 and 10 days after treatment for each of the 1st, 2nd and 3rd sprays on a visual rating basis. A visual rating of 1 to 10 was made based on percent leaf injury (0 to 10%—rating 1; 11 to 20%—rating 2; 21 to 30%—rating 3; 31 to 40%—rating 4; 41 to 50%—rating 5; 51 to 60%—rating 6; 60 to 70%—rating 7; 71 to 80%—rating 8; 81 to 90%—rating 9; and 91 to 100%—rating 10).
2.8. Statistical Analysis
Before performing statistical analysis, angular transformations were applied to normalize the data; however, unchanged means are accessible in the tables. Insect populations were compared with programs through one-way ANOVAs followed by Tukey’s test for multiple comparisons at p ≤ 0.05. All procedures were carried out using SPSS v. 20 software.
3. Results and Discussion
3.1. Gas Chromatography and MS/MS Analyses
A total of 59 constituents were identified from a bitter apple seed, of which seven have significant insecticidal properties: (i) 9,12-octadecadienoic acid (Z,Z)-, methyl ester; (ii) dotriacontance; (iii) 6-octadecenoic acid, methyl ester; (iv) octadecanoic acid; (v) 9, 12-octadecadienoic acid (Z,Z)-; (vi) hexatriacontane; and (vii) n-hexadecanoic acid (Table 1, Figure 3). The highest peak (23.49%) was found for 9, 12-octadecadienoic acid (Z,Z), which has antimicrobial activity and insecticide and miticide properties, followed by octadecanoic acid (23.30%), which has insecticide and miticide properties.

Table 1.
Phytochemical constituents of bitter apple (C. colocynthis) seeds with insecticidal properties for insect pest management.
Figure 3.
Chromatogram (peak identification and retention time) of 59 bioactive compounds of bitter apple (C. colocynthis).
The rudimentary extract of bitter apple leaves had occurrence of 10 chemical compounds, which made up 99.99% of the total bitter apple extract; mainly, 22.94% of 4-nitro-4-chlorodiphenylsulfoxide, 22.38% of erucylamide, 21.33% of heptasiloxane,1,1,3,3,5,5,7,7,9,9,11,11,13,13-tetradecamethyl, 17.85% of 5-methyl-2-phenylindolizine and 7.06% of octasiloxane,1,1,3,3,5,5,7,7,9,9,11,11,13,13, 15,15-hexadecamethyl were the main chemical compounds, and the remaining components were negligible compounds present in small quantities with comparative peak areas ranging from 0.98 to 3.57% [3]. The fatty acid profiling of bitter apple seed oil carried out by GC-MS/MS in our previous study confirmed that it contains unsaturated fatty acids, i.e., 70.0%, in which more than 51.0% polyunsaturated fatty acids. It primarily contains linoleic acid (50.3%), followed by oleic acid (18.0%), stearic acid (15.2%) and palmitic acid (12.4%) [14]. The promising results of using bitter apple to control spotted bollworm (Earias vittella) might be due to cucurbitacin B, which is responsible for reduced antifeedant activity and hatchability [25]. The seed extract of bitter apple through ethanol has shown antifeedant and poisonous activities against mites [26]. Moreover, Mollashahi et al. [27] reported that the effect of bitter apple fruit extract against grasshopper (Chrotogonus trachypterus) under controlled conditions showed the highest mortality rate (87.5%) at 40 mg mL−1 and the lowest (23.3%) at 10 mg mL−1 concentration. The LC50 value for the bitter apple plant on grasshopper was noted to be 18.58 mg mL−1. The toxicity possessions of bitter apple fruit, stem, leaf and root extracts were examined against Rhopalosiphum padi, and the results showed superior effectiveness of the stem extract compared to other parts of bitter apple extracts against R. padi [28]. Furthermore, the identified biomolecule from bitter apple fruits, i.e., 7,8-benzoquinoline, was the most effective against red spider mite (Tetranychus urticae). At the same time, 8-hydroxyquinoline and quinoline, 2-methyl quinoline were effective against maize weevil (Sitophilus zeamais) and rice weevil (S. oryzae) [29]. The water extract from bitter apple had a toxic effect on Rhopalosiphum padi [28]. Bitter apple extracted in petroleum and methanol revealed toxic effects against different mosquito species with anti-oviposition behaviors and larvicidal effects [30].
