Parents’ Selection Affects Embryo Rescue, Seed Regeneration and the Heredity of Seedless Trait in Table Grape Breeding Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site, Plant Material and Experimental Design
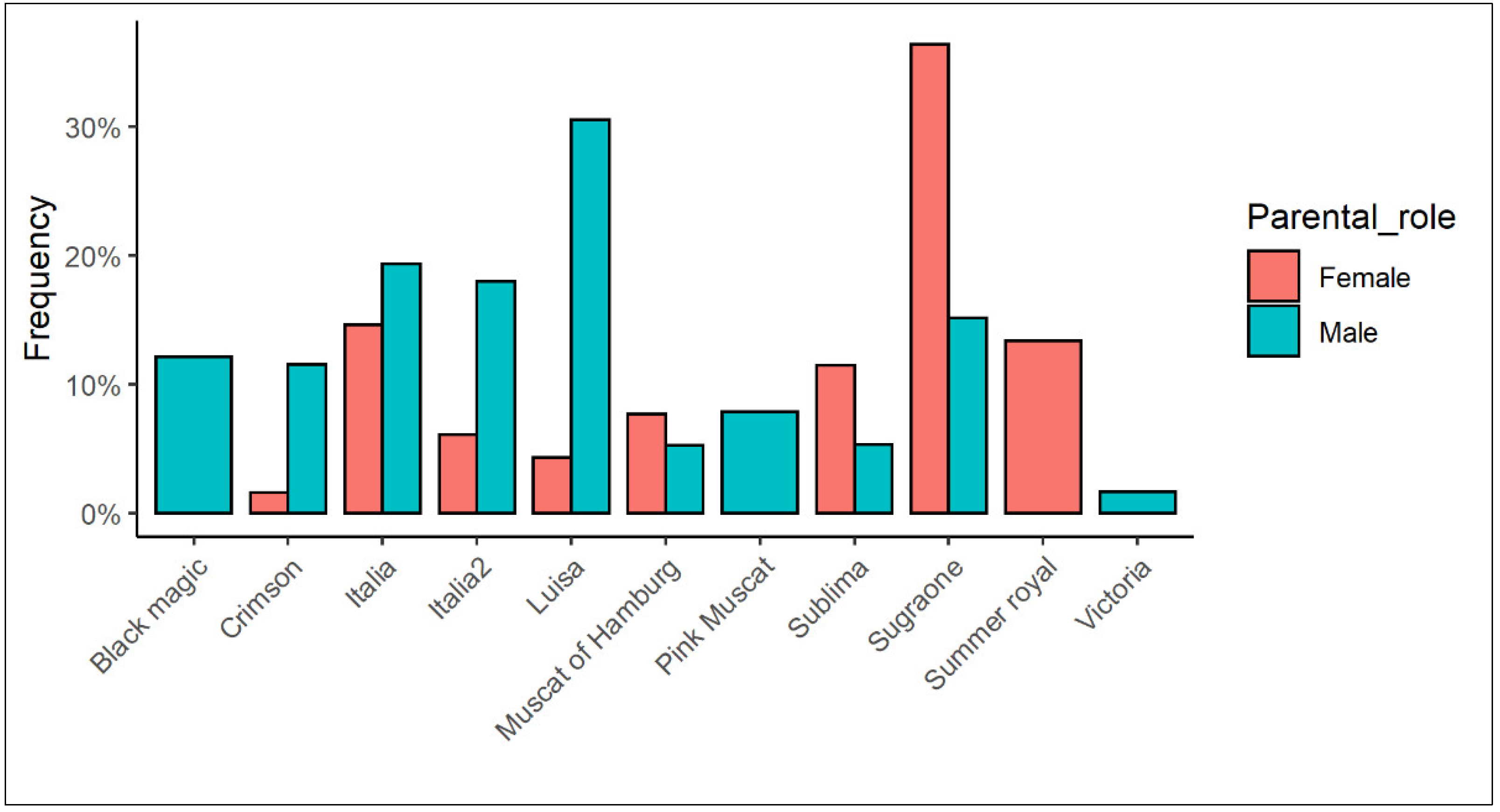
2.2. Crossing
2.3. Embryo Rescue and Plant Development
2.4. Plant Developed from Seeded Female Parent
2.5. DNA Extraction and Marker Assisted Selection (MAS)
2.6. Efficiency of the Cross Pollination and Statistical Analysis
3. Results and Discussion
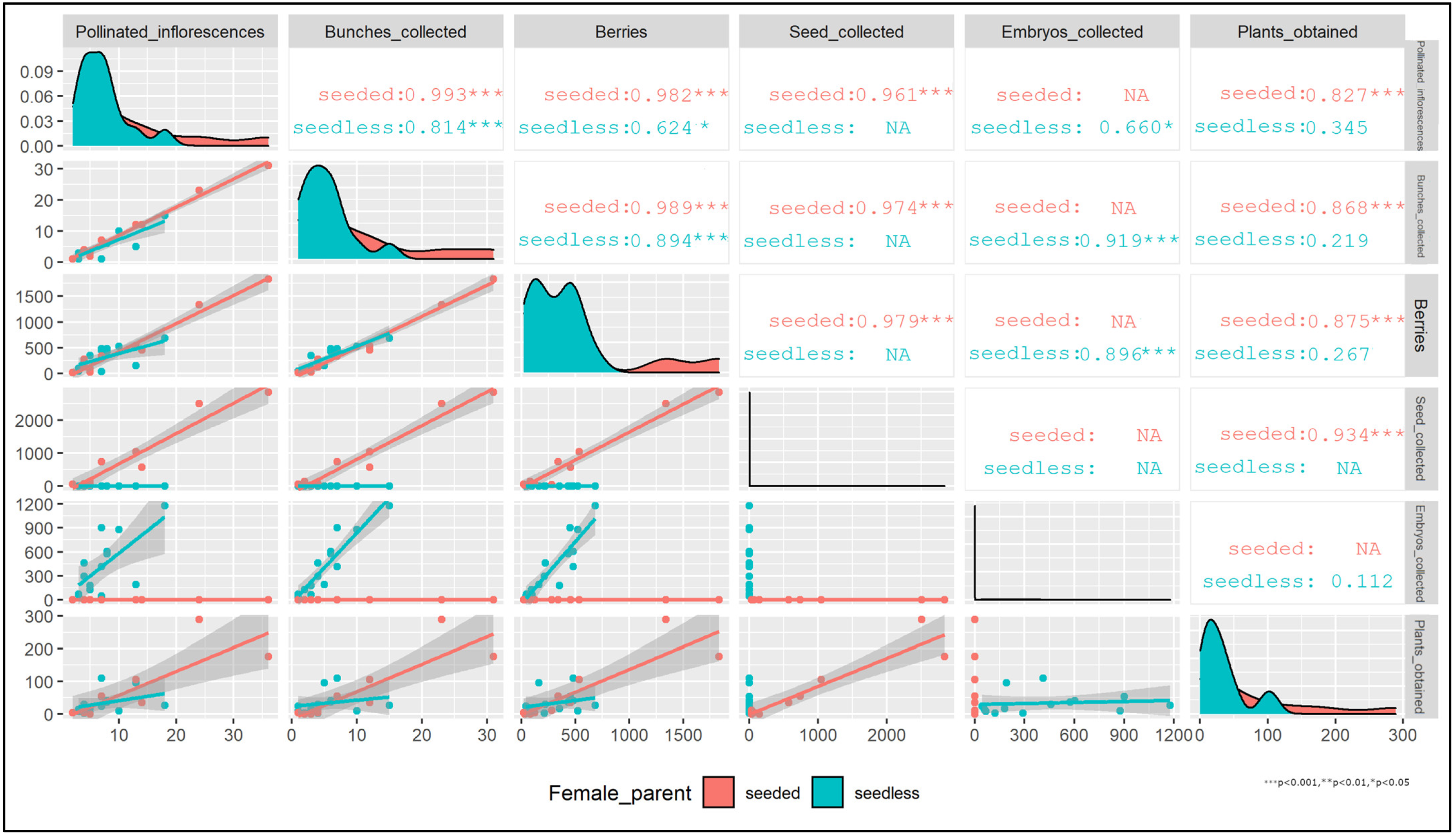
3.1. Fertilization and Seed/Fruit Set Evaluation
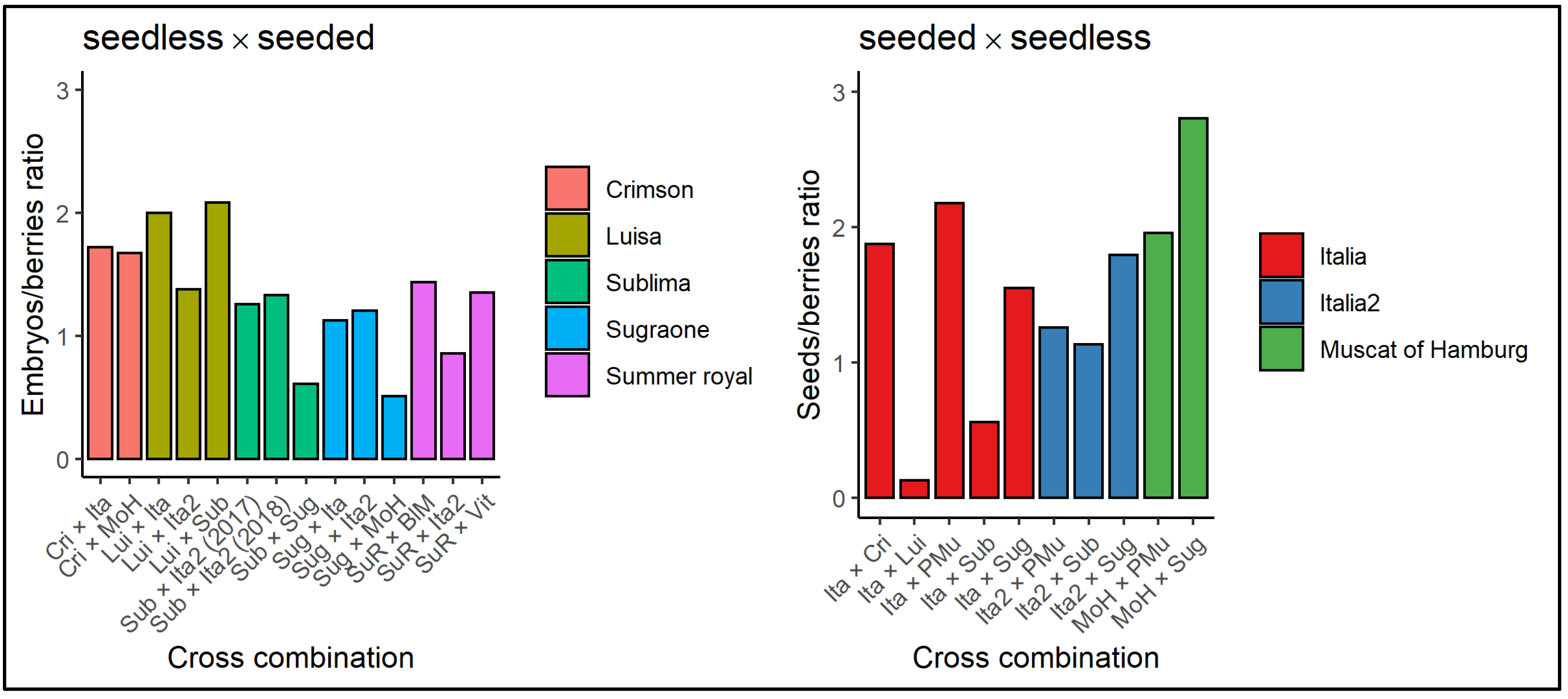
3.2. Embryo and Seed Plant Regeneration Efficiency
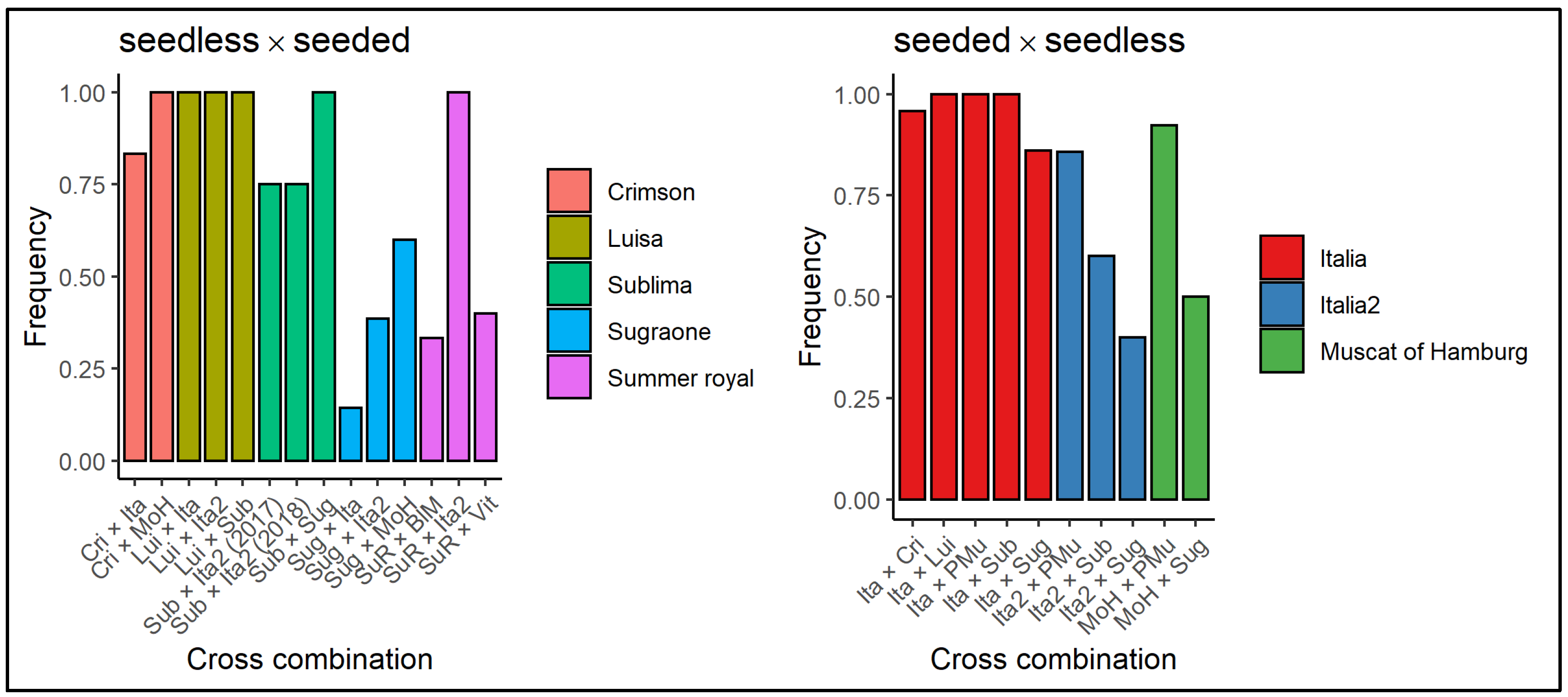
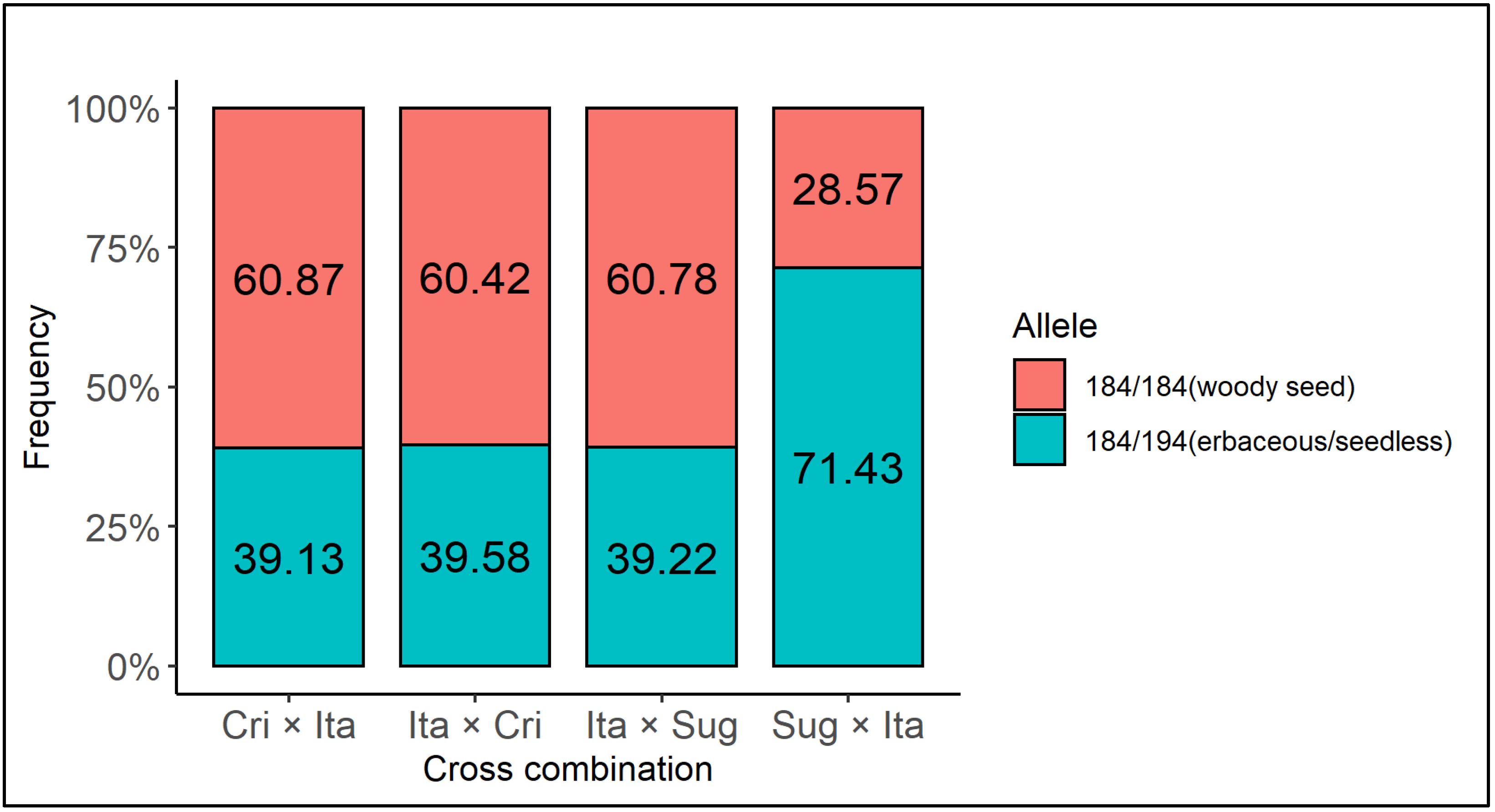
3.3. Validation of the Molecular Marker for Seedlessness According to the Different Parental Lines and the Direction of the Cross
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akkurt, M.; Tahmaz, H.; Veziroğlu, S. Recent Developments in Seedless Grapevine Breeding. S. Afr. J. Enol. Vitic. 2019, 40, 2. [Google Scholar] [CrossRef]
- Karaagac, E.; Vargas, A.M.; de Andrés, M.T.; Carreño, I.; Ibáñez, J.; Carreño, J.; Martínez-Zapater, J.M.; Cabezas, J.A. Marker Assisted Selection for Seedlessness in Table Grape Breeding. Tree Genet. Genomes 2012, 8, 1003–1015. [Google Scholar] [CrossRef] [Green Version]
- Nicolosi, E.; Ferlito, F.; Amenta, M.; Rapisarda, P. Shelf-Life of Minimally Processed Table Grapes Packed in Snack-Size Containers. Acta Hortic. 2018, 1209, 417–424. [Google Scholar] [CrossRef]
