A Gas Diffusion Analysis Method for Simulating Surface Nitrous Oxide Emissions in Soil Gas Concentrations Measurement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of the Diffusion Coefficient of the Silicone Membrane
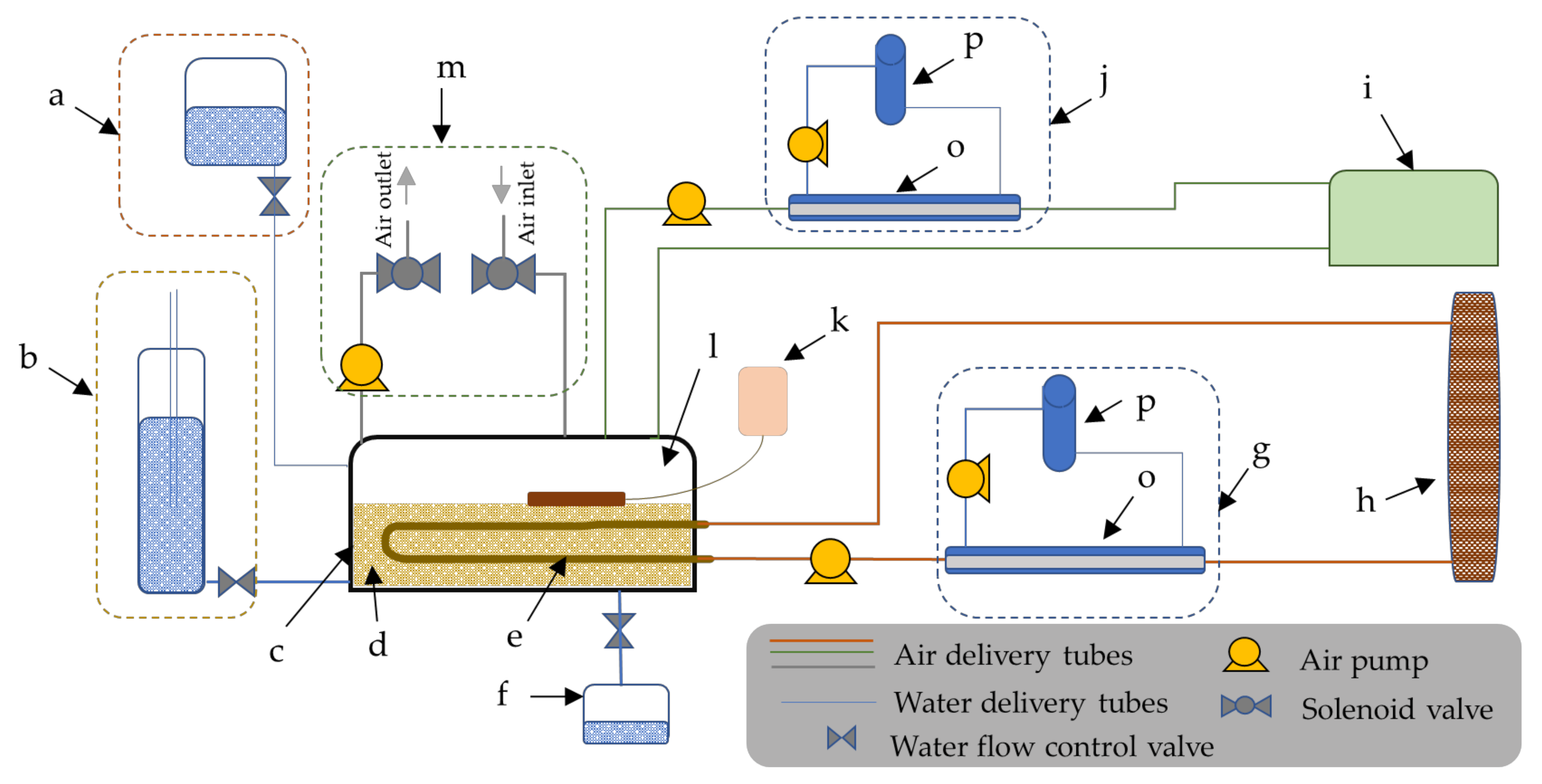
2.2. The Experimental Setup for Monitoring the N2O Flux and Soil Atmospheric N2O Gas Concentrations
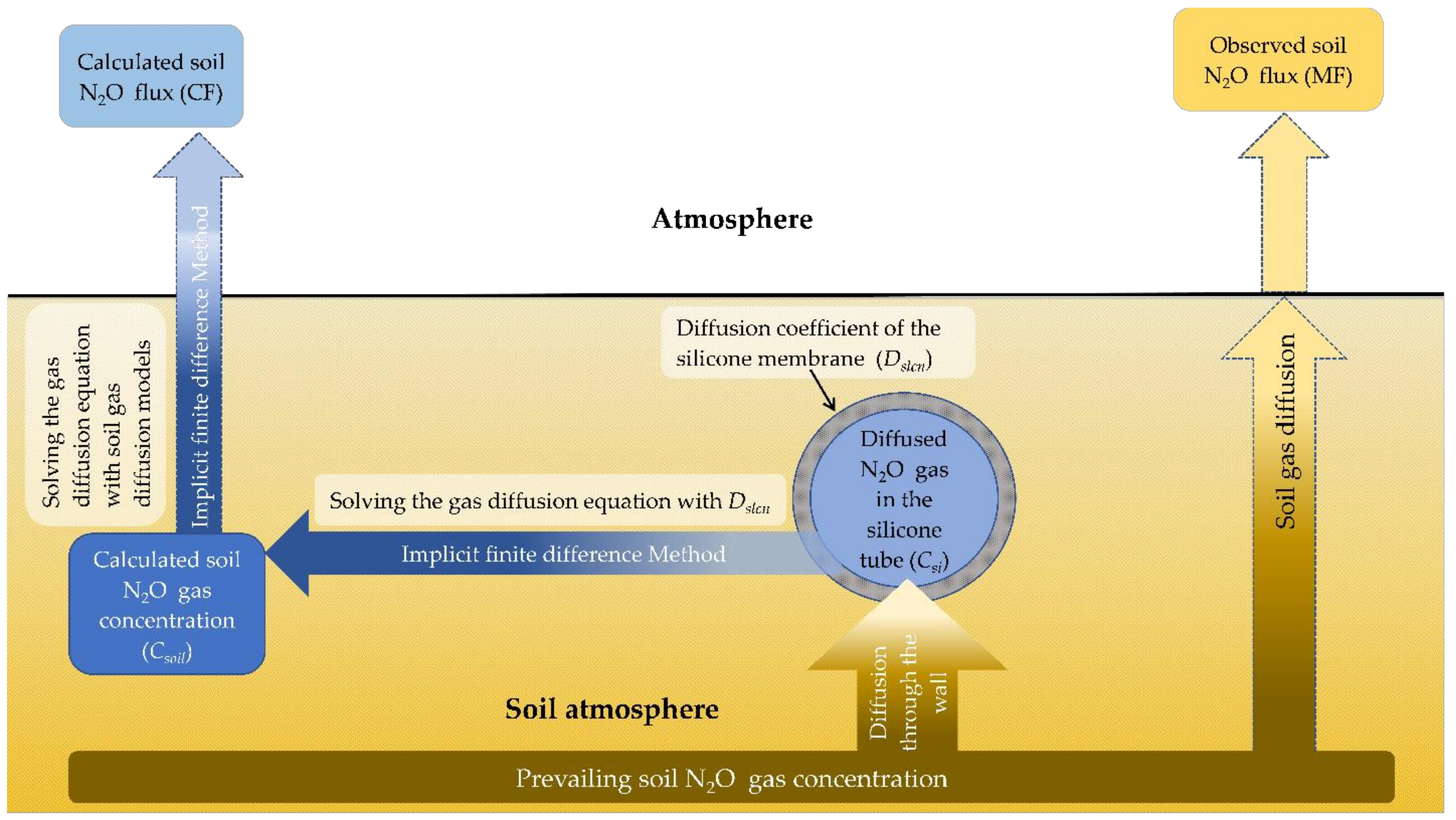
2.3. Simulation Steps for Predicted N2O Surface Flux (CF)
2.3.1. Simulation Steps for Predicting the Soil N2O Gas Level from the Measured Gas in Silicone Diffusion Cell
2.3.2. Steps for Simulating the Predicted N2O Flux from the Soil Surface
3. Results and Discussion
