Utilizing Urea–Chitosan Nanohybrid for Minimizing Synthetic Urea Application and Maximizing Oryza sativa L. Productivity and N Uptake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Chitosan–Urea Nanohybrid
2.3. Field Experimental Details
2.4. Experimental Design and Treatments
2.5. Experimental Details
2.6. Measurements
2.6.1. Growth Traits
2.6.2. Yield and Its Components
2.6.3. Nutrients Uptake
2.7. Statistical Analysis
3. Results
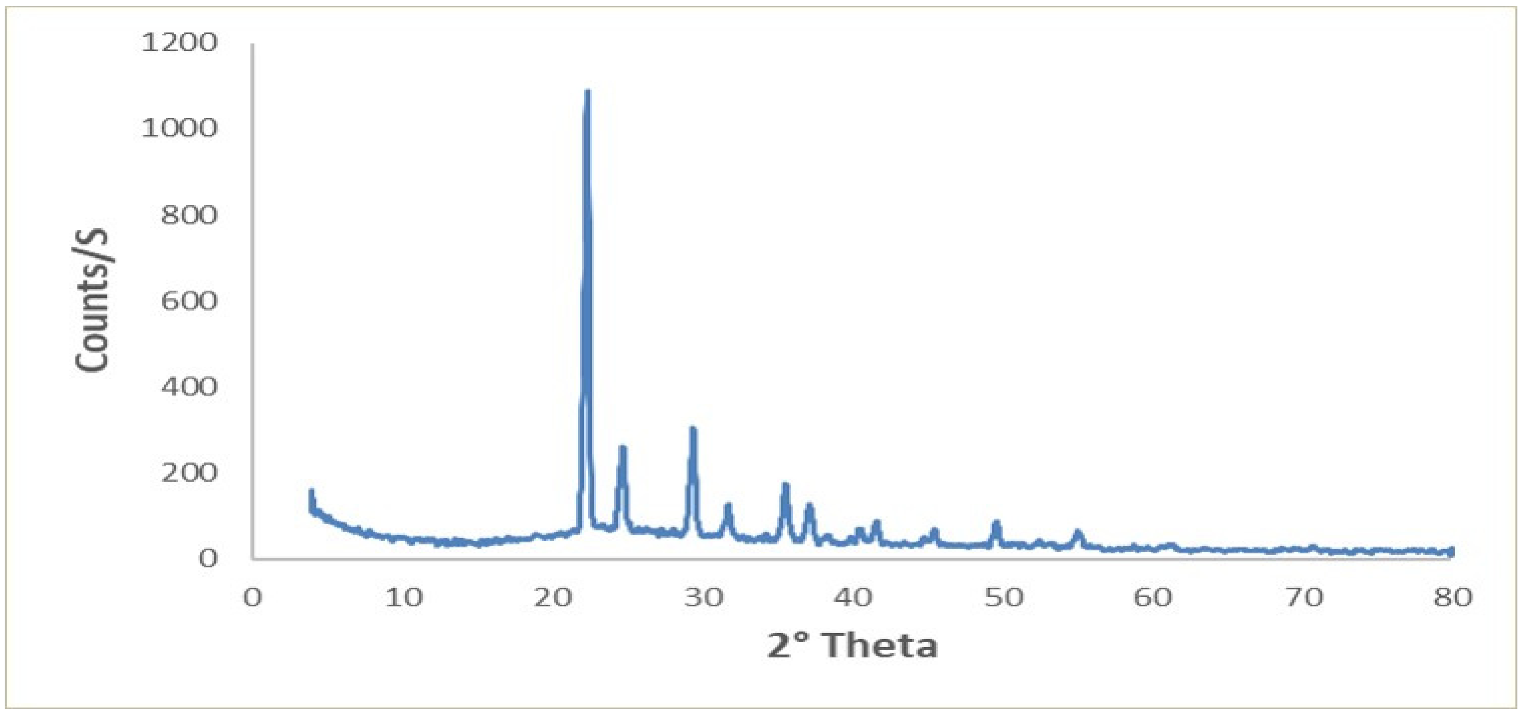
3.1. X-ray Diffraction (XRD) Pattern of CS–Urea NC
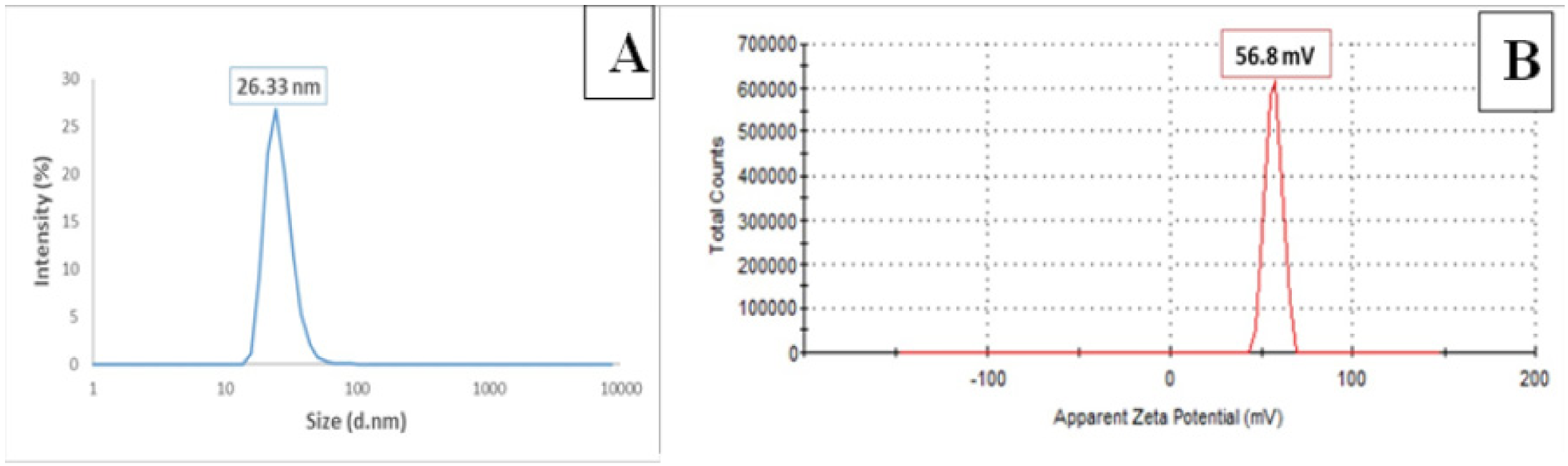
3.2. Dynamic Light Scattering (DLS) Analysis
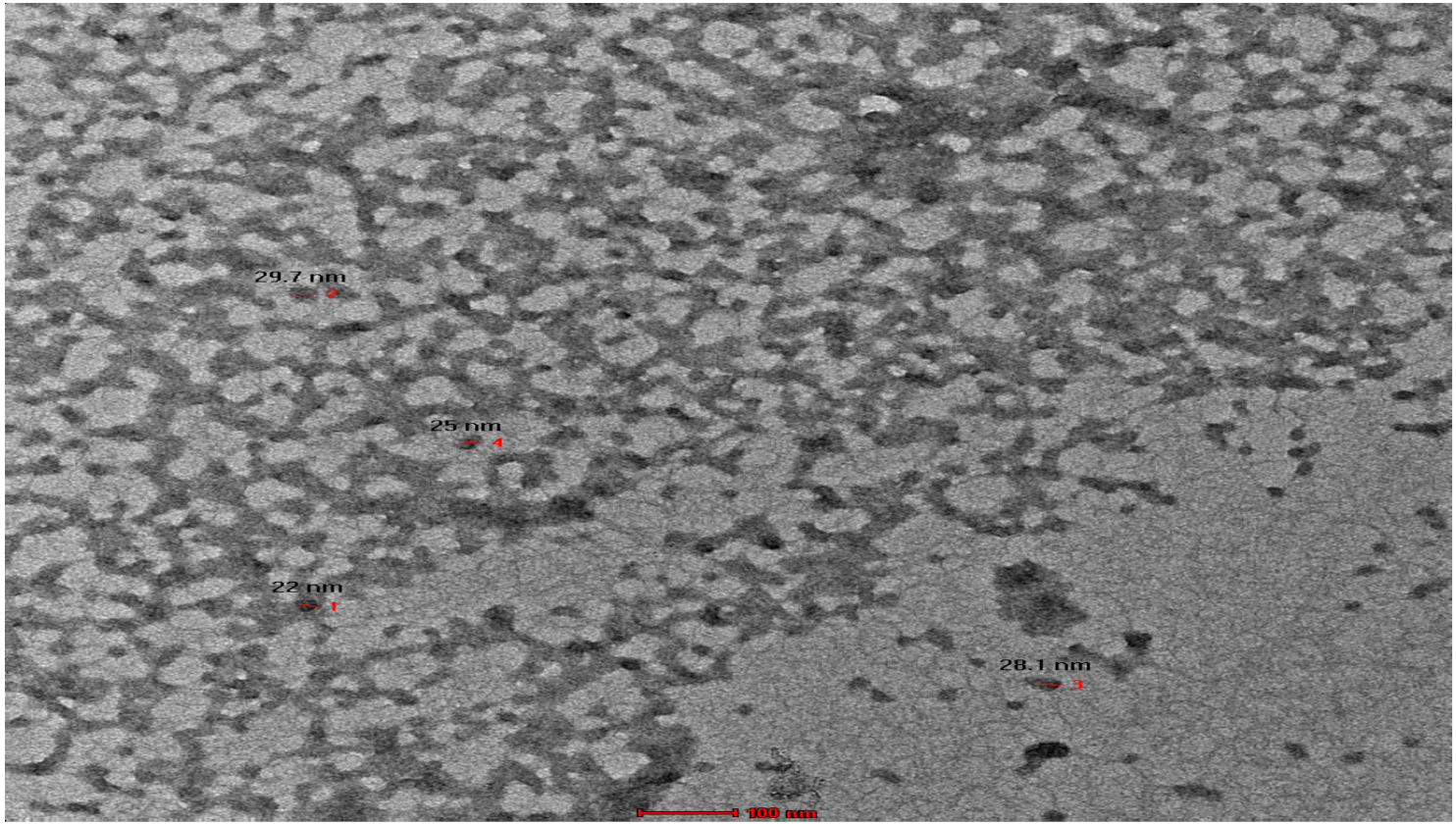
3.3. TEM Analysis Result
3.4. Effects of Different Rates of Classical Urea Fertilizer, Urea Chitosan Nanohybrid and Their Combinations on Rice Growth Traits
3.5. Effects of Different Rates of Classical Urea Fertilizer, Urea Chitosan Nanohybrid, and Their Combinations on Rice Yield and Related Traits
3.6. Effects of Different Rates of Classical Urea Fertilizer, Urea Chitosan Nanohybrid, and Their Combinations on N Content and Uptake
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borlaug, N.E. The Green Revolution Revisited and the Road Ahead; Nobelprize.org: Stockholm, Sweden, 2002. [Google Scholar]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2021, 10, 2. [Google Scholar] [CrossRef]
- Eid, M.A.M.; Abdel-Salam, A.A.; Salem, H.M.; Mahrous, S.E.; Seleiman, M.F.; Alsadon, A.A.; Solieman, T.H.I.; Ibrahim, A.A. Interaction Effects of Nitrogen Source and Irrigation Regime on Tuber Quality, Yield, and Water Use Efficiency of Solanum tuberosum L. Plants 2020, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Soloveichik, G. Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process. Nat. Catal. 2019, 2, 377–380. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Mahmood, A.; Awan, M.I.; Barbanti, L.; Seleiman, M.F.; Bakhsh, G.; Alkharabsheh, H.M.; Babur, E.; Shao, J.; et al. Management Strategies to Mitigate N2O Emissions in Agriculture. Life 2022, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Ladha, J.; Tirol-Padre, A.; Reddy, C.; Cassman, K.; Verma, S.; Powlson, D.; Van Kessel, C.; Richter, D.d.B.; Chakraborty, D.; Pathak, H. Global nitrogen budgets in cereals: A 50-year assessment for maize, rice and wheat production systems. Sci. Rep. 2016, 6, 19355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Kobayashi, K.; Loladze, I.; Zhu, J.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, eaaq1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Omara, P.; Aula, L.; Oyebiyi, F.; Raun, W.R. World cereal nitrogen use efficiency trends: Review and current knowledge. Agrosystems Geosci. Environ. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.A.; Mahdi, A.H.A.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of Nano Silicon and Nano Selenium on Root Characters, Growth, Ion Selectivity, Yield, and Yield Components of Rice (Oryza sativa L.) under Salinity Conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Hussain, S.; Huang, J.; Huang, J.; Ahmad, S.; Nanda, S.; Anwar, S.; Shakoor, A.; Zhu, C.; Zhu, L.; Cao, X. Rice production under climate change: Adaptations and mitigating strategies. In Environment, Climate, Plant and Vegetation Growth; Springer: Berlin/Heidelberg, Germany, 2020; pp. 659–686. [Google Scholar]
- Madruga, D.G. Importance of air quality networks in controlling exposure to air pollution. In Environmental Emissions; IntechOpen: London, UK, 2020. [Google Scholar]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Mohanty, S.; Wassmann, R.; Nelson, A.; Moya, P.; Jagadish, S. Rice and Climate Change: Significance for Food Security and Vulnerability; The International Rice Research Institute: Los Banos, Philippines, 2013; Volume 14, pp. 1–14. [Google Scholar]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Hafez, E.M. Optimizing inputs management for sustainable agricultural development. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt; Springer: Berlin/Heidelberg, Germany, 2021; pp. 487–507. [Google Scholar]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Zhang, R.; Wang, W.; Zhao, X.; Du, Y.; Yin, H. Effects of chitosan oligosaccharides on the yield components and production quality of different wheat cultivars (Triticum aestivum L.) in Northwest China. Field Crops Res. 2015, 172, 11–20. [Google Scholar] [CrossRef]
- Kumar, V.; Sangeetha, K.; Ajitha, P.; Aisverya, S.; Sashikala, S.; Sudha, P. Chitin and Chitosan: The Defense Booster in Agricultural Field. In Handbook of Biopolymers; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2018; pp. 93–134. [Google Scholar]
- Guan, G.; Azad, M.; Kalam, A.; Lin, Y.; Kim, S.W.; Tian, Y.; Liu, G.; Wang, H. Biological effects and applications of chitosan and chito-oligosaccharides. Front. Physiol. 2019, 10, 516. [Google Scholar] [CrossRef] [Green Version]
- Elshayb, O.M.; Nada, A.M.; Sadek, A.H.; Ismail, S.H.; Shami, A.; Alharbi, B.M.; Alhammad, B.A.; Seleiman, M.F. The Integrative Effects of Biochar and ZnO Nanoparticles for Enhancing Rice Productivity and Water Use Efficiency under Irrigation Deficit Conditions. Plants 2022, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Kalita, R.; Saha, O.; Rahman, N.; Tiwari, S.; Phukon, M. Nanotechnology in Agriculture. In Nanobiotechnology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 101–116. [Google Scholar]