- Nicolosi, E.; Ferlito, F.; Amenta, M.; Russo, T.; Rapisarda, P. Changes in the Quality and Antioxidant Components of Minimally Processed Table Grapes during Storage. Sci. Hortic. 2018, 232, 175–183. [Google Scholar] [CrossRef]
- Ponce, M.T.; Agüero, C.B.; Gregori, M.T.; Tizio, R. Factors Affecting the Development of Stenospermic Grape (Vitis vinifera) Embryos Cultured in Vitro. Acta Hortic. 2000, 528, 667–671. [Google Scholar] [CrossRef]
- Nookaraju, A.; Barreto, M.S.; Karibasappa, G.S.; Agrawal, D.C. Synergistic Effect of CPPU and Benzyladenine on Embryo Rescue in Six Stenospermocarpic Cultivars of Grapevine. Vitis-J. Grapevine Res. 2007, 46, 188–191. [Google Scholar] [CrossRef]
- Stout, A. Seedlessness in Grapes; Technical Bulletin 238; New York State Agricultural Experiment Station: Geneva, NY, USA, 1936. [Google Scholar]
- Mejía, N.; Hinrichsen, P. A New, Highly Assertive Scar Marker Potentially Useful to Assist Selection for Seedlessness in Table Grape Breeding. Acta Hortic. 2003, 603, 559–564. [Google Scholar] [CrossRef]
- Ramming, D.W.; Emershad, R.L.; Spiegel-Roy, P.; Sahar, N.; Baron, I. Embryo Culture of Early Ripening Seeded Grape (Vitis vinifera) Genotypes. HortScience 1990, 25, 339–342. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.; Martinez, A.; Garcia De La Calera, E.; Perez, L.J.; Cenis, J.L.; Carrefio, J. In Vitro Culture of Ovules and Embryos of Grape for the Obtention of New Seedless Table Grape Cultivars. Acta Hortic. 2000, 528, 663–666. [Google Scholar] [CrossRef]