3.2. Bioassay against H. armigera
The bio-efficacy of different concentrations of “Thar Jaivik 41 EC” and other biopesticides was evaluated against gram pod borer, H. armigera, on cucurbits. The neem-based formulations, i.e., neem seed kernel extract at 5% and neem oil at 4 mL L−l of water, were least effective in tumbling the borer. The highest mortality in borer infestation was recorded with the “Thar Jaivik 41 EC” application sprayed at either 4 mL L−l (85.40 and 91.08%) or at 3 mL L−1 of water (84.25 and 90.72%), while the lowest mortality was recorded in NSKE at 5% (50.47 and 52.43%) or neem oil at 4 mL L−l of water (56.55 and 58.51%) after one day and three days of application under laboratory conditions. Likewise, in the field conditions, the highest mortality of H. armigera was attained by application of “Thar Jaivik 41 EC” at 3 mL (75.08 and 82.63%) or 4 mL L−l of water (75.77 and 78.80%). At the same time, the mortality of H. armigera was observed in NSKE at 5% (43.72 and 48.27%) and neem oil at 4 mL L−l of water (50.32 and 54.38%) (Table 2; Figure 4).

Table 2.
Bio-efficacy of “Thar Jaivik 41 EC” and other biopesticides used at different concentrations against H. armigera on cucurbits (pooled data, 2018 and 2019).
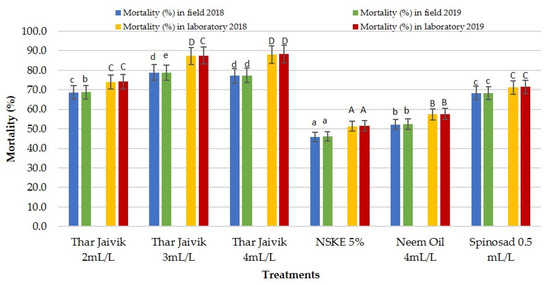
Figure 4.
Efficacy of different concentrations of “Thar Jaivik 41 EC” and other biopesticides against H. armigera on cucurbits during 2018 and 2019 (mortality (%) mean of 1 and 3 days after application). NSKE: neem seed kernel extract. Different lowercase letters and uppercase letters indicate significant differences in treatments within field and laboratory conditions, respectively.
Among the different bioformulations tested against H. armigera, a spray of azadirachtin 3% WSP at 400 g ha−1 caused the lowest larval borer population with minimum pod damage (9.55%) and maximum grain yield (1596 kg ha−1). Afterwards, azadirachtin 6% at 200 g ha−1 was found to be better in reducing the larval population at seven days after the first, second and third sprays, respectively, with a resulting grain yield of 1515.36 kg ha−1 [31]. In tomato, the highest result was due to the highest reduction in Heliothis population with NSKE compared to the other extracts; the reverse was true for the control treatment. This justifies the better efficacy of NSKE as a biopesticide against gram pod borer. Furthermore, the variation in plant height was associated with the range of insects feeding on them after the spraying of biopesticides. Tomato plants face more herbivory pressure due to higher infestation of borers and, thus, have a lower plant height than those under lesser herbivory stress [32]. Compared to panchagavya and cow urine, dasagavya was found to be abstemiously dynamic against Plutella xylostella. This was likely due to the secondary metabolites released from botanical plants or increased microbial activity when combined with different ingredients. Panchagavya combined with NSKE exhibited more antifeedant activity against Spodoptera litura than the combination of Adathoda vesica and panchagavya [33]. As per some research, cow urine and cow dung are reasonably effective against P. xylostella on cabbage [34]. A combination of cow urine with NSKE, Argemone mexicana, A. vasica, C. gigantean and V. negundo showed more promising effects than their sole applications against Spodoptera litura [33]. Likewise, a combination of cow urine with NSKE, A. vera, Pongamia pinnata and V. negundo against H. armigera and S. litura was demonstrated earlier [35]. The potential of Peumus boldus liquid extract as an insecticide was seen against maize earworm (H. zea) and the fall armyworm (S. frugiperda) at different doses ranging from 0.25 to 8.0% (v/v). After seven days of the diet, the 8.0% concentration extract was highly efficient in managing H. zea, recording a mortality rate of 30 ± 7.2% while causing the highest mortality of 75 ± 6.5% to S. frugiperda [36]. The mating, fecundity and egg capability in E. vittella were significantly affected by C. colocynthis seed extract applied at lethal and sub-lethal concentrations [37]. The intrinsic increase and net reproductive rate in the LC30 and LC80 concentrations represented a lower larvae population than the control treatment. Further, the esterase action and protein contents declined in the LC30- and LC80-treated samples compared to in the control. As per Gulzar et al. [38], ethanol-based followed by ethyl-acetate-based extracts are mainly helpful in controlling H. armigera. The ethanol extract of C. colocynthis increased larval and pupal duration compared with the non-treated control.