- Mahdavi, F.; Abdul, R.S.; Khanif, Y.M. Intercalation of urea into kaolinite for preparation of controlled release fertilizer. Chem. Ind. Chem. Eng. Q. 2014, 20, 207–213. [Google Scholar] [CrossRef]
- Roshanravan, B.; Soltani, S.M.; Rashid, S.A.; Mahdavi, F.; Yusop, M.K. Enhancement of nitrogen release properties of urea–kaolinite fertilizer with chitosan binder. Chem. Speciat. Bioavailab. 2015, 27, 44–51. [Google Scholar] [CrossRef]
- Kondal, R.; Kalia, A.; Krejcar, O.; Kuca, K.; Sharma, S.P.; Luthra, K.; Dheri, G.S.; Vikal, Y.; Taggar, M.S.; Abd-Elsalam, K.A. Chitosan-Urea Nanocomposite for Improved Fertilizer Applications: The Effect on the Soil Enzymatic Activities and Microflora Dynamics in N Cycle of Potatoes (Solanum tuberosum L.). Polymers 2021, 13, 2887. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.M.A.; Hasaneen, M.N.; Omer, A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span. J. Agric. Res. 2016, 14, 17. [Google Scholar]
- Modi, S.; Kumar, S.; Dubey, P. Dynamics of chitosan based NPK-nanofertilizers in greenhouse cucumber production system. J. Environ. Biol. 2021, 42, 162–168. [Google Scholar] [CrossRef]
- Elshamy, M.T.; Husseiny, S.M.; Farroh, K.Y. Application of nano-chitosan NPK fertilizer on growth and productivity of potato plant. J. Sci. Res. Sci. 2019, 36, 424–441. [Google Scholar] [CrossRef] [Green Version]
- Corradini, E.; De Moura, M.; Mattoso, L. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Black, C.A.; Evans, D.D.; Dinauer, R.C. Methods of Soil Analysis; Madison: Madison, WI, USA, 1965. [Google Scholar]
- Radford, P. Growth analysis formulae-their use and abuse 1. Crop Sci. 1967, 7, 171–175. [Google Scholar] [CrossRef]
- Yoshida, S.; Forno, D.A.; Cock, J.H. Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Los Banos, Philippines, 1971; p. 61. [Google Scholar]
- Cock, J.; Yoshida, S.; Forno, D.A. Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Los Banos, Philippines, 1976. [Google Scholar]
- Yoshida, S. Fundamentals of Rice Crop Science; The International Rice Research Institute: Los Banos, Philippines, 1981. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Hsu, J. Multiple Comparisons: Theory and Methods; CRC: New York, NY, USA, 1996; p. 296. [Google Scholar]
- Jatav, G.; Nirmal, D. Application of nanotechnology in soil-plant system. J. Soil Sci. 2013, 8, 176–184. [Google Scholar]
- Mahil, E.; Kumar, B. Foliar application of nanofertilizers in agricultural crops—A review. J. Farm Sci. 2019, 32, 239–249. [Google Scholar]
- Hussain, M.R.; Devi, R.R.; Maji, T.K. Controlled release of urea from chitosan microspheres prepared by emulsification and cross-linking method. Iran. Polym. J. 2012, 21, 473–479. [Google Scholar] [CrossRef]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014, 641759. [Google Scholar] [CrossRef]