- Perl, A.; Sahar, N.; Spiegel-Roy, P.; Gavish, S.; Elyasi, R.; Orr, E.; Bazak, H. Conventional and Biotechnological Approaches in Breeding Seedless Table Grapes. Acta Hortic. 2000, 528, 613–618. [Google Scholar] [CrossRef]
- Ferrara, G.; Mazzeo, A. Potential and Actual Bud Fruitfulness: A Tool for Predicting and Managing the Yield of Table Grape Varieties. Agronomy 2021, 11, 841. [Google Scholar] [CrossRef]
- Dangi, G.S.; Mendum, M.L.; Prins, B.H.; Walker, M.A.; Meredith, C.P.; Simon, C.J. Simple se-quence repeat analysis of a clonally propagated species: A tool for managing a grape germplasm collection. Genome 2001, 44, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, J.; Vargas, A.M.; Palancar, M.; Borrego, J.; De Andrés, M.T. Genetic relationships among table-grape varieties. Am. J. Enol. Vitic. 2009, 60, 35–42. [Google Scholar]
- Ramming, D.W.; Tarailo, R.E.; Sayed, A.B. ‘Crimson Seedless’: A New Late-maturing, Red Seedless Grape. Hortscience 1995, 30, 1473–1474. [Google Scholar] [CrossRef] [Green Version]
- Perl, A.; Sahar, N.; Eliassi, R.; Baron, P.; Spiegel-Roy, P.; Bazak, H. Breeding of new seedless table grapes in Israel conventional and biotechnological approach. Acta Hortic. 2003, 623, 185–187. [Google Scholar] [CrossRef]
- Emershad, R.L.; Ramming, D.W. In-ovulo embryo culture of Vitis vinifera L. cv. ‘Thompson Seedless’. HortScience 1982, 17, 576. [Google Scholar]
- Cain, D.W.; Emershad, R.L.; Tarailo, R.E. In Ovulo Embryo Culture and Seedling Development of Seeded and Seedless Grapes (Vitis vinifera L.). Vitis 1983, 22, 9–14. [Google Scholar]