3.3. Bioassay against T. citricida
The bio-efficacy of different concentrations of “Thar Jaivik 41 EC” and other biopesticides was tested against T. citricida on citrus plant leaves under laboratory and field conditions. The highest reduction of aphids was recorded with the spray of “Thar Jaivik 41 EC” applied either at 4 mL L−l (87.75 and 94.88%) or 3 mL L−l of water (87.45 and 94.65%). The lowest mortality was recorded with a spray of NSKE 5% (50.93 and 56.37%) and spinosad at 0.5 mL L−l of water (54.37 and 56.92%) after 1 and 3 days of application under laboratory conditions. Likewise, in field conditions, “Thar Jaivik 41 EC” spray at either 4 mL or 3 mL L−l of water was the most effective bioformulation in controlling aphid population with noted mortality of 78.67 and 87.15% and 78.53 and 86.42%, respectively, whereas, the lowest mortality in the aphid population was noted with the application of NSKE 5% (46.57 and 50.97%) or spinosad at 0.5 mL L−l of water (50.20 and 53.43%) after 1 and 3 days of application under field conditions (Table 3; Figure 5). Thus, “Thar Jaivik 41 EC” at 3 mL L−1 of water can be considered the most appropriate for managing aphids.

Table 3.
Efficacy of “Thar Jaivik 41 EC” and other biopesticides used at different concentrations against T. citricida on citrus plant (pooled data, 2018 and 2019).
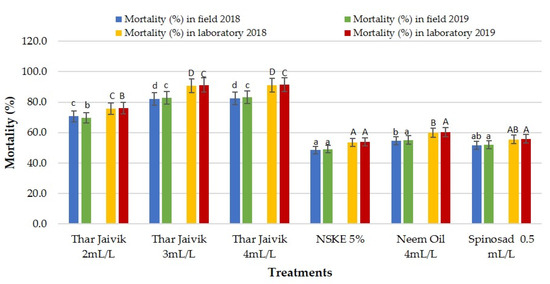
Figure 5.
Efficacy of different concentrations of “Thar Jaivik 41 EC” and other biopesticides against T. citricida on citrus plants during 2018 and 2019 (mortality mean one and three days after application). NSKE: neem seed kernel extract. Different lowercase letters and uppercase letters indicate significant differences in treatments within field and laboratory conditions, respectively.
The toxicity results revealed that the LC50 value of 3.91 mg mL−1 of A. argyi was more toxic to B. brassicae, followed by the LC50 values of 10.04 mg and 6.26 mL−1 of C. indica and C. colocynthis, respectively. The LC50 values were 0.22, 1.96 and 2.87 mg mL−1 of C. colocynthis, C. indica and A. argyi, respectively, during the residual assay [3]. It was revealed that Ageratum conyzoides and Cassia sophera extracts against B. brassicae induced mortality that was equivalent to the synthetic insecticide emamectin benzoate [39]. Pissinati and Ventura [40] also observed that using A. indica oil against B. brassicae as a suitable alternative to synthetic insecticides caused a noticeable reduction in their population. Iqbal et al. [41] noticed that the application of orange peel extracts before and after applications at 48, 72 and 144 h showed 20.7%, 44.3% and 65.7% mortality in the aphid population, respectively, whereas marigold extract was least effective on wheat aphid control. Santos et al. [42] reported that A. indica powdered seeds were used in distilled water at assorted concentrations of 23.8, 122.0, 410.0 and 1410.0 mg 100 mL−1 against A. gossypii control. The result showed that the aqueous extracts may reduce the endurance period of A. gossypii with high deliberation, reducing the insects’ life expectancy from 17.4 to 2.5 days.
