Abstract
Many effects of polyamines (PAs) are well known in plant developmental processes; however, the significance of their catabolism is not well understood. Copper amine oxidase (CuAO) is involved in the degradation of diamine putrescine (Put). The genotype dependence and time-dependent effects of this enzyme are rarely examined, so this study aims to discover the role of CuAO in tomato genotypes in different stages of seedling development. Exogenously applied L-aminoguanidine (AG), a CuAO inhibitor, was used to decrease the activity of CuAOs. Based on our results, it can be concluded that there is a genotype dependence of Put degradation, and AG treatment caused a long-term shift of PA catabolism by changing the activities of polyamine oxidase (PAO), catalyzing the degradation of higher PAs. Our results demonstrate that the modification of PA catabolism could have long-term results in polyamine metabolism in different tomato genotypes.
1. Introduction
The tomato (Solanum lycopersicum Mill.L.) is the second most important and widely consumed vegetable crop plant next to the potato in the world [1]. Its importance is not only for the food industry but also in human medicine. The biodiversity of tomatoes is extremely high because of the huge numbers of genotypes [2]. Despite the enormous amount of data and the number of studies that have been generated about the tomato stress responses, the genotype-dependent growth responses are rarely examined.
Polyamines are essential polycationic molecules contributing to the growth and development of plants. Their metabolism plays a significant role in plant cells acting similar to a hub [3,4,5].
PA catabolism is one of the most complex processes of these plant-growth-promoting compounds [6]. Two types of enzymes catalyze the PA degradation: the copper-containing diamine oxidase (DAO or CuAOs, EC 1.4.3.6) and the FAD-containing polyamine oxidase (PAO, EC 1.5.3.11) [7]. During catabolic processes, hydrogen peroxide (H2O2) could be synthesized as a by-product contributing to inducing signal pathways involved in plant development [8,9].
CuAOs degrade putrescine into reactive aldehydes, ammonia, and H2O2, contributing to the biosynthesis of gamma amino butyric acid (GABA). Reduced CuAO activities could alter the development of seedlings, as Alharbi et al. [10] showed in Arabidopsis thaliana.
Recently, Fraudentali et al. [11] summarized and characterized four members of the CuAO gene family in Arabidopsis thaliana and revealed the developmental, hormonal, and stress modulation of their gene expression. However, the participation of CuAOs in early plant growth and development remains elusive. Additionally, the question of how reduced CuAO activity could affect the further development of plants remains unanswered.
We aimed to compare three commercially available tomato cultivars in the case of their polyamine catabolism and investigate the involvement of copper amine oxidase in these alterations. Our work could enhance our knowledge about the polyamine catabolism of tomatoes to make them efficient for further agricultural practices and build their resistance to stress factors.
2. Materials and Methods
2.1. Plant Materials and Inhibitor Application
Plants of commercial tomato cultivars (Solanum lycopersicum L. cvar Rio Fuego, Tigerella, and Romus) were grown in a hydroponic system under greenhouse conditions at the Department of Plant Biology, University of Szeged. The cultivars used for this study are different in their growth type (Rio Fuego—determinate, Tigerella—half-determinate, Romus—indeterminate) from Rédei Kertimag (Budapest, Hungary). Seeds of tomato cultivars were germinated at 26 °C for 3 d in dark, and seedlings were subsequently transferred to perlite for 1 week [12] and grown in a controlled environment under 200 µmol m−2 s−1 photon flux density (F36W/GRO lamps, OSRAM SYLVANIA, Danvers, MA, USA), with 12/12 h light/dark period, a day/night temperatures of 24/22 °C, and relative humidity of 55–60%. The modified Hoagland nutrient solution was used for irrigation; the pH was 5.8. Samples were taken from seedlings at 3 days after germination (DAG) and at 10 DAG grown in hydroponic culture in the greenhouse. The experiments were repeated three times. L-aminoguanidine (AG) (Sigma-Aldrich, Merck Gmbh, Germany) was used as DAO inhibitor and was applied to the growth medium at germination for 3 days [13,14,15]. The concentration of AG was 1 mM based on our previous experiments without any toxic effects.
2.2. Biomass Production and Primary Root Growth of Tomato Genotypes
Biomass of plants grown with AG at 3DAG and 10DAG was measured by analytical scale (Adam Equipment NBL2541, Milton Keynes, UK), and the samples were kept at −20 °C for biochemical analyses. The growth of tomato genotypes was monitored by image capture (Canon EOS 700D, Canon, UK) (Figure 1). The primary root growth, shoot length, and root length was analyzed by ruler. The experiments were repeated at least three times.