- Tian, L.; Wang, Y.; Niu, L.; Tang, D. Breeding of Disease-Resistant Seedless Grapes Using Chinese Wild Vitis Spp. I. In Vitro Embryo Rescue and Plant Development. Sci. Hortic. 2008, 117, 136–141. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, X.; Wang, Y. Embryo Rescue Technique and Its Applications for Seedless Breeding in Grape. Plant Cell Tissue Organ Cult. 2015, 120, 861–880. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, Z.; Xu, K.; Guo, Y.; Zhang, C.; Li, T.; Jiang, Y.; Liu, G.; Xu, Y. Study on Improving Plantlet Development and Embryo Germination Rates in in Vitro Embryo Rescue of Seedless Grapevine. N. Z. J. Crop Hortic. Sci. 2017, 46, 39–53. [Google Scholar] [CrossRef]
- Li, T.; Li, Z.; Yin, X.; Guo, Y.; Wang, Y.; Xu, Y. Improved in Vitro Vitis vinifera L. Embryo Development of F1 Progeny of ‘Delight’ × ‘Ruby Seedless’ Using Putrescine and Marker-Assisted Selection. Vitr. Cell. Dev. Biol.-Plant 2018, 54, 291–301. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, B.; Li, P.; Shu, X.; Zhang, X.; Zhang, J. New Cold-Resistant, Seedless Grapes Developed Using Embryo Rescue and Marker-Assisted Selection. Plant Cell Tissue Organ Cult. 2019, 140, 551–562. [Google Scholar] [CrossRef]
- Kebeli, N.; Boz, Y.; Özer, C. Studies on the Applying of Embryo Culture in Breeding New Hybrids by Crossing Seedless Grape Cultivars. Acta Hortic. 2003, 625, 279–281. [Google Scholar] [CrossRef]
- Valdez, J.G.; Ulanovsky, S.M. In Vitro Germination of Stenosperrnie Seeds from Reciprocal Crosses (Vitis vinifera L.) Applying Different Techniques. Vitis 1997, 36, 51–58. [Google Scholar]