- Narayanan, A.; Dhamodharan, R. Super water-absorbing new material from chitosan, EDTA and urea. Carbohydr. Polym. 2015, 134, 337–343. [Google Scholar] [CrossRef]
- Chandrkrachang, S. The application of chitin and chitosan in agriculture in Thailand. Adv. Chitin Sci. 2002, 5, 458–462. [Google Scholar]
- Behboudi, F.; Tahmasebi-Sarvestani, Z.; Kassaee, M.Z.; Modarres-Sanavy, S.A.M.; Sorooshzadeh, A.; Mokhtassi-Bidgoli, A. Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 2019, 42, 1439–1451. [Google Scholar] [CrossRef]
- Mae, T. Physiological nitrogen efficiency in rice: Nitrogen utilization, photosynthesis, and yield potential. Plant Soil 1997, 196, 201–210. [Google Scholar] [CrossRef]
- Chamnanmanoontham, N.; Pongprayoon, W.; Pichayangkura, R.; Roytrakul, S.; Chadchawan, S. Chitosan enhances rice seedling growth via gene expression network between nucleus and chloroplast. Plant Growth Regul. 2015, 75, 101–114. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M.; Liang, R. Preparation and properties of a double-coated slow-release NPK compound fertilizer with superabsorbent and water-retention. Bioresour. Technol. 2008, 99, 547–554. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Fageria, N.; Santos, A.D.; Cutrim, V.A. Dry matter and yield of lowland rice genotypes as influence by nitrogen fertilization. J. Plant Nutr. 2008, 31, 788–795. [Google Scholar] [CrossRef]
- Qiao, J.; Yang, L.; Yan, T.; Xue, F.; Zhao, D. Rice dry matter and nitrogen accumulation, soil mineral N around root and N leaching, with increasing application rates of fertilizer. Eur. J. Agron. 2013, 49, 93–103. [Google Scholar] [CrossRef]
- Lough, T.J.; Lucas, W.J. Integrative plant biology: Role of phloem long-distance macromolecular trafficking. Annu. Rev. Plant Biol. 2006, 57, 203–232. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xie, X.; Zhao, J.; Liu, X.; Feng, W.; White, J.C.; Xing, B. Xylem-and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environ. Sci. Technol. 2012, 46, 4434–4441. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Alotaibi, M.A.; Alhammad, B.A.; Alharbi, B.M.; Refay, Y.; Badawy, S.A. Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy 2020, 10, 790. [Google Scholar] [CrossRef]
- Hasaneen, M.; Abdel-Aziz, H.; El-Bialy, D.; Omer, A.M. Preparation of chitosan nanoparticles for loading with NPK fertilizer. Afr. J. Biotechnol. 2014, 13, 3158–3164. [Google Scholar] [CrossRef] [Green Version]
- Hasaneen, M.; Abdel-Aziz, H.M.M.; Omer, A.M. Effect of foliar application of engineered nanomaterials: Carbon nanotubes NPK and chitosan nanoparticles NPK fertilizer on the growth of French bean plant. Biochem. Biotechnol. Res. 2016, 4, 68–76. [Google Scholar]