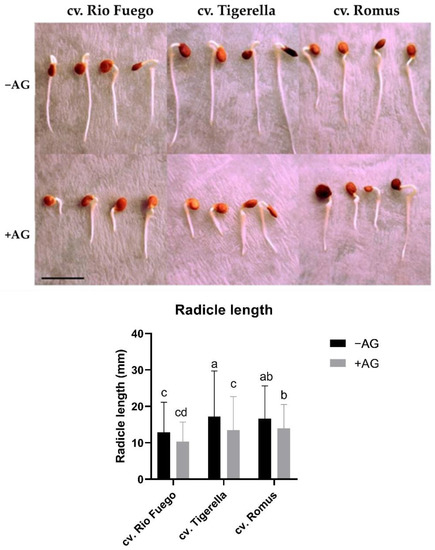
Figure 1.
Germination of tomato seeds of three different cultivars. Representative image of seed and radicles of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 3 DAG. Scale bar = 1 cm. Data of radicle length are the mean ± SD of at least three biological replicates, n = 30. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test, p < 0.05).
2.3. Free Polyamines Analysis by HPLC
The levels of free Put, Spd, and Spm were determined by high-performance liquid chromatography (HPLC) separation of benzoyl-derivatized PAs, as described by Szepesi et al. [12]. In brief, leaf or root samples were homogenized in 5% (v/v) perchloric acid. After centrifugation, the supernatant was neutralized with 2 M NaOH; then, the PAs were derivatized with benzoyl chloride to produce benzoyl-polyamines. Diethyl ether was added to the aqueous phase to obtain organic phase for dryness by vacuum evaporator. Acetonitrile was pipetted to the dry sample and was injected into JASCO HPLC system (JASCO, Tokyo, Japan). Separation was made in a reverse-phase C18 column (250 × 4.6 mm internal diameter, 5 µm particle size; Phenomenex, Torrance, CA, USA). Detection of benzoyl-PAs was made by UV/VIS detector (JASCO HPLC System, Japan) with a wavelength 254 nm. The mobile phase was water/CAN in 55:45 (v:v) ratio with flow rate 0.5 mL min−1. The applied standards were Put, Spd, and Spm in the form of hydrochlorides from Sigma-Aldrich, Merck GMBH, Germany. The results are the means of three independent biological samples expressed in µmol g−1 fresh weight−1.
2.4. Polyamine Catabolism: Diamine (DAO, EC 1.4.3.6) and Polyamine Oxidase (PAO, EC 1.4.3.4)
Copper amine oxidase (CuAO or DAO, EC 1.4.3.6) and polyamine oxidase (PAO, EC 1.4.3.4) activities were analyzed by a spectrophotometric method as described by Moschou et al. [16] with some modification. Fresh plant tissues were ground in liquid N2 to fine powder, and extraction buffer was added to each sample in ratio of 1:3. The extraction buffer contained 0.2 M TRIS (hydroxymethyl)aminomethane (pH 8.0); 10% glycerol; 0.25% Triton X-100; 0.5 mM phenylmethanesulfonyl fluoride (PMSF); 0.01 mM leupeptin. The homogenates were left on ice for 20 min and centrifuged for 10 min at 7000× g at 4 °C (Eppendorf centrifuge 5424R, Eppendorf GMBH, Germany). The reaction mixture contained supernatant and 100 mM potassium phosphate buffer (pH 6.6); then, the reaction was started by adding 1 M Put for DAO or 1 M Spd for PAO activity measurements. The reaction mixture was incubated for 1.5 h at 37 °C, and after, the reaction was stopped by adding 20% (w/v) trichloroacetic acid. To analyze the content of Δ1-pyrroline, degradation products of enzymes, 2-aminobenzaldehyde (from 10 mg mL−1 stock solution) was pipetted to the reaction mixture. After centrifugation, absorbance of the supernatant was determined at 430 nm (KONTRON, Milano, Italy). The enzyme activity was expressed as the specific activity (U g−1 FW), where one unit (U) represents the amount of enzyme catalyzing the formation of 1 µmol of Δ1-pyrroline min−1.
2.5. Hydrogen Peroxide (H2O2) Content Measurements
H2O2 levels of tomato leaf and root tissues were measured by the method of Horváth et al. [17]. A total of 100 mg of leaf or root samples was homogenized in 0.75 mL of ice-cold, 0.1% trichloroacetic acid. After centrifugation, 0.5 mL of supernatant was used for the measurement. The reaction mixture contained 0.5 mL 50 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M potassium iodide (KI). The reaction was started with the addition of the supernatant followed by incubation for 10 min at 25 °C. The absorbance values were recorded by spectrophotometer (KONTRON, Milano, Italy) at 390 nm. A standard curve was prepared by the H2O2 standard. The results were expressed as µmol H2O2 g−1 FW.