- Yamashita, H.; Horiuchi, S.; Taira, T. Development of Seeds and the Growth of Triploid Seedlings Obtained from Reciprocal Crosses between Diploids and Tetraploids Grapes. J. Jpn. Soc. Hortic. Sci. 1993, 62, 249–255. [Google Scholar] [CrossRef]
- Doligez, A.; Adam-Blondon, A.F.; Cipriani, G.; Di Gaspero, G.; Laucou, V.; Merdinoglu, D.; Meredith, C.P.; Riaz, S.; Roux, C.; This, P. An Integrated SSR Map of Grapevine Based on Five Mapping Populations. Theor. Appl. Genet. 2006, 113, 369–382. [Google Scholar] [CrossRef]
- Bennici, S.; Di Guardo, M.; Distefano, G.; La Malfa, S.; Puglisi, D.; Arcidiacono, F.; Ferlito, F.; Deng, Z.; Gentile, A.; Nicolosi, E. Influence of the Genetic Background on the Performance of Molecular Markers Linked to Seedlessness in Table Grapes. Sci. Hortic. 2019, 252, 316–323. [Google Scholar] [CrossRef]
- Bennici, S.; Las Casas, G.; Distefano, G.; Guardo, M.D.; Continella, A.; Ferlito, F.; Gentile, A.; La Malfa, S. Elucidating the Contribution of Wild Related Species on Autochthonous Pear Germplasm: A Case Study from Mount Etna. PLoS ONE 2018, 13, e0198512. [Google Scholar] [CrossRef] [Green Version]
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martinez-Zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic Diversity and Population Structure Assessed by SSR and SNP Markers in a Large Germplasm Collection of Grape. BMC Plant Biol. 2013, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, D.; Brancadoro, L.; Lorenzis, G. De Genetic Diversity and Population Structure in a Vitis spp. Core Collection Investigated by SNP Markers. Diversity 2020, 12, 103. [Google Scholar] [CrossRef] [Green Version]