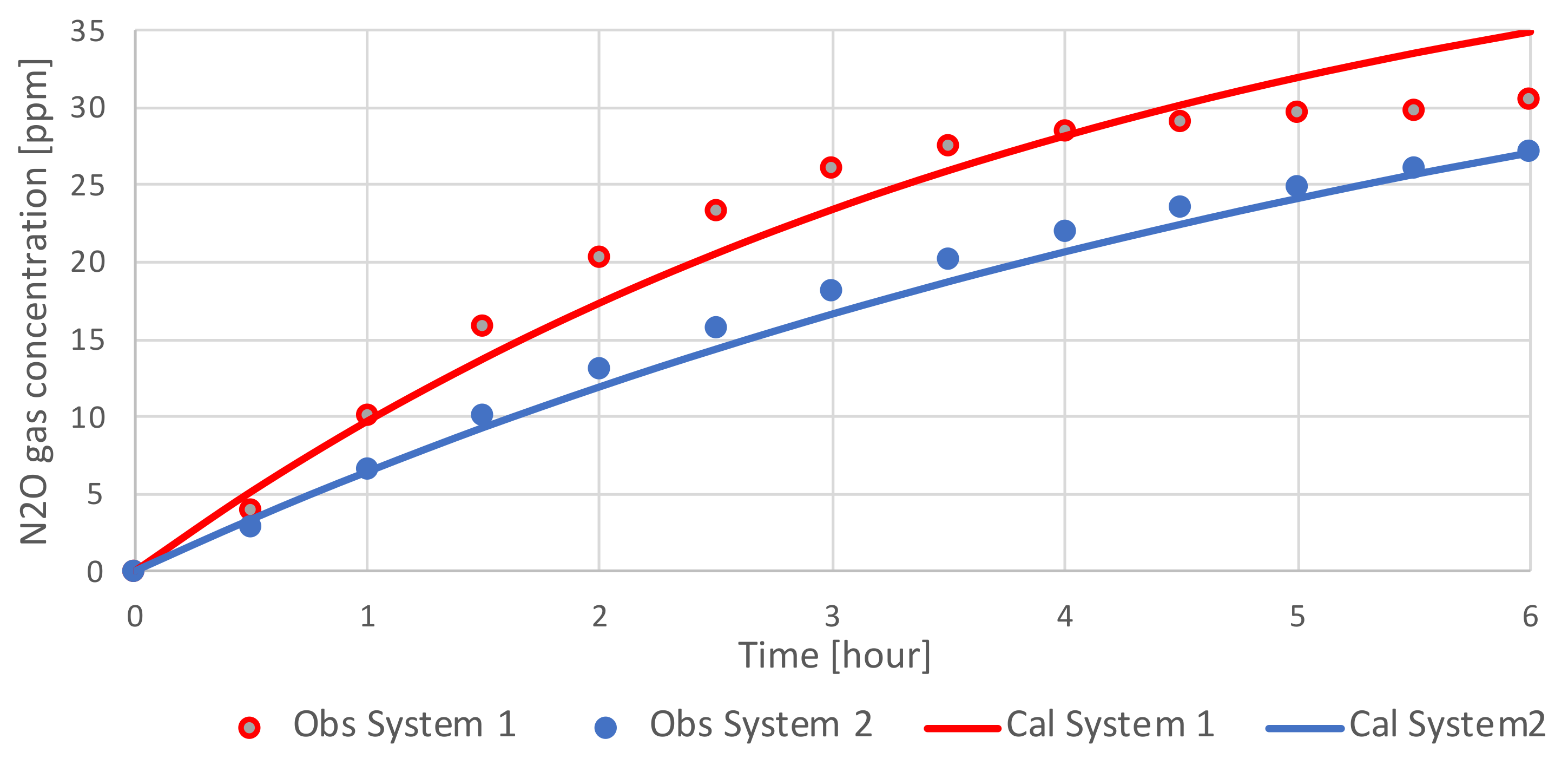
3.1. Diffusion Coefficient of the Silicone Membrane
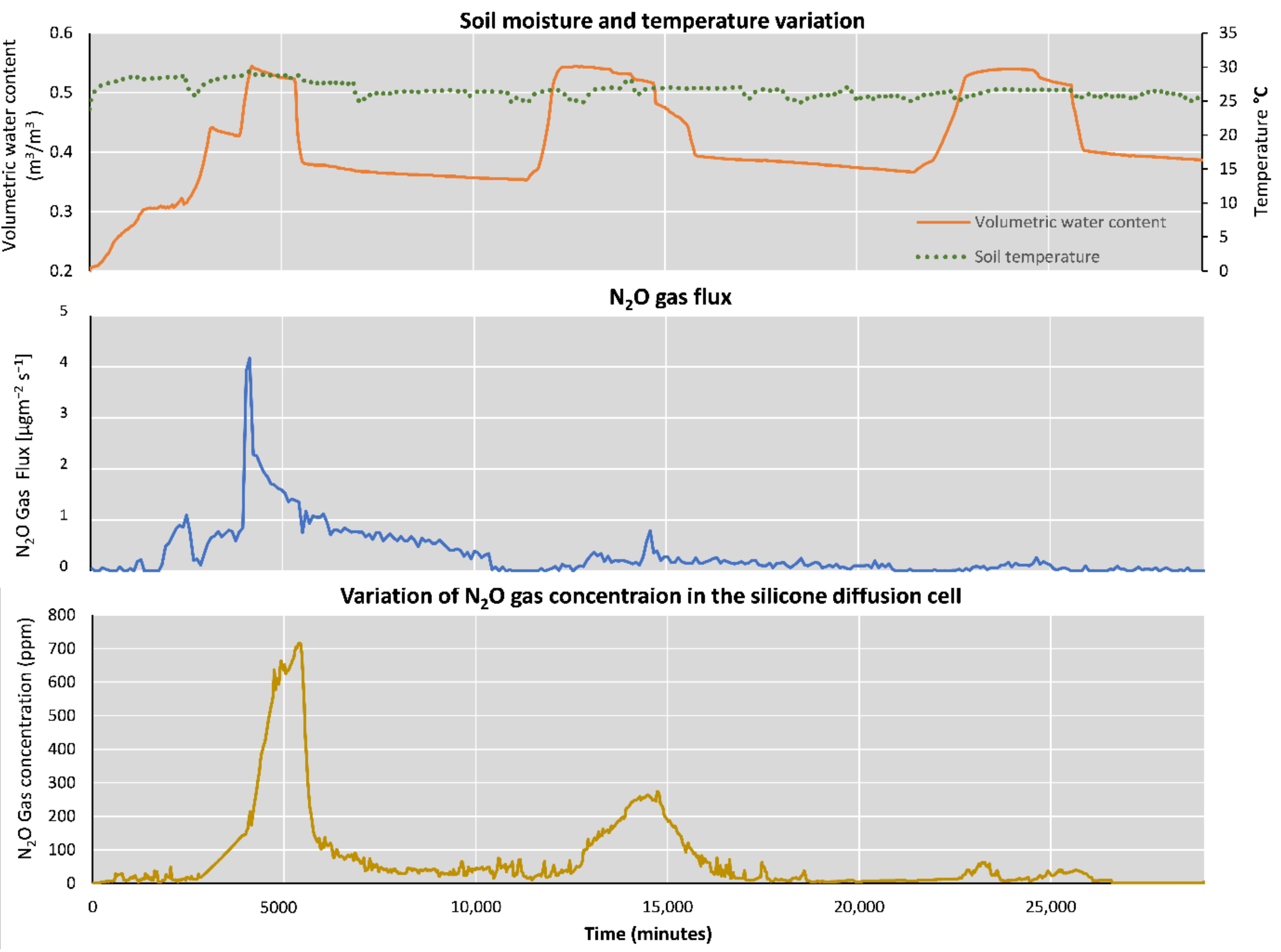
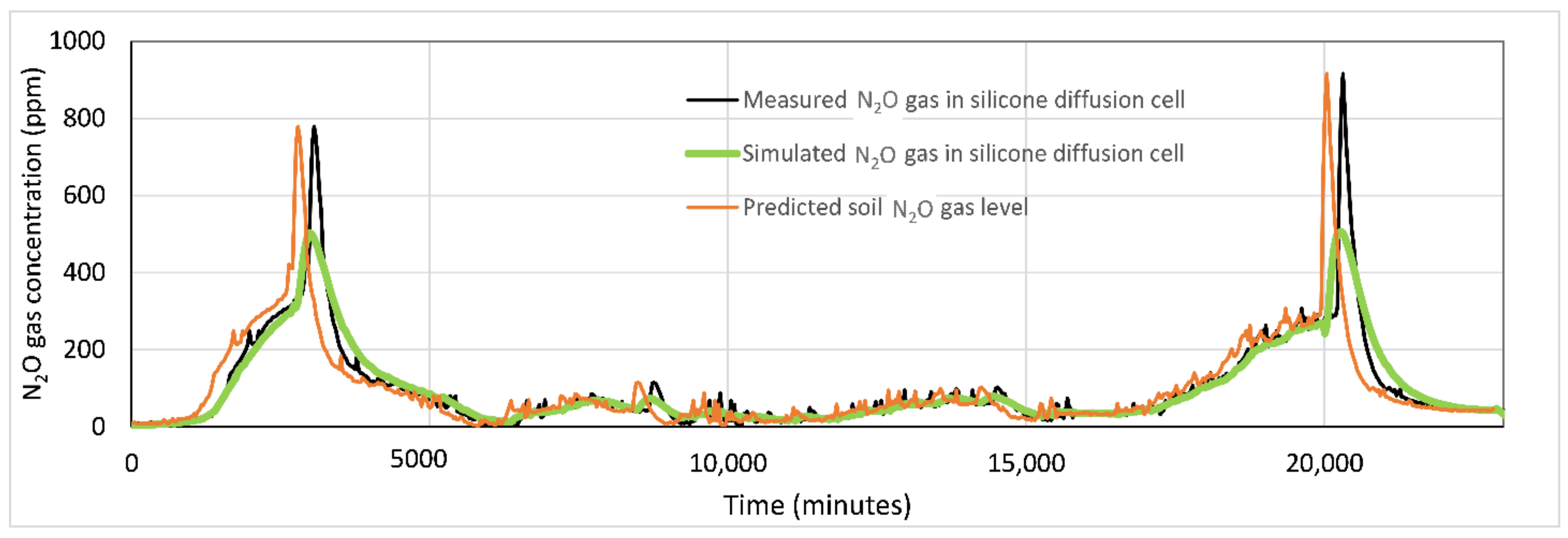
3.2. Results of the Variations in N2O Gas Concentration in the Silicone diffusion cell and N2O Gas flux of the Headspace of Experiment 1 and 2
3.3. Results of the Simulation Steps of the Soil N2O Gas Level Prediction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skiba, U.; Rees, B. Nitrous oxide, climate change and agriculture. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2014, 9, 1–7. [Google Scholar] [CrossRef]