- Martens, D.; Westermann, D. Fertilizer applications for correcting micronutrient deficiencies. Micronutr. Agric. 1991, 4, 549–592. [Google Scholar]
- Alloway, B.J. Micronutrients and crop production: An introduction. In Micronutrient Deficiencies in Global Crop Production; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–39. [Google Scholar]
- Hasegawa, H. High-yielding rice cultivars perform best even at reduced nitrogen fertilizer rate. Crop Sci. 2003, 43, 921–926. [Google Scholar] [CrossRef]
- Pal, R.; Mahajan, G.; Sardana, V.; Chauhan, B. Impact of sowing date on yield, dry matter and nitrogen accumulation, and nitrogen translocation in dry-seeded rice in North-West India. Field Crops Res. 2017, 206, 138–148. [Google Scholar] [CrossRef]
- Gewaily, E.; Hamad, H.S.; Arafat, E. Optimizing Sowing Date and Nitrogen Fertilizer Level for the New Rice Variety Sakha Super 300. J. Plant Prod. 2019, 10, 777–784. [Google Scholar] [CrossRef]
- Peng, J.; Feng, Y.; Wang, X.; Li, J.; Xu, G.; Phonenasay, S.; Luo, Q.; Han, Z.; Lu, W. Effects of nitrogen application rate on the photosynthetic pigment, leaf fluorescence characteristics, and yield of indica hybrid rice and their interrelations. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Masoni, A.; Ercoli, L.; Mariotti, M.; Arduini, I. Post-anthesis accumulation and remobilization of dry matter, nitrogen and phosphorus in durum wheat as affected by soil type. Eur. J. Agron. 2007, 26, 179–186. [Google Scholar] [CrossRef]
- Dordas, C. Dry matter, nitrogen and phosphorus accumulation, partitioning and remobilization as affected by N and P fertilization and source–sink relations. Eur. J. Agron. 2009, 30, 129–139. [Google Scholar] [CrossRef]
- Ntanos, D.A.; Koutroubas, S. Dry matter and N accumulation and translocation for Indica and Japonica rice under Mediterranean conditions. Field Crops Res. 2002, 74, 93–101. [Google Scholar] [CrossRef]
- Kamiji, Y.; Yoshida, H.; Palta, J.A.; Sakuratani, T.; Shiraiwa, T. N applications that increase plant N during panicle development are highly effective in increasing spikelet number in rice. Field Crops Res. 2011, 122, 242–247. [Google Scholar] [CrossRef]
- Xiong, J.; Ding, C.Q.; Wei, G.B.; Ding, Y.F.; Wang, S.H. Characteristic of dry-matter accumulation and nitrogen-uptake of super-high-yielding early rice in China. Agron. J. 2013, 105, 1142–1150. [Google Scholar] [CrossRef]
- Reddy, N.P.; Rao, C.B.B.; Surekha, K.; Hussain, S. Growth of Transplanted Rice as Influenced by Enriched Nitrogen Sources at Different Levels. Int. Res. J. Pure Appl. Chem. 2020, 21, 114–119. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Lieffering, M.; Kobayashi, K.; Okada, M.; Mitchell, M.W.; Gumpertz, M. Effects of free-air CO2 enrichment and nitrogen supply on the yield of temperate paddy rice crops. Field Crops Res. 2003, 83, 261–270. [Google Scholar] [CrossRef]