3.4. Bioassay against Bioagent C. septempunctata
Furthermore, the different concentrations of “Thar Jaivik 41 EC” and other biopesticides/insecticides were evaluated against coccinellids on citrus plants. Insecticide acephate at 1.5 g L−l of water showed the highest mortality of coccinellids, with a high reduction of coccinellids (79.07%) after one day of treatment. Among the different biopesticides, “Thar Jaivik 41 EC” applied at 4 mL L−l of water reduced the population of coccinellids, but its 2 and 3 mL L−l rates had a minor effect when the evaluation was made 1 day after the treatment. Again, the highest mortality of coccinellids was recorded after three days of treatment with acephate (85.40%) and the lowest with NSKE after 3 days of treatment (16.48%), with intermediate values for “Thar Jaivik41 EC” at 2 and 3 mL L−l of water (Table 4; Figure 6).

Table 4.
Efficacy of different concentrations of “Thar Jaivik 41 EC” and other biopesticides against coccinellids on citrus plant in field conditions (pooled data, 2018 and 2019).
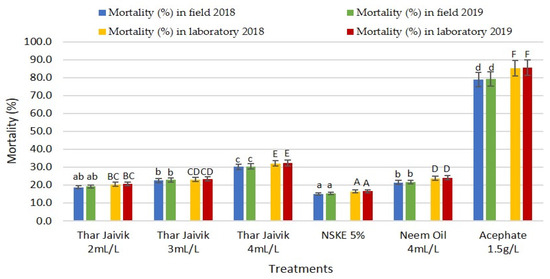
Figure 6.
Efficacy of different concentrations of “Thar Jaivik 41 EC” and other biopesticides against coccinellids on citrus plant in field conditions during 2018 and 2019. NSKE: neem seed kernel extract. Different lowercase letters and uppercase letters indicate significant differences after one and three days, respectively, within field and laboratory conditions.
Simmonds et al. [43] reported that all the evaluated botanical pesticides influenced the foraging behavior of C. montrouzieri. The botanicals BTG 504 and neem kernel extract influenced bioagents’ larval and adult foraging behavior. The larvae fed on a smaller quantity of mealy bugs when treated with BTG 504 and BTG 505 compared to untreated mealy bugs. Similarly, Duraimurugan and Regupathy [44] reported that NSKE was applied to cotton. The predator populations were stirred from cotton to trap crops (red gram and okra), and the occurrence ratio was distorted towards trap crops. The better incidence ratios of coccinellids varied from 1:0.76 to 1:0.78 and from 1:1.09 to 1:1.26 for cotton:bhindi and cotton:red gram, respectively. The incidence ratio of spiders changed to 1:0.86–1.33 and 1:1.10–1:1.98 for cotton:okra and cotton:red gram, respectively. Hwang et al. [45] observed that plant extract contains like matrine and neem products, which are less lethal to predatory and parasitic natural enemies according to the standard of IOBC. Tian et al. [46] reported that botanical pesticides such as azadirachtin and matrine did not affect the growth of beneficial arthropods, including coccinellids, spiders and parasitoids, in a field of tea. The low toxicological hazard of matrine for the biocontrol agents may be due to the freely biodegradable characteristic shown under natural conditions. In the meantime, matrine was also a little toxic to non-target organisms, such as pollinators, in the tea environment and had no teratogenesis, carcinogenesis or mutagenesis [47]. Phyto-constituents such as essential oils, fatty acids, esters, glycosides, alkaloids and flavonoids have insecticidal properties and are used as an alternative to chemical compounds to control insects in different ways as they are toxicants, growth retardants, feeding deterrents/antifeedants, repellents, attractants and chemosterilants. Botanical insecticides affect only target insects and do not harm beneficial natural enemies and provide residue-free food and are safe for the environment. Therefore, using botanical insecticides as an integrated insect management program can greatly help reduce the use of synthetic insecticides [48]. Botanical pesticides, as well as being efficacious in managing pests, are non-toxic to beneficial insects such as pollinators and are also inexpensive and easily biodegradable and, hence, are safe for the environment [49].