- Mosier, A.R.; Syers, J.K.; Freney, J.R. Nitrogen fertilizer: An essential component of increased food, feed, and fiber production. In Agriculture and the Nitrogen Cycle: Assessing the Impacts of Fertilizer Use on Food Production and the Environment; Mosier, A.R., Syers, J.K., Freney, J.R., Eds.; SCOPE 65 Island Press: Washington, DC, USA, 2004; pp. 3–15. [Google Scholar]
- Kaur, R.; Kaur, S. Biological alternates to synthetic fertilizers: Efficiency and future scopes. Indian J. Agric. Res. 2018, 52, 587–595. [Google Scholar] [CrossRef]
- Gollner, G.; Starz, W.; Friedel, J.K. Crop performance, biological N fixation and pre-crop effect of pea ideotypes in an organic farming system. Nutr. Cycl. Agroecosyst. 2019, 115, 391–405. [Google Scholar] [CrossRef] [Green Version]
- USGCRP (U.S. Global Change Research Program). Climate Science Special Report: Fourth National Climate Assessment; Wuebbles, D.J., Fahey, D.W., Hibbard, K.A., Dokken, D.J., Stewart, T.K., Maycock, B.C., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2017; Volume I. [Google Scholar] [CrossRef] [Green Version]
- Global Annual Surface Man Abundances (2020) and Trends of Key Greenhouse Gases from the GAW In-Situ Observational Network for GHG. The Averaging Method is Described in GAW Report No. 184 9. Available online: https://gaw.kishou.go.jp/static/publications/bulletin/Bulletin2019/ghg-bulletin-16.pdf (accessed on 20 April 2022).