2.6. Determination of Photosynthetic Pigment Contents
The photosynthetic pigments were determined by the method of Faragó et al. [18]. Fresh plant tissues were ground by ethanol and homogenate was centrifuged (Eppendorf centrifuge 5424R, Eppendorf GMBH, Germany) at 12,000 rpm, 4 °C, for 10 min. The supernatants were analyzed at wavelengths 664, 648, and 470 nm (Synergy HTX plate reader, BioTek Instruments, Winooski, VT, USA). Pigment contents were normalized for 1 g fresh weight after indicated calculations in Faragó et al., 2018.
2.7. Total Soluble Protein Content
Homogenization of plant tissues and analysis of total soluble protein contents was performed by method of Bradford [19]. Samples were ground by ice-cold phosphate buffer (KH2PO4 and Na2HPO4, 50 mM, pH 7.0) and centrifuged by Eppendorf centrifuge (Eppendorf 5424R, Eppendorf GMBH, Germany) for 10 min at 4 °C. After adding Bradford reagent, the supernatant was measured at 595 nm (Synergy HTX plate reader, BioTek Instruments, Winooski, VT, USA).
2.8. Statistical Analysis
Data presented here are the mean values from at least three independent experiments. Statistical analysis of two-way analysis of variance (ANOVA) was carried out with GraphPad Prism version 8.0.1.244 for Windows (GraphPad Software, La Jolla, CA, USA). Different letters on the bars denote significant differences (p < 0.05) based on Tukey’s post hoc test for multiple comparisons.
3. Results
3.1. Phenotypic Alterations by L-Aminoguanidine (AG) in Tomato Genotypes
3.2. Germination Time Courses of Tomato Seeds
We examined the germination time courses of the tomato genotypes. Rio Fuego and Romus showed better germination time than Tigerella (Figure 2). Supplying AG did not induce any significant changes in germination time, suggesting that AG did not affect the early growth and development of tomato genotypes.
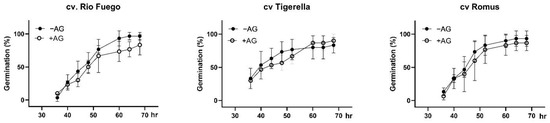
Figure 2.
Germination time courses of tomato seeds. Germination time courses for tomato seeds imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after indicated time points of germination. Germination was scored every 4 h from 30 till 70 h, and results are presented as the germination percentages. Data are the mean ± SD of the three biological replicates of 30 seeds each, n = 30. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05.
3.3. Biomass and Protein Contents of Tomato Genotypes
After three days of germination, the biomass and protein contents were investigated. It can be seen in Figure 3 that there was no difference between biomasses of genotypes; however, in the case of protein contents, Rio Fuego showed the highest protein contents compared to Tigerella and Romus. AG treatment did not induce a significant decrease in biomass. In the case of Rio Fuego, the protein content significantly decreased after AG treatment (Figure 3).
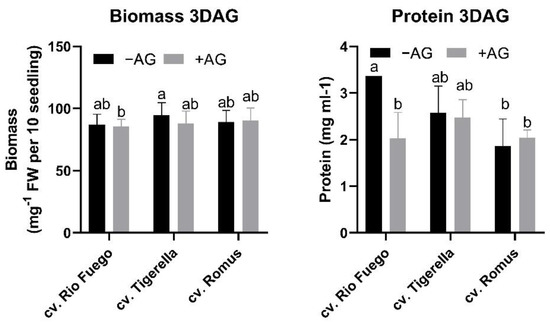
Figure 3.
Biomass and protein levels of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 3 DAG. Data of biomass and protein levels are the mean ± SD of at least three biological replicates, n = 30. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
3.4. Free Polyamine Contents of Tomato Genotypes
As polyamines are essential polycations involved in plant growth and development and stress responses, we analyzed the free polyamine contents of tomato genotypes after three days of germination. Romus has the highest polyamine contents in all three polyamines, putrescine (Put), spermidine (Spd), spermine (Spm) (Figure 4). After AG treatment, only in Romus was there a significant decrease in Put and Spd, which resulted in lower total PA contents in these seedlings (Figure 4 and Figure 5). However, the decrease in Put induced a shift in the ratio of higher PAs to Put, demonstrating that Romus is the most sensitive for AG-induced DAO decreases (Figure 5).
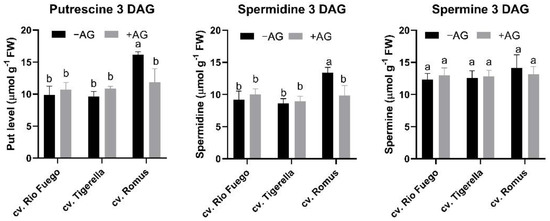
Figure 4.