3.5. Phytotoxicity Effect of “Thar Jaivik 41 EC”
The phytotoxicity effect of “Thar Jaivik 41 EC” on plants was evaluated, and no symptoms were evident when it was applied at dosages up to ten times higher than the recommended dose (Table 5).

Table 5.
Phytotoxic effect of different concentrations of “Thar Jaivik 41 EC” on cucurbit plants (snap melon, muskmelon and kachri).
Werrie et al. [50] mentioned that the impending phytotoxicity of Cinnamomum cassia EO (CEO) on apple trees, M. domestica, was evaluated in terms of damage (malondialdehyde) and glutathione redox state (oxidative burst). A 2% concentration of CEO, which minimized the glutathione in leaf content from 269.6 ± 45.8 to 143 ± 28.4 nmol g−1 FW after 30 min, illustrated a quick and robust oxidative rupture. The malondialdehyde improved significantly at 24 h post application to 10.7 ± 3.05 nmol g−1 FW. Plant protection stimulation was earlier alleged after applying trans-cinnamaldehyde (CEO main compound). Therefore, the elicitor perspective was evaluated through qRT-PCR on the appearance level of 29 genes associated with chief defense pathways (oxidative stress, secondary metabolism, PR protein and parietal modification). Pavela [51] reported that, when the dosage concentration was used at the highest limit, no symptoms of phytotoxicity were observed in the treated plants during the study.
4. Conclusions
In conclusion, over-reliance on chemical insecticides is discouraged due to their harmful stress on human health, environmental effect, soil health and resistance development to pest and pathogen strains. The depressing effects of synthetic pesticides on human health has attracted attention to botanical insecticides due to their lower costs and slight or no ecological damage. From this perspective, we developed a novel biopesticide formulation for the first time from the Thar desert endemic species C. colocynthis, named “Thar Jaivik 41 EC”. “Thar Jaivik 41 EC” has insecticidal properties and is an adequate substitute to chemical pesticides (feeding deterrents, toxicants, repellents and bunging the respiration). Overall, “Thar Jaivik 41 EC” application at a dose of 3 mL L−1 can be considered optimal for managing the insect pests of different horticultural crops and assures residue-free food, as well as causes less harm to beneficial natural enemies such as coccinellids. Therefore, we suggest using “Thar Jaivik 41 EC” as an integral part of the IPM program, which can significantly decrease the use of synthetic insecticides, or even for organic farming, which is based on the increasing demand for organically produced foods in the future and is the reason why alternative approaches are required for biopesticides.
Author Contributions
Conceptualization, S.M.H.; methodology, S.M.H., R.B. and M.K.B.; validation, S.M.H. and M.K.B.; formal analysis and investigation, S.M.H., R.B. and M.K.B.; supervision, S.M.H., D.S. and M.K.B. writing—original draft preparation, S.M.H.; writing—review and editing, P.L.S., J.S.G., R.K., P.K., D.K.S., Y.R. and C.E.-N.; validation, S.M.H., R.B.; P.L.S., J.S.G., R.K., P.K., D.K.S., D.S., Y.R. and C.E.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Acknowledgments
The authors thankfully acknowledge the Director of the ICAR-Central Institute for Arid Horticulture, Bikaner, India, for providing experiment facilities and guidance for experimentation and R. Swaminathan, College of Agriculture, MPUAT, Udaipur, and Manjeet Singh, SKRAU, Bikaner, for critical suggestions in performing the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haldhar, S.M.; Bhargava, R.; Berwal, M.K.; Saroj, P.L. Noval biopesticide compositions and formulation from bitter apple (Citrullus colocynthis) for insect control. Pat. Off. J. 2019, 20, 20435. [Google Scholar]