- Harada, J.; Arima, S.; Shibayama, H.; Kabashima, R. Effect of chitosan application on growth and seed yield of soybean. Mar. Highl. Biosci. Cent. Rep. 1995, 2, 15–19. [Google Scholar]
- Sultanaa, S. Summaries of Country Reports on Radiation Processing and Application of Chitosan. Annex 2010, 4, 1. [Google Scholar]
- Wei, H.; Li, H.; Cheng, J.; Zhang, H.; Huo, Z.; Xu, K.; Guo, B.; Hu, Y.; Cui, P. Effects of slow/controlled release fertilizer types and their application regime on yield in rice with different types of panicle. Acta Agron. Sin. 2017, 43, 730–740. [Google Scholar] [CrossRef]
- Gharieb, A. NPK Compound Fertilizer Foliar Application Impacts Productivity and Grain Quality of Rice. Alex. J. Agric. Sci. 2021, 66, 13–21. [Google Scholar] [CrossRef]
| Minimum Temperature (°C) | Maximum Temperature (°C) | Relative Humidity (%) | |
|---|---|---|---|
| first (2020) season | |||
| June | 23.8 | 32.0 | 56.7 |
| July | 27.3 | 33.7 | 67.7 |
| August | 28.2 | 34.6 | 67.5 |
| September | 27.1 | 34.2 | 67.2 |
| October | 24.6 | 31.5 | 65.9 |
| second (2021) season | |||
| June | 25.0 | 33.1 | 60.0 |
| July | 26.4 | 34.2 | 67.9 |
| August | 28.8 | 34.3 | 66.8 |
| September | 27.4 | 33.8 | 68.1 |
| October | 23.8 | 31.2 | 66.4 |
| Character | 2020 | 2021 |
|---|---|---|
| physical analysis | ||
| Texture | Clay | Clay |
| Sand (%) | 12.80 | 12.60 |
| Silt (%) | 32.00 | 31.40 |
| Clay (%) | 55.20 | 56.00 |
| Chemical analysis | ||
| pH (1:2.5 soil extract) | 8.30 | 8.11 |
| EC (dSm−1) | 2.03 | 2.0 |
| Organic matter % | 1.70 | 1.53 |
| NH4 * (ppm) | 12.60 | 15.70 |
| NO3 * (ppm) | 11.50 | 10.30 |
| P * (ppm) | 12.60 | 14.00 |
| K * (ppm) | 351.20 | 350.40 |
| Zn * (ppm) | 0.90 | 0.95 |
| Mn * (ppm) | 3.81 | 3.88 |
| Fe * (ppm) | 2.96 | 2.94 |
| Treatments | Soil Application Rate of Classical Urea (%) | Exogenous Urea Chitosan Nanohybrid (mg N L−1) |
|---|---|---|
| CU | 0% | 0 mg N L−1 |
| U1 | 100% (165 Kg N ha−1) | 0 mg N L−1 |
| U2 | 80% (132 Kg N ha−1) | 250 mg N L−1 |
| U3 | 80% (132 Kg N ha−1) | 500 mg N L−1 |
| U4 | 60% (99 Kg N ha−1) | 250 mg N L−1 |
| U5 | 60% (99 Kg N ha−1) | 500 mg N L−1 |
| U6 | 40% (66 Kg N ha−1) | 250 mg N L−1 |
| U7 | 40% (66 Kg N ha−1) | 500 mg N L−1 |
| Traits | Chlorophyll Content | Leaf Area Index | Dry Matter Production (g/hill) | ||||
|---|---|---|---|---|---|---|---|
| Treatments | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | |
| CU | 36.11 c | 34.45 d | 5.64 d | 5.35 c | 26.36 d | 25.74 d | |
| U1 | 45.76 a | 43.60 a | 7.54 a | 7.38 a | 51.84 a | 50.69 a | |