- Laucou, V.; Launay, A.; Bacilieri, R.; Lacombe, T.; Adam-Blondon, A.F.; Bérard, A.; Chauveau, A.; De Andrés, M.T.; Hausmann, L.; Ibáñez, J.; et al. Extended Diversity Analysis of Cultivated Grapevine Vitis vinifera with 10K Genome-Wide SNPs. PLoS ONE 2018, 13, e0192540. [Google Scholar] [CrossRef] [PubMed]
- Mejía, N.; Soto, B.; Guerrero, M.; Casanueva, X.; Houel, C.; de los Ángeles Miccono, M.; Ramos, R.; Le Cunff, L.; Boursiquot, J.M.; Hinrichsen, P.; et al. Molecular, Genetic and Transcriptional Evidence for a Role of VvAGL11 in Stenospermocarpic Seedlessness in Grapevine. BMC Plant Biol. 2011, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamini, C.; Cardone, M.F.; Anaclerio, A.; Perniola, R.; Pichierri, A.; Genghi, R.; Alba, V.; Forleo, L.R.; Caputo, A.R.; Montemurro, C.; et al. Validation Assay of P3-VvAGL11 Marker in a Wide Range of Genetic Background for Early Selection of Stenospermocarpy in Vitis vinifera L. Mol. Biotechnol. 2013, 54, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Malabarba, J.; Buffon, V.; Mariath, J.E.A.; Maraschin, F.S.; Margis-Pinheiro, M.; Pasquali, G.; Revers, L.F. Manipulation of VviAGL11 Expression Changes the Seed Content in Grapevine (Vitis vinifera L.). Plant Sci. 2018, 269, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlito, F.; Distefano, G.; Gentile, A.; Allegra, M.; Lakso, A.N.; Nicolosi, E. Scion–Rootstock Interactions Influence the Growth and Behaviour of the Grapevine Root System in a Heavy Clay Soil. Aust. J. Grape Wine Res. 2020, 26, 68–78. [Google Scholar] [CrossRef]