- Ravishankara, A.R.; Daniel, J.; Portmann, R. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Zschornack, T.; Rosa, C.; Reis, C.; Pedroso, G.; Camargo, E.; Santos, D.; Boeni, M.; Bayer, C. Soil CH4 and N2O Emissions from Rice Paddy Fields in Southern Brazil as Affected by Crop Management Levels: A Three-Year Field Study. Rev. Bras. Ciênc. Solo 2018. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Brewer, P.E.; Shugart, H.H.; Lerdau, M.T.; Allison, S.D. Soil aggregates as biogeochemical reactors and implications for soil-atmosphere exchange of greenhouse gases—A concept. Glob. Chang. Biol. 2019, 25, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Chesworth, W. Encyclopedia of Soil Science; Springer: Dorderecht, The Netherlands, 2009; p. 902. [Google Scholar]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2018, 69, 2–4. [Google Scholar] [CrossRef]
- Barnard, R.; Leadley, P.W.; Hungate, B.A. Global change, nitrification, and denitrification: A review. Glob. Biogeochem. Cycles. 2005, 19, GB1007. [Google Scholar] [CrossRef]
- Van Groenigen, K.J.; Osenberg, C.W.; Hungate, B.A. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 2011, 475, 214–216. [Google Scholar] [CrossRef]
- Wolf, B.; Chen, W.; Brüggemann, N.; Zheng, X.; Pumpanen, J.; Butterbach-Bahl, K. Applicability of the soil gradient method for estimating soil-atmosphere CO2, CH4, and N2O fluxes for steppe soils in Inner Mongolia. J. Plant Nutr. Soil Sci. 2011, 174, 359–372. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Prior, S.A.; Runion, G.B.; Torbert, H.A.; Tian, H.Q.; Lu, C.Q.; Venterea, R.T. Effects of elevated carbon dioxide and increased temperature on methane and nitrous oxide fluxes: Evidence from field experiments. Front. Ecol. Environ. 2012, 10, 520–527. [Google Scholar] [CrossRef]
- Ma, F.; Li, M.; Wei, N.; Dong, L.; Zhang, X.; Han, X.; Li, K.; Guo, L. Impacts of Elevated Atmospheric CO2 and N Fertilization on N2O Emissions and Dynamics of Associated Soil Labile C Components and Mineral N in a Maize Field in the North China Plain. Agronomy 2022, 12, 432. [Google Scholar] [CrossRef]
- Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/climate-change/ (accessed on 15 April 2022).
- Denmead, O.T. Approaches to measuring fluxes of methane and nitrous oxide between landscapes and the atmosphere. Plant Soil. 2008, 309, 5–24. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Sander, B.O.; Pelster, D.; Díaz-Pinés, E. Quantifying Greenhouse Gas Emissions from Managed and Natural Soils. In Methods for Measuring Greenhouse Gas Balances and Evaluating Mitigation Options in Smallholder Agriculture; Rosenstock, T., Rufino, M., Butterbach-Bahl, K., Wollenberg, L., Richards, M., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef] [Green Version]
- Maljanen, M.; Liikanen, A.; Silvola, J.; Martikainen, P.J. Measuring N2O emissions from organic soils by closed chamber or soil/snow N2O gradient methods. Eur. J. Soil Sci. 2003, 54, 625–631. [Google Scholar] [CrossRef]
- Martikainen, P.J.; Nykänen, H.; Crill, P.; Silvola, J. Effect of a lowered water table on nitrous oxide fluxes from northern peatlands. Nature 1993, 366, 51–53. [Google Scholar] [CrossRef]
- Nykänen, H.; Alm, J.; Lång, K.; Silvola, J.; Martikainen, P.J. Emissions of CH4, N2O and CO2 from a virgin fen and a fen drained for grassland in Finland. J. Biogeogr. 1995, 22, 351–357. [Google Scholar] [CrossRef]
- Flessa, H.; Wild, U.; Klemisch, M.; Pfadenhauer, J. Nitrous oxide and methane fluxes from organic soils under agriculture. Eur. J. Soil Sci. 1998, 49, 327–335. [Google Scholar]
- Chikowo, R.; Zingore, S.; Snapp, S.; Johnston, A. Farm typologies, soil fertility variability and nutrient management in smallholder farming in Sub-Saharan Africa. Nutr. Cycl. Agroecosyst. 2014, 100, 1–18. [Google Scholar] [CrossRef]