- Fetoh, B.E.-S.A.; Asiry, K.A. Toxicological and larvicidal activities of Alzanzalakhet, Melia azedarach against cucurbit fly, Dacus ciliatus at Hail Province in Saudi Arabia. Toxicol. Environ. Chem. 2012, 94, 1350–1356. [Google Scholar] [CrossRef]
- Ahmed, M.; Peiwen, Q.; Gu, Z.; Liu, Y.; Sikandar, A.; Hussain, D.; Javeed, A.; Shafi, J.; Iqbal, M.F.; An, R. Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.). Sci. Rep. 2020, 10, 522. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Koul, O.; Walia, S.; Dhaliwal, G. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Naumann, K.; Isman, M.B. Toxicity of a neem (Azadirachta indica A. Juss) insecticide to larval honey bees. Am. Bee J. 1996, 136, 518–520. [Google Scholar]
- Lal, C.; Verma, L. Use of certain bio-products for insect-pest control. Indian J. Tradit. Knowl. 2006, 5, 79–82. [Google Scholar]
- Chandrashekharaiah, M.; Satish, S.; Vasudev, K.; Arya, V.V.; Narasimhamurthy, G. Efficacy of plant and aboriginal preparations against diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). J. Entomol. Zool. Stud. 2015, 3, 18–23. [Google Scholar]
- Bozsik, A. Studies on aphicidal efficiency of different stinging nettle extracts. Anz. Schädlingskunde Pflanzenschutz Umweltschutz 1996, 69, 21–22. [Google Scholar] [CrossRef]
- Macêdo, M.E.; Consoli, R.A.; Grandi, T.S.; dos Anjos, A.M.; de Oliveira, A.B.; Mendes, N.M.; Queiróz, R.O.; Zani, C.L. Screening of Asteraceae (Compositae) plant extracts for larvicidal activity against Aedes fluviatilis (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz 1997, 92, 565–570. [Google Scholar] [CrossRef]
- Perry, A.S.; Yamamoto, I.; Ishaaya, I.; Perry, R.Y. Insecticides in Agriculture and Environment: Retrospects and Prospects; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ranga Rao, G.; Rupela, O.; Wani, S.; Rahman, S.; Jyothsna, J.; Rameshwar Rao, V.; Humayun, P. Bio-intensive pest management reduces pesticide use in India. Pestic. News 2007, 76, 16–17. [Google Scholar]
- Bikdeloo, M.; Colla, G.; Rouphael, Y.; Hassandokht, M.R.; Soltani, F.; Salehi, R.; Kumar, P.; Cardarelli, M. Morphological and physio-biochemical responses of watermelon grafted onto rootstocks of wild watermelon [Citrullus colocynthis (L.) Schrad] and commercial interspecific cucurbita hybrid to drought stress. Horticulturae 2021, 7, 359. [Google Scholar] [CrossRef]
- Berwal, M.K.; Ram, C.; Gurjar, P.S.; Gora, J.S.; Kumar, R.; Verma, A.K.; Singh, D.; Basile, B.; Rouphael, Y.; Kumar, P. The Bioactive Compounds and Fatty Acid Profile of Bitter Apple Seed Oil Obtained in Hot, Arid Environments. Horticulturae 2022, 8, 259. [Google Scholar] [CrossRef]
- Javadzadeh, H.R.; Davoudi, A.; Davoudi, F.; Valizadegan, G.; Goodarzi, H.; Mahmoodi, S.; Ghane, M.R.; Faraji, M. Citrullus colocynthis as the Cause of Acute Rectorrhagia. Case Rep Emerg. Med. 2013, 2013, 652192. [Google Scholar]
- Tarraf, W.; Laquale, S.; De Mastro, G.; D’Addabbo, T. The potential of Citrullus colocynthis oil as a biocide against phytoparasitic nematodes. Crop Protect. 2019, 124, 104843. [Google Scholar] [CrossRef]
- Meena, N.; Singh, B.; Lal, G.; Kant, K.; Meena, R. Sustainable management of aphid in coriander (Coriandrum sativum L.) through botanicals and bio-pesticides. Int. J. Seed Spices 2016, 6, 25–31. [Google Scholar]
- Prabuseenivasan, S.; Jayakumar, M.; Raja, N.; Ignacimuthu, S. Effect of bitter apple, Citrullus colocynthis (L.) Schrad seed extracts against pulse beetle, Callosobruchus maculatus Fab. (Cole-optera: Bruchidae). Entomon-Trivandrum 2004, 29, 81–84. [Google Scholar]