- Rana, G.; Katerji, N.; Introna, M.; Hammami, A. Microclimate and Plant Water Relationship of the “Overhead” Table Grape Vineyard Managed with Three Different Covering Techniques. Sci. Hortic. 2004, 102, 105–120. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono and Dicotyledonous Plants; BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry: Berlin/Braunschweig, Germany, 2001. [Google Scholar]
- Emershad, R.; Ramming, D. Somatic Embryogenesis and Plant Development from Immature Zygotic Embryos of Seedless Grapes (Vitis vinifera L.). Plant Cell Rep. 1994, 14, 6–12. [Google Scholar] [CrossRef]
- Wang, W.Q.; Song, S.Q.; Li, S.H.; Gan, Y.Y.; Wu, J.H.; Cheng, H.Y. Quantitative Description of the Effect of Stratification on Dormancy Release of Grape Seeds in Response to Various Temperatures and Water Contents. J. Exp. Bot. 2009, 60, 3397–3406. [Google Scholar] [CrossRef] [Green Version]
- Ergenoglu, F.; Tangolar, S.; Gok, S. The Effects of Some Pre-Treatments for Promoting Germination of Grape Seeds. Acta Hortic. 1997, 441, 207–212. [Google Scholar] [CrossRef]
- Doyle, J. DNA Protocols for Plants. In Molecular Techniques in Taxonomy; NATO ASI Series; Springer: Berlin, Germany, 1991; Volume 57, pp. 283–293. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; R Package Version 2.2.5.; Northwestern University: Evanston, IL, USA, 2020; Available online: https://cran.r-project.org/package=psych (accessed on 1 March 2022).