- Breuer, L.; Papen, H.; Butterbach-Bahl, K. N2O-emission from tropical forest soils of Australia. J. Geophys. Res. 2000, 105, 26353–26378. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Papen, H.; Rennenberg, H. Impact of gas transport through rice cultivars on methane emission from rice paddy fields. Plant Cell Environ. 1997, 20, 1175–1183. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Gasche, R.; Breuer, L.; Papen, H. Fluxes of NO and N2O from temperate forest soils: Impact of forest type, N deposition and of liming on the NO and N2O emissions. Nutr. Cycl. Agroecosyst. 1997, 48, 79–90. [Google Scholar] [CrossRef]
- Bandara, K.M.T.S.; Sakai, K.; Nakandakari, T.; Yuge, K. A Low-Cost NDIR-Based N2O Gas Detection Device for Agricultural Soils: Assembly, Calibration Model Validation, and Laboratory Testing. Sensors 2021, 21, 1189. [Google Scholar] [CrossRef]
- Pihlatie, M.; Pumpanen, J.; Rinne, J.; Ilvesniemi, H.; Simojoki, A.; Hari, P.; Vesala, T. Gas concentration driven fluxes of nitrous oxide and carbon dioxide in boreal forest soil. Tellus B Chem. Phys. Meteorol. 2007, 59, 458–469. [Google Scholar] [CrossRef]
- Goffin, S.; Longdoz, B.; Maier, M.; Schack-Kirchner, H.; Aubinet, M. Soil Respiration in forest Ecosystems: Combination of a multilayer Approach and an Isotopic Signal Analysis. Commun. Agric. Appl. Biol. Sci. 2012, 72, 139–144. [Google Scholar]
- Maier, M.; Longdoz, B.; Laemmel, T.; Schack-Kirchner, H.; Lang, F. 2D profiles of CO2, CH4, N2O and gas diffusivity in a well aerated soil: Measurement and Finite Element Modeling. Agric. Met. 2017, 247, 21–33. [Google Scholar] [CrossRef]
- Jacinthe, P.-A.; Dick, W.A. Use of silicone tubing to sample nitrous oxide in the soil atmosphere. Soil Biol. Biochem. 1996, 28, 721–726. [Google Scholar] [CrossRef]
- Jacinthe, P.-A.; Groffman, P.M. Silicone rubber sampler to measure dissolved gases in saturated soils and waters. Soil Biol. Biochem. 2001, 33, 907–912. [Google Scholar] [CrossRef]
- Kammann, C.; Grünhage, L.; Jäger, H.J. A new sampling technique to monitor concentrations of CH4, N2O and CO2 in air at well-defined depths in soils with varied water potential. Eur. J. Soil Sci. 2001, 52, 297–303. [Google Scholar] [CrossRef]
- Maier, M.; Machacova, K.; Lang, F.; Svobodova, K.; Urban, O. Combining soil and tree-stem flux measurements and soil gas profiles to understand CH4 pathways in Fagus sylvatica forests. J. Plant Nutr. Soil Sci. 2018, 181, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Maier, M.; Gartiser, V.; Schengel, A.; Lang, V. Long Term Soil Gas Monitoring as Tool to Understand Soil Processes. Appl. Sci. 2020, 10, 8653. [Google Scholar] [CrossRef]
- Marinov, M.; Nikolov, G.; Gieva, E.; Ganev, B. Improvement of NDIR carbon dioxide sensor accuracy. In Proceedings of the 38th International Spring Seminar on Electronics Technology (ISSE), Eger, Hungary, 6–10 May 2015; pp. 466–471. [Google Scholar]
- Yasuda, T.; Yonemura, S.; Tani, A. Comparison of the characteristics of small commercial NDIR CO2 sensor models and development of a portable CO2 measurement device. Sensors 2012, 12, 3641–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honeycutt, W.T.; Ley, M.T.; Materer, N.F. Precision and Limits of Detection for Selected Commercially Available, Low-Cost Carbon Dioxide and Methane Gas Sensors. Sensors 2019, 19, 3157. [Google Scholar] [CrossRef] [Green Version]
- Hodgkinson, J.; Smith, R.; Wo, H.; Saffell, J.R.; Tatam, R.P. A low cost, optically efficient carbon dioxide sensor based on nondispersive infra-red (NDIR) measurement at 4.2 μm. In Optical Sensing and Detection II; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; Volume 8439, p. 843919. [Google Scholar]