Free polyamine contents of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 3 DAG. Data of free polyamines are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
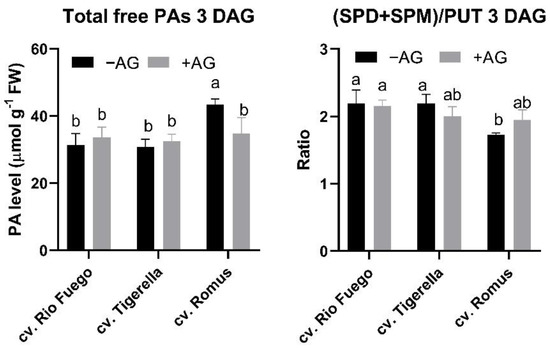
Figure 5.
The ratio of PAs and total free PA contents of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 3 DAG. Data of ratios and total free PAs are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
3.5. Polyamine Catabolism of Tomato Genotypes at 3DAG
AG is reported to induce inhibition of copper amine oxidase (DAO or CuAO). We investigated the activities of enzymes involved in PA degradation in tomato genotypes. It was not a surprise that in all genotypes, AG drastically decreased the DAO enzyme activities. However, PAO activities for degradation of higher polyamines showed the opposite effect in Tigerella and Romus but not in Rio Fuego. It is suggested that the compensation of PA-degradation enzymes could be a genotype-dependent response (Figure 6).
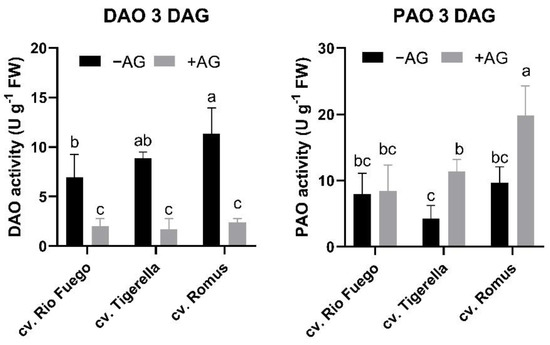
Figure 6.
Polyamine catabolism (DAO and PAO) of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 3 DAG. Data of enzyme activities are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
3.6. Biochemical Parameters of Tomato Seedlings at 10 Days after Germination
To check how AG-induced changes in early development worked as a long-term effect on the growth of tomato genotypes, we grew seedlings in optimal conditions in perlite irrigated with nutrient solution for 1 week. The fresh weight of the tomato genotypes was only altered in the roots of Rio Fuego and in shoots in the case of Tigerella, while the lengths of the shoots and roots did not change significantly irrespective of genotypes (Table 1).

Table 1.
Growth parameters of tomato cultivars after 10 DAG.
3.7. Protein Contents of Tomato Seedlings at 10 Days after Germination
Protein contents from seedlings at 10 days after germination showed some differences between genotypes (Figure 7). In the case of shoots, Rio Fuego indicated higher protein contents; however, roots of Romus had higher protein contents after AG treatment. Interestingly, Tigerella did not show any significant protein level increase compared to the control (Figure 7).
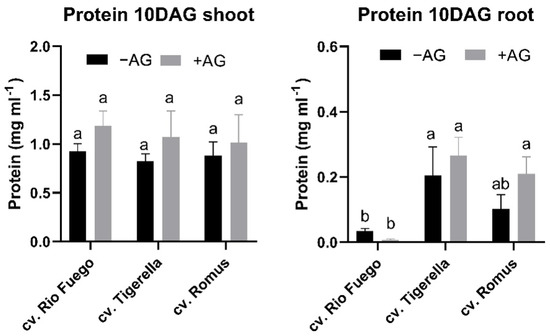
Figure 7.
Protein levels of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 10 DAG. Data of protein levels are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
3.8. Hydrogen Peroxide Contents of Tomato Seedlings at 10 Days after Germination
We observed the contents of H2O2 to investigate any oxidative responses to AG treatment. As can be seen in Figure 8, the H2O2 levels were constant compared to those for AG treatment both in the shoots and roots, indicating that no oxidative stress could occur in these seedlings (Figure 8).
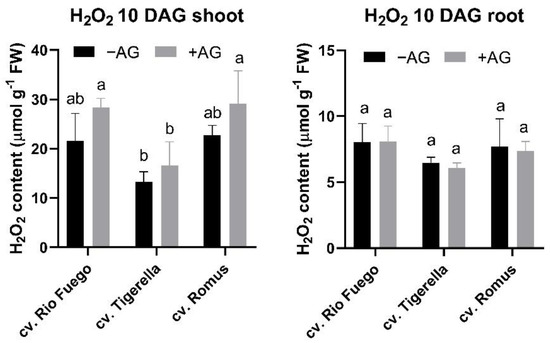
Figure 8.