- Li, Z.; Li, T.; Wang, Y.; Xu, Y. Breeding New Seedless Grapes Using in Ovulo Embryo Rescue and Marker-Assisted Selection. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 241–248. [Google Scholar] [CrossRef]
- Motosugi, H.; Naruo, T. Production of Triploid Grape Rootstocks by Embryo Culture and Their Growth Characteristics. J. Jpn. Soc. Hortic. Sci. 2003, 72, 107–115. [Google Scholar] [CrossRef] [Green Version]

| Female Parent | Male Parent | Cross Acronym | |
|---|---|---|---|
| seedless female parent | Sublima | Sugraone | SubxSug |
| Sublima | Italia2 | SubxIta2 (2017) | |
| SubxIta2 (2018) | |||
| Sugraone | Italia2 | SugxIta2 | |
| Sugraone | Italia | SugxIta | |
| Sugraone | Muscat of Hamburg | SugxMoH | |
| Crimson | Muscat of Hamburg | CrixMoH | |
| Crimson | Italia | CrixIta | |
| Summer royal | Black magic | SuRxBlM | |
| Summer royal | Victoria | SuRxVit | |
| Summer royal | Italia2 | SuRxIta2 | |
| Luisa | Sublima | LuixSub | |
| Luisa | Italia2 | LuixIta2 | |
| Luisa | Italia | LuixIta | |
| seeded female parent | Italia | Sublima | ItaxSub |
| Italia | Luisa | ItaxLui | |
| Italia | Sugraone | ItaxSug | |
| Italia | Crimson | ItaxCri | |
| Italia | Pink muscat | ItaxPMu | |
| Italia2 | Sublima | Ita2xSub | |
| Italia2 | Sugraone | Ita2xSug | |
| Italia2 | Pink Muscat | Ita2xPMu | |
| Muscat of Hamburg | Sugraone | MoHxSug | |
| Muscat of Hamburg | Pink muscat | MoHxPMu |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puglisi, D.; Las Casas, G.; Ferlito, F.; Nicolosi, E.; Di Guardo, M.; Scollo, F.; Saitta, G.; La Malfa, S.; Gentile, A.; Distefano, G. Parents’ Selection Affects Embryo Rescue, Seed Regeneration and the Heredity of Seedless Trait in Table Grape Breeding Programs. Agriculture 2022, 12, 1096. https://doi.org/10.3390/agriculture12081096
Puglisi D, Las Casas G, Ferlito F, Nicolosi E, Di Guardo M, Scollo F, Saitta G, La Malfa S, Gentile A, Distefano G. Parents’ Selection Affects Embryo Rescue, Seed Regeneration and the Heredity of Seedless Trait in Table Grape Breeding Programs. Agriculture. 2022; 12(8):1096. https://doi.org/10.3390/agriculture12081096
Chicago/Turabian StylePuglisi, Damiano, Giuseppina Las Casas, Filippo Ferlito, Elisabetta Nicolosi, Mario Di Guardo, Francesco Scollo, Giuseppe Saitta, Stefano La Malfa, Alessandra Gentile, and Gaetano Distefano. 2022. "Parents’ Selection Affects Embryo Rescue, Seed Regeneration and the Heredity of Seedless Trait in Table Grape Breeding Programs" Agriculture 12, no. 8: 1096. https://doi.org/10.3390/agriculture12081096
APA StylePuglisi, D., Las Casas, G., Ferlito, F., Nicolosi, E., Di Guardo, M., Scollo, F., Saitta, G., La Malfa, S., Gentile, A., & Distefano, G. (2022). Parents’ Selection Affects Embryo Rescue, Seed Regeneration and the Heredity of Seedless Trait in Table Grape Breeding Programs. Agriculture, 12(8), 1096. https://doi.org/10.3390/agriculture12081096