- Maier, M.; Schack-Kirchner, H. Using the gradient method to determine soil gas flux: A review. Agric. For. Meteorol. 2014, 192–193, 78–95. [Google Scholar] [CrossRef]
- Atsuhiro, T.; Tetsuya, H.; Hiroshi, A.T.; Yoshihiro, F. Analytical estimation of the vertical distribution of CO2 production within soil: Application to a Japanese temperate forest. Agric. For. Meteorol. 2004, 126, 223–235. [Google Scholar] [CrossRef]
- Schack-Kirchner, H.; Kublin, E.; Hildebrand, E.E. Finite-Element Regression to Estimate Production Profiles of Greenhouse Gases in Soils. Vadose Zone J. 2011, 10, 169–183. [Google Scholar] [CrossRef]
- Buckingham, E. Contributions to Our Knowledge of the Aeration of Soils; USDA Burr Soil Bulletin, 25; US Government Printing Office: Washington, DC, USA, 1904. [Google Scholar]
- Millington, R.J.; Quirk, J.M. Transport in porous media. In Transactions of the 7th International Congress of Soil Science; Van Beren, F.A., Ed.; Elsevier: Amsterdam, The Netherlands, 1960; pp. 97–106. [Google Scholar]
- Millington, R.J.; Quirk, J.M. Permeability of porous solids. Trans. Faraday Soc. 1961, 57, 1200–1207. [Google Scholar] [CrossRef]
- Moldrup, P.T.; Olesen, J.; Gamst, P.; Schjønning, P.; Yamaguchi, T.; Rolston, D.E. Predicting the gas diffusion coefficient in repacked soil: Water-induced linear reduction model. Soil Sci. Soc. Am. J. 2000, 64, 1588–1594. [Google Scholar] [CrossRef]
- Moldrup, P.T.; Olesen, P.; Schjønning, P.; Yamaguchi, T.; Rolston, D.E. Predicting the gas diffusion coefficient in undisturbed soil from soil water characteristics. Soil Sci. Soc. Am. J. 2000, 64, 94–100. [Google Scholar] [CrossRef]
- Moldrup, P.; Kruse, C.W.; Rolston, D.E.; Yamaguchi, T. Modelling diffusion and reaction in soils: III. Predicting gas diffusivity from the Campbell soil water retention model. Soil Sci. 1996, 161, 366–375. [Google Scholar] [CrossRef]
- Williams, E.J.; Hutchinson, G.L.; Fehsenfeld, F.C. NOx and N2O emissions from soil. Glob. Biogeochem. Cycles 1992, 4, 351–388. [Google Scholar] [CrossRef]
- Poth, M.; Focht, D.D. 15N kinetic analysis of N2O production by Nitrosomonas europea: An examination of nitrifier denitrification. Appl. Environ. Microbiol. 1985, 49, 1134–1141. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A. Sources of nitric oxide and nitrous oxide following wetting of dry soil. Soil Sci. Soc. Am. J. 1992, 56, 95–102. [Google Scholar] [CrossRef]
- Davidson, E.A. Soil water content and the ratio of nitrous oxide to nitric oxide emitted from soil. In The Biogeochemistry of Global Change: Radiatively Active Trace Gases; Oremland, R.S., Ed.; Chapman & Hall: New York, NY, USA, 1992. [Google Scholar]
- Xunhua, Z.; Mingxing, W.; Yuesi, W.; Renxing, S.; Gou, J.; Jing, L.; Jisheng, J.; Laotu, L. Impacts of soil moisture on nitrous oxide emission from croplands: A case study on the rice-based agro-ecosystem in Southeast China. Chemosphere—Glob. Chang. Sci. 2000, 2, 207–224. [Google Scholar]
- Wang, J.M.; Murphy, J.G.; Geddes, J.A.; Winsborough, C.L.; Basiliko, N.; Thomas, S.C. Methane fluxes measured by eddy covariance and static chamber techniques at a temperate forest in central Ontario, Canada. Biogeosciences 2013, 10, 4371–4382. [Google Scholar] [CrossRef] [Green Version]
- Kroon, P.S.; Hensen, A.; Jonker, H.J.J.; Ouwersloot, H.G.; Vermeulen, A.T.; Bosveld, F.C. Uncertainties in eddy covariance flux measurements assessed from CH4 and N2O observations. Agric. For. Meteorol. 2010, 150, 806–816. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Xu, X.; Long, R.; Wang, Q.; Wang, C.; Du, Y.; Zhao, X. Methane emissions by alpine plant communities in the Qinghai-Tibet Plateau. Biol. Lett. 2008, 4, 681–684. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Nepstad, D.C.; Ishida, F.Y.; Brando, P.M. Effects of an experimental drought and recovery on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Glob. Chang. Biol. 2008, 14, 2582–2590. [Google Scholar] [CrossRef]