Hydrogen peroxide levels of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 10 DAG. Data of H2O2 levels are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
3.9. Photosynthetic Pigment Contents of Tomato Seedlings at 10 Days after Germination
Additionally, similar to hydrogen peroxide contents, photosynthetic pigment contents, such as chlorophyll-a, chlorophyll-b, or carotenoids, did not show any significant differences, except for Chl-b in Rio Fuego (Figure 9).
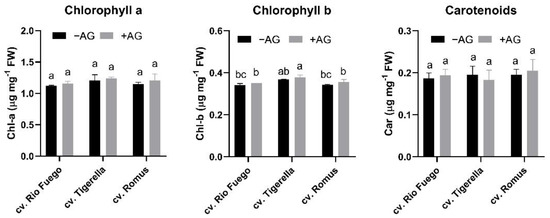
Figure 9.
Pigment contents of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 10 DAG. Data of pigment contents are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
3.10. Free polyamine Contents of Tomato Seedlings at 10 Days after Germination
We investigated how AG treatment could induce long-term alterations in tomato genotypes. Contents of free PAs changed in a genotype-dependent manner at 10 days after germination. AG induced an increase in Put and Spm levels in Tigerella in shoots, but in roots, only Romus showed a Spd increase, but in Tigerella, surprisingly, the Spm levels were eliminated (Figure 10).
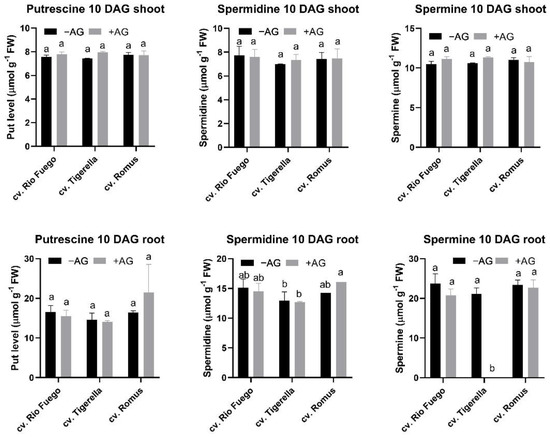
Figure 10.
Free polyamine contents of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 10 DAG. Data of PA contents are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
As can be seen in Figure 11, only Tigerella showed altered total free PAs compared to the control at 10 days of germination.
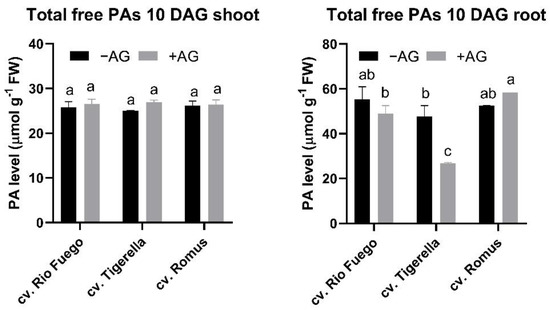
Figure 11.
Total free PA contents of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 10 DAG. Data of ratios and total free PAs are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
3.11. The Ratio of Higher PAs to Put in Tomato Seedlings at 10 Days after Germination
If the ratio of higher PAs to Put is analyzed, AG treatment caused a significant decrease in this ratio in the case of Tigerella, while in Romus, an increase occurred in this ratio (Figure 12).
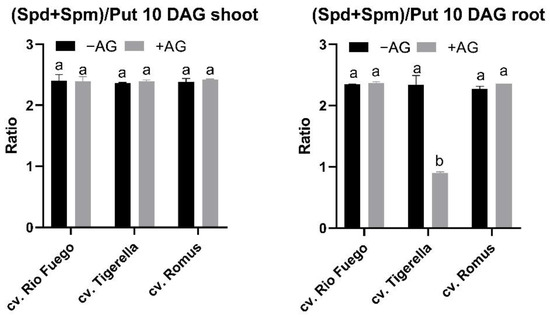
Figure 12.
Ratios of (Spd+Spm)/Put of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 10 DAG. Data of ratios are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
3.12. Polyamine Catabolism of Tomato Seedlings at 10 Days after Germination
To observe the long-term effect of AG on polyamine catabolism, DAO and PAO were investigated in shoots and roots. DAO activities of Rio Fuego remained higher than control shoots (Figure 13), but in PAO activities, more alterations could be seen in Figure 14. Increased PAO in shoots could be seen only in the case of Tigerella, while in roots, the other two genotypes, Rio Fuego and Romus, demonstrated higher PAO activities after AG treatment (Figure 14).