- Korat, D.; Dengale, K. Cow urine-A natural animal by product for insect pest suppression. Green Farm. 2011, 2, 502–504. [Google Scholar]
- Miah, M.N.A.; Miah, M.R.U.; Hossain, M.M.; Haque, M.E. Insecticidal effects of cattle urine and indigenous plant extracts against sugarcane mealybugs. Am. J. Zool. 2018, 1, 35–39. [Google Scholar]
- Chauhan, R.S. Immunomodulatory properties of indigenous cow urine. MOJ Immunol. 2018, 6, 302–303. [Google Scholar] [CrossRef]
- Randhava, G.K.; Sharma, R. Chemotherapeutic potential of cow urine: A review. J. Intercult Ethnopharmacol. 2015, 4, 180–186. [Google Scholar] [CrossRef]
- Naseri, B.; Fathipour, Y.; Moharramipour, S.; Hosseininaveh, V. Comparative life history and fecundity of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) on different soybean varieties. Entomol. Sci. 2009, 12, 147–154. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Tallamy, D.W.; Stull, J.; Ehresman, N.P.; Gorski, P.M.; Mason, C.E. Cucurbitacins as feeding and oviposition deterrents to insects. Environ. Entomol. 1997, 26, 678–683. [Google Scholar] [CrossRef]
- Mansour, F.; Azaizeh, H.; Saad, B.; Tadmor, Y.; Abo-Moch, F.; Said, O. The potential of middle eastern flora as a source of new safe bio-acaricides to control Tetranychus cinnabarinus, the carmine spider mite. Phytoparasitica 2004, 32, 66–72. [Google Scholar] [CrossRef]
- Mollashahi, H.; Mirshekari, A.; Ghorbani, M.; Tarrah, A. Insecticidal effect of the fruit extract bitter melon (Citrullus colocynthis) on locust Chrotogonus trachypterus (Orth: Pyrgomorphidae). Biosci. Biotechnol. Res. Asia 2017, 14, 1285–1289. [Google Scholar] [CrossRef]
- Asiry, K.A. Aphidicidal activity of different aqueous extracts of bitter apple Citrullus colocynthis (L.) against the bird cherry-oat aphid, Rhopalosiphum padi (L.) (Homoptera: Aphididae) under laboratory conditions. S J Anim. Plant Sci. 2015, 25, 456–462. [Google Scholar]
- Jeon, J.-H.; Lee, H.-S. Biofunctional constituent isolated from Citrullus colocynthis fruits and structure–Activity relationships of its analogues show acaricidal and insecticidal efficacy. J. Agric. Food Chem. 2014, 62, 8663–8667. [Google Scholar] [CrossRef]
- Rahuman, A.A.; Gopalakrishnan, G.; Venkatesan, P.; Geetha, K. Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2008, 102, 867–873. [Google Scholar] [CrossRef]
- Shekhara, C.; Rachappa, V.; Yelshetty, S.; Sreenivas, A. Biorationals for eco-friendly management of gram pod borer, Helicoverpa armigera (Hubner) on chickpea. J. Exp. Zool. India 2014, 17, 679–682. [Google Scholar]
- Ali, S.; Li, Y.; Haq, I.U.; Abbas, W.; Shabbir, M.Z.; Khan, M.M.; Mamay, M.; Niaz, Y.; Farooq, T.; Skalicky, M. The impact of different plant extracts on population suppression of Helicoverpa armigera (Hub.) and tomato (Lycopersicon esculentum Mill) yield under field conditions. PLoS ONE 2021, 16, e0260470. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Das, S.K.; Mishra, A.; Rattan, S.R.P.; Raddy, S.G.; Arya, H.P.S. Validation of Indigenous Technical Knowledge in Agriculture; Document; ICAR: New Delhi, India, 2004; Volume 4, pp. 78–85. [Google Scholar]
- Bharati, S. Role of Organics and Indigenous Components against Spodoptera litura (Fab.) in Groundnut and Soybean. M.Sc. (Ag.) Thesis, University of Agricultural Sciences, Dharwad, India, 2005; pp. 1–132. [Google Scholar]
- Barapatre, A.; Lingappa, S. Larvicidal and antifeedant activity of indigenous plant protection practices for Helicoverpa armigera (Hub). In In Proceedings of the Frontier Areas of Entomological Research; Indian Agricultural Research Institute: New Delhi, India, 2003; pp. 335–336. [Google Scholar]