- Davidson, E.A.; Savage, K.; Verchot, L.V.; Navarro, R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric. For. Meteorol. 2002, 113, 21–37. [Google Scholar] [CrossRef]
- Conen, F.; Smith, K.A. An explanation of linear increases in gas concentration under closed chambers used to measure gas exchange between soil and the atmosphere. Eur. J. Soil Sci. 2000, 51, 111–117. [Google Scholar] [CrossRef]
- Longdoz, B.; Yernaux, M.; Aubinet, M. Soil CO2 efflux measurements in a mixed forest: Impact of chamber disturbances, spatial variability and seasonal evolution. Glob. Chang. Biol. 2000, 6, 907–917. [Google Scholar] [CrossRef]
- Lund, C.P.; Riley, W.J.; Pierce, L.L.; Field, C.B. The effects of chamber pressurization on soil-surface CO2 flux and the implications for NEE measurements under elevated CO2. Glob. Chang. Biol. 1999, 5, 269–281. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Zheng, X. Static opaque chamber-based technique for determination of net exchange of CO2 between terrestrial ecosystem and atmosphere. Chin. Sci. Bull. 2004, 49, 381. [Google Scholar] [CrossRef]
- Junwei, L.; Jianghua, W. Gross photosynthesis explains the ‘artificial bias’ of methane fluxes by static chamber (opaque versus transparent) at the hummocks in a boreal peatland. Environ. Res. Lett. 2014, 9, 105005. [Google Scholar]
- Lei, M.; Wei, Z.; Xunhua, Z.; Zhisheng, Y.; Han, Z.; Rui, W.; Bo, Z.; Kai, W.; Chunyan, L.; Guangmin, C.; et al. Attempt to correct grassland N2O fluxes biased by the DN-based opaque static chamber measurement. Atmos. Environ. 2021, 264, 118687. [Google Scholar] [CrossRef]





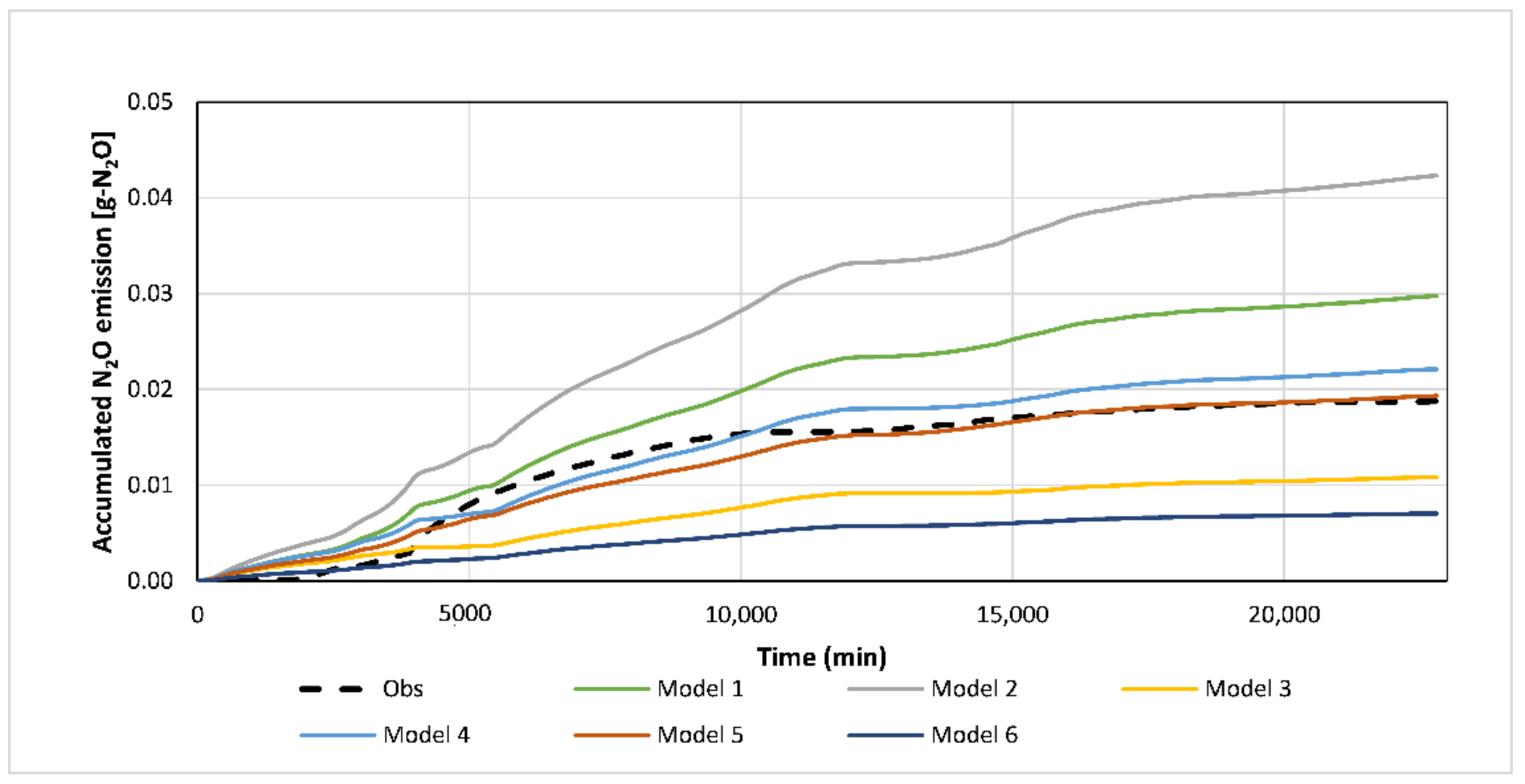
| Experiment | Accuracy Test | Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| 1 | SMAPE (%) | 16.45 | 31.85 | 20.02 | 8.18 | 10.18 | 19.72 | |||
| d | 0.9954 | 0.9905 | 0.9965 | 0.9996 | 0.9994 | 0.9964 | ||||
| 2 | SMAPE (%) | 12.63 | 27.89 | 18.18 | 10.73 | 8.02 | 20.65 | |||
| d | 0.9947 | 0.9844 | 0.9924 | 0.9992 | 0.9997 | 0.9893 | ||||
| Color scale of SMAPE: Symmetric mean absolute percentage error (%). | ||||||||||
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | |||
| Color scale of d: Willmott’s agreement index (goodness of fit (scale 0–1) of the model’s output (CF) to the measured cumulative N2O flux (MF)). | ||||||||||
| 0.9 | 0.92 | 0.94 | 0.96 | 0.98 | 1 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandara, K.M.T.S.; Sakai, K.; Nakandakari, T.; Yuge, K. A Gas Diffusion Analysis Method for Simulating Surface Nitrous Oxide Emissions in Soil Gas Concentrations Measurement. Agriculture 2022, 12, 1098. https://doi.org/10.3390/agriculture12081098
Bandara KMTS, Sakai K, Nakandakari T, Yuge K. A Gas Diffusion Analysis Method for Simulating Surface Nitrous Oxide Emissions in Soil Gas Concentrations Measurement. Agriculture. 2022; 12(8):1098. https://doi.org/10.3390/agriculture12081098
Chicago/Turabian StyleBandara, K. M. T. S., Kazuhito Sakai, Tamotsu Nakandakari, and Kozue Yuge. 2022. "A Gas Diffusion Analysis Method for Simulating Surface Nitrous Oxide Emissions in Soil Gas Concentrations Measurement" Agriculture 12, no. 8: 1098. https://doi.org/10.3390/agriculture12081098
APA StyleBandara, K. M. T. S., Sakai, K., Nakandakari, T., & Yuge, K. (2022). A Gas Diffusion Analysis Method for Simulating Surface Nitrous Oxide Emissions in Soil Gas Concentrations Measurement. Agriculture, 12(8), 1098. https://doi.org/10.3390/agriculture12081098