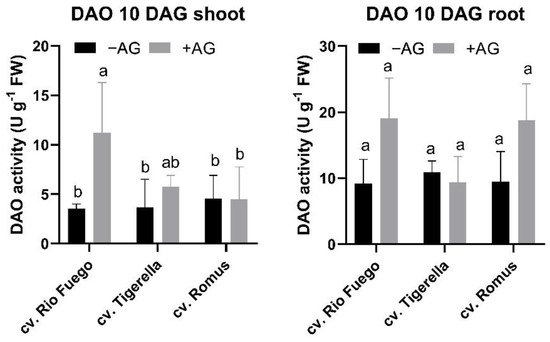
Figure 13.
Diamine oxidase (DAO) activities of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 10 DAG. Data of enzyme activities are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
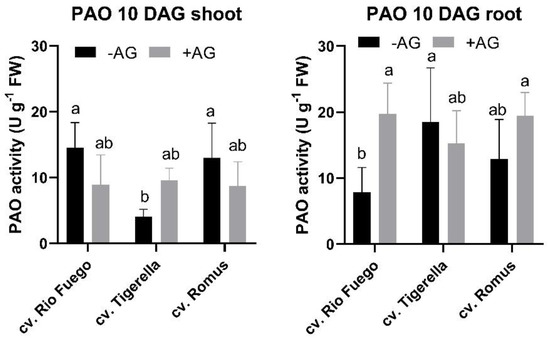
Figure 14.
Polyamine oxidase (PAO) activities of three tomato cultivars (Solanum lycopersicum cv. Rio Fuego, Tigerella, Romus) imbibed in water (−AG) or in 1 mM L-aminoguanidine (+AG) after 10 DAG. Data of enzyme activities are the mean ± SD of at least three biological replicates, n = 4. Different letters denote significant differences (two-way ANOVA, Tukey’s post hoc test p < 0.05).
4. Discussion
The tomato is a diverse plant species with many cultivars and genotypes. Its development shows some genotype-dependent features and processes, but sometimes, it is a difficult task to investigate the associations of phenotypes and genotypes [20]. Recent studies mainly focus on their stress tolerance, while the differences in their developmental processes sometimes remain explored.
In this study, we compared three tomato genotypes to enhance our knowledge about the early development of tomato seedlings based on their polyamine catabolism.
Copper amine oxidases (CuAOs) play a role in plant growth and development [9]. It was reported earlier that in root cell expansion, CuAOs have been reported to be involved in soybean seedlings [21]. To investigate the cultivar dependence of PA catabolism, especially CuAOs, we applied AG, a CuAO inhibitor. After 3 days of germination, we provided evidence that AG could induce reduced root growth and development (Figure 1) depending on the cultivar; reports in the literature indicate that root system architecture is characterized morphologically by the diverse tomato genotypes at early development [22]. Moreover, AG caused a slight delay of germination in all cultivars (Figure 2), which is in agreement with the results of CuAO mutant Arabidopsis thaliana with reduced CuAO activities [10].
Figure 3 shows that biomass was unaffected by AG; however, in the case of protein contents, only Rio Fuego showed decreased levels.
From investigated cultivars, Romus showed the highest values of Put and Spd, but not Spm (Figure 4); however, after AG application, the contents of these PAs significantly reduced compared to other cultivars. Our findings reveal that Romus was the most sensitive to AG treatment. AG treatment led to a reduction in total free PAs in the case of Romus; however, the ratio of higher PAs was higher than Put in this genotype (Figure 5).
In line with earlier results from AG-induced DAO inhibition [13,23], AG successfully reduced CuAO activities in all cases irrespective of tomato genotypes (Figure 6). Surprisingly, we found that PAOs showed higher activities after AG treatment, except for Rio Fuego, suggesting a potential compensation of the missing CuAO activities (Figure 6). A similar mechanism has been suggested by Sequera-Mutiozabal et al. [4], who demonstrated that in case of reduced PAO activities, more Put could be accumulated in AtPAO4 mutants in dark-induced senescence in Arabidopsis thaliana. Unfortunately, CuAO activities were not investigated in that study, which would further support our hypothesis.
To examine the long-term effect of reduced CuAO activities, tomato seedlings were grown in optimal nutrient solution for 1 week to check that tomato cultivars could show any recovery effect from AG-induced disturbed PA catabolism. To our best knowledge, this is the first study showing the relationship between the recovery of PA catabolism and cultivars. As Table 1 demonstrates, there were no significant changes between growth parameters of different tomato cultivars after AG treatment.
From studied biochemical parameters, protein contents were similar in the case of shoots in all cultivars; however, only the roots of Romus exhibited higher protein contents after AG treatment (Figure 7).
It was reported earlier that hydrogen peroxide and PAs could act as a double-edged sword in plant abiotic stress responses [24]. H2O2 and photosynthetic pigment contents remained constant compared to control, indicating that reduced CuAO in early development did not cause any significant decrease in photosynthesis or induce any oxidative stress response. Only the cultivar dependence could be seen (Figure 8 and Figure 9).