| U2 | 44.51 a | 45.32 a | 7.11 ab | 6.93 ab | 49.31 a | 47.80 ab | |
| U3 | 45.26 a | 44.20 a | 7.68 a | 7.71 a | 52.32 a | 51.04 a | |
| U4 | 42.32 ab | 40.86 bc | 6.90 b | 6.64 ab | 43.63 ab | 42.36 bc | |
| U5 | 44.25 a | 43.10 a | 7.22 ab | 7.10 ab | 48.86 a | 48.14 ab | |
| U6 | 37.83 c | 36.92 cd | 6.01 d | 5.45 c | 33.65 c | 33.47 cd | |
| U7 | 38.64 bc | 39.62 bc | 6.63 b | 6.15 b | 38.23 b | 37.01 cd | |
| F. test | ** | ** | ** | ** | ** | ** | |
| Traits | Plant Height (cm) | Number of Panicles per Hill | Panicle Weight (gm) | ||||
|---|---|---|---|---|---|---|---|
| Treatments | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | |
| CU | 98.6 d | 96.9 d | 13.26 cd | 12.18 c | 2.51 c | 2.32 bc | |
| U1 | 123.8 ab | 124.9 a | 24.16 a | 24.77 a | 3.94 ab | 4.07 a | |
| U2 | 122.7 ab | 123.7 a | 22.76 ab | 21.07 ab | 3.48 ab | 3.67 a | |
| U3 | 126.4 a | 123.7 a | 25.34 a | 22.16 ab | 4.31 a | 4.20 a | |
| U4 | 120.2 ab | 119.8 ab | 19.02 bc | 17.27 b | 3.22 b | 3.13 ab | |
| U5 | 122.2 ab | 121.9 a | 22.40 ab | 20.57 ab | 3.68 ab | 3.60 a | |
| U6 | 107.6 c | 108.5 c | 15.10 cd | 13.24 c | 2.88 bc | 2.57 b | |
| U7 | 111.4 c | 113.7 c | 17.15 bc | 16.38 b | 3.01 bc | 2.91 b | |
| F. test | ** | ** | ** | ** | ** | ** | |
| Traits | Panicle Length (cm) | Number of Filled Grains/Panicle | Number of Empty Grains/Panicle | ||||
|---|---|---|---|---|---|---|---|
| Treatments | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | |
| CU | 17.10 c | 16.66 c | 110.6 c | 107.8 d | 17.0 a | 19.6 a | |
| U1 | 22.84 a | 23.37 a | 151.8 a | 150.1 a | 7.5 c | 8.3 c | |
| U2 | 21.23 ab | 20.78 ab | 149.1 a | 146.5 a | 6.5 c | 7.4 c | |
| U3 | 23.72 a | 23.48 a | 156.3 a | 150.7 a | 5.1 c | 6.0 cd | |
| U4 | 19.38 b | 19.44 b | 132.2 c | 131.4 b | 9.2 bc | 10.8 bc | |
| U5 | 21.08 ab | 21.35 ab | 148.1 a | 147.3 a | 8.3 bc | 8.7 c | |
| U6 | 18.65 bc | 17.31 bc | 121.8 b | 119.6 c | 12.8 b | 14.2 b | |
| U7 | 19.30 b | 17.92 bc | 130.6 b | 127.3 b | 11.6 b | 11.3 b | |
| F. test | ** | ** | ** | ** | ** | ** | |
| Traits | 1000-Grain Weight (g) | Grain Productivity (t ha−1) | Straw Yield (t ha−1) | ||||
|---|---|---|---|---|---|---|---|
| Treatments | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | |
| CU | 28.23 c | 28.04 c | 8.10 c | 7.87 cd | 10.82 c | 9.91 c | |
| UI | 31.08 a | 30.65 ab | 12.03 a | 12.40 a | 14.63 a | 15.10 a | |
| U2 | 30.28 a | 30.41 ab | 11.78 ab | 11.40 ab | 13.92 ab | 13.61 ab | |
| U3 | 30.94 a | 31.23 a | 12.58 a | 12.45 a | 15.45 a | 15.18 a | |
| U4 | 29.43 b | 29.13 b | 10.84 b | 10.53 b | 13.34 b | 12.84 b | |
| U5 | 30.12 ab | 30.10 ab | 11.34 ab | 11.23 ab | 14.10 ab | 13.65 ab | |