- Silva, G.; Rodríguez, J.C.; Blanco, C.A.; Lagunes, A. Bioactivity of a water extract of boldus (Peumus boldus Molina) against Spodoptera frugiperda (JE Smith) and Helicoverpa zea Boddie (Lepidoptera: Noctuidae). Chilean J. Agric. Res. 2013, 73, 135–141. [Google Scholar] [CrossRef]
- Hassam, U.; Gulzar, A.; Rasool, B.; Zafar, S.; Younis, T.; Shakeel, M.; Khan, D.; Ullah, S.; Khaliq, S.; Ahmad, S. Efficacy of Citrullus colocynthis seed extract on Earias vittella, Fabricius, (Lepidoptera: Noctuidae): Environment sustainable approach. Braz. J. Biol. 2022, 84, e254479. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, A.; Maqsood, A.; Munir, A.; Tariq, M.; Ali, M.; Qureshi, R. Toxicity, antifeedant and sub-lethal effects of Citrullus colocynthis extracts on cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Pak. J. Zool. 2017, 49, 2019–2026. [Google Scholar] [CrossRef]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Nicol, H.I.; Munyakazi, L.; Stevenson, P.C. Tri-trophic insecticidal effects of African plants against cabbage pests. PLoS ONE 2013, 8, e78651. [Google Scholar] [CrossRef]
- Pissinati, A.; Ventura, M. Control of cabbage aphid, Brevicoryne brassicae (L.) using kaolin and neem oil. J. Entomol. 2015, 12, 48–54. [Google Scholar] [CrossRef]
- Iqbal, M.; Kahloon, M.; Nawaz, M.; Javaid, M. Effectiveness of some botanical extracts on wheat aphids. J. Anim. Plant Sci. 2011, 21, 114–115. [Google Scholar]
- Santos, T.M.d.; Costa, N.P.; Torres, A.L.; Boiça Júnior, A.L. Effect of neem extract on the cotton aphid. Pesqui. Agropec. Bras. 2004, 39, 1071–1076. [Google Scholar] [CrossRef]
- Simmonds, M.; Manlove, J.; Blaney, W.; Khambay, B. Effect of Botanical Insecticides on the Foraging and Feeding Behavior of the Coccinellid Predator Cryptolaemus montrouzieri. Phytoparasitica 2000, 28, 99–107. [Google Scholar] [CrossRef]
- Duraimurugan, P.; Regupathy, A. Influence of trap crops and application of neem seed kernel extract on the occurrence of natural enemies in cotton ecosystem. Resist. Pest Manag. Newsl. 2005, 15, 7–9. [Google Scholar]
- Hwang, I.-C.; Kim, J.; Kim, H.-M.; Kim, D.-I.; Kim, S.-G.; Kim, S.-S.; Jang, C. Evaluation of toxicity of plant extract made by neem and matrine against main pests and natural enemies. Korean J. Appl. Entom. 2009, 48, 87–94. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, Z.; Huang, X.; Zhang, L.; Zhang, Z. Evaluation of Botanicals for Management of Piercing–Sucking Pests and the Effect on Beneficial Arthropod Populations in Tea Trees Camellia sinensis (L.) O. Kuntze (Theaceae). J. Insect Sci. 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yan, H.; Shi, X.; Liu, B.; Ma, Z.; Zhang, X. Comprehensive evaluation of effective constituents in total alkaloids from Sophora alopecuroides L. and their joint action against aphids by laboratory toxicity and field efficacy. Ind. Crops Prod. 2018, 111, 149–157. [Google Scholar] [CrossRef]
- Hikal, W.-M.; Baeshen, R.-S.; Hussein, A.-H.S. Botanical insecticide as simple extractives for pest control. Cogent Biol 2017, 3, 1. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Werrie, P.-Y.; Juillard, A.; Heintz, C.; Brisset, M.-N.; Fauconnier, M.-L. Phytotoxicity and Plant Defence Induction by Cinnamomum cassia Essential Oil Application on Malus domestica Tree: A Molecular Approach. Agronomy 2022, 12, 512. [Google Scholar] [CrossRef]
- Pavela, R. Effectiveness of some botanical insecticides against Spodoptera littoralis Boisduvala (Lepidoptera: Noctudiae), Myzus persicae Sulzer (Hemiptera: Aphididae) and Tetranychus urticae Koch (Acari: Tetranychidae). Plant Protect. Sci. 2009, 45, 161–167. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).