Total free PAs demonstrated alterations between genotypes (Figure 11). Tigerella showed higher Put and Spm levels in the shoot of AG-treated seedlings, while in roots, Spm was under the limit of detection (Figure 10). Therefore, the contents of total free PAs were the lowest in the case of this genotype. This evidence is reflected by the ratio of PAs in the roots of Tigerella (Figure 12).
Regarding CuAO activities, it remains to be clarified why the CuAO activity of Rio Fuego increased after AG treatment in shoots during recovery (Figure 13). Interestingly, PAO activities were highest in Tigerella shoots, while in roots, Rio Fuego and Romus showed higher activities than the control (Figure 14).
Our results point out the necessity of understanding the cultivar dependence of PA catabolism and related mechanisms not only in growth and development but also in stress responses.
A recent review suggests that plant CuAOs could act as key components in hormone signaling leading to stress-induced phenotypic plasticity [25]. Additionally, cell wall amine oxidases could be attributed to root xylem differentiation in root development and stress conditions [26,27].
Cultivar dependence of tomatoes is reported under drought stress by Montesinos-Pereira et al. [28], suggesting that genotype dependence strongly connects to optimal and sustainable agriculture.
Deciphering the natural biodiversity of plant species and cultivars and the involvement of these findings in agricultural practices could enhance the effectiveness and sustainable application of these cultivars [29].
5. Conclusions
The current study has compared tomato cultivars at early developmental stages to study the involvement of polyamine catabolism, especially copper amine oxidases. By exogenously adding a CuAO inhibitor, AG, cultivar dependence was suggested based on our results. Additionally, we proved evidence about the long-term effect of disturbed polyamine catabolism in tomatoes elucidating the potential cooperation between CuAOs and PAOs in early development. Future experiments are needed to evaluate the potential application of selected cultivars for breeding programs to enhance stress tolerance by adjusting polyamine catabolism.
Author Contributions
Conceptualization, Á.S. (Ágnes Szepesi), L.B. and Z.M.K.; methodology, Á.S. (Ágnes Szepesi) and Z.M.K.; formal analysis, Á.S. (Ágnes Szepesi), Z.M.K., H.K. and Á.S. (Árpád Szilágyi); writing—original draft paper, Á.S. (Ágnes Szepesi), L.B., H.K. and Z.M.K.; writing and editing, Á.S. (Ágnes Szepesi), L.B., Z.M.K. and Á.S. (Árpád Szilágyi); funding acquisition, Á.S. (Ágnes Szepesi). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NRDI (National Research, Development and Innovation) Office by the Hungarian Ministry grant number FK129061 to Á.S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors thank the excellent help of Etelka Bécsné Kozma for laboratory assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- FAOSTAT. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 December 2021).
- Roohanitaziani, R.; de Maagd, R.A.; Lammers, M.; Molthoff, J.; Meijer-Dekens, F.; van Kaauwen, M.P.W.; Finkers, R.; Tikunov, Y.; Visser, R.G.F.; Bovy, A.G. Exploration of a Resequenced Tomato Core Collection for Phenotypic and Genotypic Variation in Plant Growth and Fruit Quality Traits. Genes 2020, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Sequera-Mutiozabal, M.; Antoniou, C.; Tiburcio, A.F.; Alcázar, R.; Fotopoulos, V. Polyamines: Emerging Hubs Promoting Drought and Salt Stress Tolerance in Plants. Curr. Mol. Biol. Rep. 2017, 3, 28–36. [Google Scholar] [CrossRef]
- Szepesi, Á. Chapter 22 Molecular Mechanisms of Polyamines-Induced Abiotic Stress Tolerance in Plants. In Book Approaches for Enhancing Abiotic Stress Tolerance in Plants, 1st ed.; Hasanuzzaman, M., Nahar, K., Fujita, M., Oku, H., Islam, T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; p. 18. [Google Scholar]
- Wang, W.; Paschalidis, K.; Feng, J.C.; Song, J.; Liu, J.H. Polyamine Catabolism in Plants: A Universal Process with Diverse Functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef] [Green Version]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper-containing amine oxidases contribute to terminal polyamine oxidation in peroxisomes and apoplast of Arabidopsis thaliana. BMC Plant Biol. 2013, 13, 109. [Google Scholar] [CrossRef] [Green Version]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of amine oxidases in plant development and defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef]