| U6 | 28.87 c | 28.44 bc | 9.10 c | 8.79 c | 11.42 c | 11.18 bc | |
| U7 | 29.21 b | 29.03 b | 10.22 b | 9.95 b | 12.78 bc | 12.37 b | |
| F. test | ** | ** | ** | ** | ** | ** | |
| Traits | N Content in Grain Yield (%) | N Content in Straw Yield (%) | |||
|---|---|---|---|---|---|
| Treatments | Season 1 | Season 2 | Season 1 | Season 2 | |
| CU | 0.964 c | 0.927 d | 0.301 c | 0.283 c | |
| UI | 1.397 a | 1.337 ab | 0.434 a | 0.416 ab | |
| U2 | 1.348 ab | 1.328 ab | 0.410 ab | 0.393 ab | |
| U3 | 1.421 a | 1.447 a | 0.484 a | 0.481 a | |
| U4 | 1.226 bc | 1.203 bc | 0.378 b | 0.338 bc | |
| U5 | 1.278 abc | 1.276 ab | 0.391 ab | 0.382 ab | |
| U6 | 1.027 bc | 0.993 c | 0.336 bc | 0.296 c | |
| U7 | 1.082 bc | 1.051 bc | 0.367 b | 0.331 bc | |
| F. test | ** | ** | ** | ** | |
| Traits | N Uptake in Grain Yield (kg ha−1) | N Uptake in Straw Yield (kg ha−1) | |||
|---|---|---|---|---|---|
| Treatments | Season 1 | Season 2 | Season 1 | Season 2 | |
| CU | 78.1 c | 72.9 d | 32.5 c | 28.0 c | |
| UI | 168.0 a | 162.5 ab | 63.4 ab | 62.8 ab | |
| U2 | 152.8 ab | 149.1 ab | 57.8 ab | 53.6 ab | |
| U3 | 178.8 a | 180.1 a | 74.7 a | 73.0 a | |
| U4 | 132.8 abc | 126.6 bc | 45.1 bc | 43.4 bc | |
| U5 | 144.9 ab | 143.2 ab | 55.2 ab | 52.1 ab | |
| U6 | 93.5 bc | 87.2 c | 38.4 bc | 33.1 c | |
| U7 | 110.6 bc | 104.5 cd | 46.9 bc | 40.9 bc | |
| F. test | ** | ** | ** | ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshayb, O.M.; Nada, A.M.; Farroh, K.Y.; AL-Huqail, A.A.; Aljabri, M.; Binothman, N.; Seleiman, M.F. Utilizing Urea–Chitosan Nanohybrid for Minimizing Synthetic Urea Application and Maximizing Oryza sativa L. Productivity and N Uptake. Agriculture 2022, 12, 944. https://doi.org/10.3390/agriculture12070944
Elshayb OM, Nada AM, Farroh KY, AL-Huqail AA, Aljabri M, Binothman N, Seleiman MF. Utilizing Urea–Chitosan Nanohybrid for Minimizing Synthetic Urea Application and Maximizing Oryza sativa L. Productivity and N Uptake. Agriculture. 2022; 12(7):944. https://doi.org/10.3390/agriculture12070944
Chicago/Turabian StyleElshayb, Omnia M., Abdelwahed M. Nada, Khaled Y. Farroh, Arwa Abdulkreem AL-Huqail, Maha Aljabri, Najat Binothman, and Mahmoud F. Seleiman. 2022. "Utilizing Urea–Chitosan Nanohybrid for Minimizing Synthetic Urea Application and Maximizing Oryza sativa L. Productivity and N Uptake" Agriculture 12, no. 7: 944. https://doi.org/10.3390/agriculture12070944
APA StyleElshayb, O. M., Nada, A. M., Farroh, K. Y., AL-Huqail, A. A., Aljabri, M., Binothman, N., & Seleiman, M. F. (2022). Utilizing Urea–Chitosan Nanohybrid for Minimizing Synthetic Urea Application and Maximizing Oryza sativa L. Productivity and N Uptake. Agriculture, 12(7), 944. https://doi.org/10.3390/agriculture12070944