- Tavladoraki, P.; Cona, A.; Angelini, R. Copper-Containing Amine Oxidases and FAD-Dependent Polyamine Oxidases Are Key Players in Plant Tissue Differentiation and Organ Development. Front. Plant Sci. 2016, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, B.; Hunt, J.D.; Dimitrova, S.; Spadafora, N.D.; Cort, A.P.; Colombo, D.; Müller, C.T.; Ghuge, S.A.; Davoli, D.; Cona, A.; et al. Mutation of Arabidopsis Copper-Containing Amine Oxidase Gene AtCuAOδ Alters Polyamines, Reduces Gibberellin Content and Affects Development. Int. J. Mol. Sci. 2020, 21, 7789. [Google Scholar] [CrossRef]
- Fraudentali, I.; Ghuge, S.A.; Carucci, A.; Tavladoraki, P.; Angelini, R.; Rodrigues-Pousada, R.A.; Cona, A. Developmental, hormone- and stress-modulated expression profiles of four members of the Arabidopsis copper-amine oxidase gene family. Plant Physiol. Biochem. 2020, 147, 141–160. [Google Scholar] [CrossRef]
- Szepesi, A.; Csiszár, J.; Gémes, K.; Horváth, E.; Horváth, F.; Simon, M.L.; Tari, I. Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation, and increases Na+ content in leaves without toxicity symptoms in Solanum lycopersicum L. J. Plant Physiol. 2009, 166, 914–925. [Google Scholar] [CrossRef]
- Longu, S.; Mura, A.; Padiglia, A.; Medda, R.; Floris, G. Mechanism-based inactivators of plant copper/quinone containing amine oxidases. Phytochemistry 2005, 66, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Guo, Q.; Gu, Z. GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem. 2013, 136, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, H.; Gu, Z. Factors influencing diamine oxidase activity and γ-aminobutyric acid content of fava bean (Vicia faba L.) during germination. J. Agric. Food Chem. 2011, 59, 11616–11620. [Google Scholar] [CrossRef]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 2008, 20, 1708–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef]
- Faragó, D.; Sass, L.; Valkai, I.; Andrási, N.; Szabados, L. PlantSize offers an affordable, non-destructive method to measure plant size and color in vitro. Front. Plant Sci. 2018, 9, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Korwin Krukowski, P.; Ellenberger, J.; Röhlen-Schmittgen, S.; Schubert, A.; Cardinale, F. Phenotyping in Arabidopsis and Crops—Are We Addressing the Same Traits? A Case Study in Tomato. Genes 2020, 11, 1011. [Google Scholar] [CrossRef]
- Delis, C.; Dimou, M.; Flemetakis, E.; Aivalakis, G.; Katinakis, P. A root- and hypocotyl-specific gene coding for copper-containing amine oxidase is related to cell expansion in soybean seedlings. J. Exp. Bot. 2006, 57, 101–111. [Google Scholar] [CrossRef]
- Alaguero-Cordovilla, A.; Gran-Gómez, F.J.; Tormos-Moltó, S.; Pérez-Pérez, J.M. Morphological Characterization of Root System Architecture in Diverse Tomato Genotypes during Early Growth. Int. J. Mol. Sci. 2018, 19, 3888. [Google Scholar] [CrossRef] [Green Version]
- Bouchereau, A.; Aziz, A.; Larher, F.; Martin-Tanguy, J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999, 140, 103–125. [Google Scholar] [CrossRef]
- Gupta, K.; Sengupta, A.; Chakraborty, M.; Gupta, B. Hydrogen Peroxide and Polyamines Act as Double Edged Swords in Plant Abiotic Stress Responses. Front. Plant Sci. 2016, 7, 1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraudentali, I.; Rodrigues-Pousada, R.A.; Angelini, R.; Ghuge, S.A.; Cona, A. Plant Copper Amine Oxidases: Key Players in Hormone Signaling Leading to Stress-Induced Phenotypic Plasticity. Int. J. Mol. Sci. 2021, 22, 5136. [Google Scholar] [CrossRef] [PubMed]
- Tisi, A.; Angelini, R.; Cona, A. Does polyamine catabolism influence root development and xylem differentiation under stress conditions? Plant Signal. Behav. 2011, 6, 1844–1847. [Google Scholar] [CrossRef] [Green Version]
- Ghuge, S.A.; Tisi, A.; Carucci, A. Rodrigues-Pousada, R.A.; Franchi, S.; Tavladoraki, P.; Angelini, R.; Cona, A. Cell Wall Amine Oxidases: New Players in Root Xylem Differentiation under Stress Conditions. Plants 2015, 4, 489–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montesinos-Pereira, D.; Barrameda-Medina, Y.; Romero, L.; Ruiz, J.M.; Sánchez-Rodríguez, E. Genotype differences in the metabolism of proline and polyamines under moderate drought in tomato plants. Plant Biol. 2014, 16, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.E.V.; Johansson, E.; Centellas Quezada, A.; Gustavsson, K.-E.; Olsson, M.E. Genotype and Maturity Stage Affect the Content and Composition of Polyamines in Tomato—Possible Relations to Plant and Human Health. Horticulturae 2021, 7, 300. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).