Biostimulants Application on Olea europaea L. in Mediterranean Conditions Increase the Production and Bioactive Compounds of Drupes and Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Biostimulants Treatments, and Experimental Design
- (1)
- Auxym (A) product derived from tropical plants extracts by Hello Nature® (Rivoli Veronese, VR, Italy). The product was used as foliar application at the dose of 1.5 L ha−1.
- (2)
- Biohelp (B) glycine betaine-based product by Biolchim SPA (Bologna, Italy), a bio-promoter of resistance to environmental stress. The product was used as foliar application at the dose of 10 kg ha−1.
- (3)
- Trianum-P (T) a product based on Trichoderma by Koppert Biological Systems (Bussolengo, Italy), with active ingredient Trichoderma harzianum Rifai strain T-22 (also known as KRL-AG2*). The product was used both as foliar application and radical application at the dose of 2.5 kg ha−1.
- (4)
- Control (C) plants were only treated with water.
2.2. Harvest Time, Production Plants−1, Maturation Index and Oil Extraction
2.3. Carotenoids Determination of the Drupes
2.4. Anthocyanins Determination of the Drupes
2.5. Fat Extraction by Drupes
2.6. Chemicals, Reagents, and Material
2.7. Ultrasound-Assisted Extraction of Polyphenolic Compounds of the Drupes
2.8. UHPLC-HRMS Analysis of Polyphenolic Compounds of the Drupes
2.9. Antioxidant Activity Evaluation of the Drupes
2.10. Quality Indices of Olive Oil
2.11. Fatty Acid Profile of Olive Oil
2.12. Total Polyphenols Content of the Oil
2.13. Polyphenols Determination by HPLC of Olive Oil
2.14. Statistical Analysis
3. Results and Discussion
3.1. Effects of Biostimulants on Production/Plant and Drupe Characteristics
3.2. Polyphenolic Compounds Analysis by UHPLC-Q-Orbitrap HRMS of Olive Drupes
3.3. Antioxidant Activity of Extracts of Olive Drupes
3.4. Quality Indices of Olive Oil
3.5. Oil Sensorial Analysis
3.6. Fatty Acids Composition of Oil
3.7. Polyphenols Content of Olive Oil
3.8. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, J.H.; Christensen, O.B. A Summary of the PRUDENCE Model Projections of Changes in European Climate by the End of This Century. Clim. Chang. 2007, 81, 7–30. [Google Scholar] [CrossRef]
- Giorgi, F. Climate Change Hot-Spots. Geophys. Res. Lett. 2006, 33, L08707. [Google Scholar] [CrossRef]
- Giannakopoulos, C.; Le Sager, P.; Bindi, M.; Moriondo, M.; Kostopoulou, E.; Goodess, C.M. Climatic Changes and Associated Impacts in the Mediterranean Resulting from a 2 °C Global Warming. Glob. Planet. Chang. 2009, 68, 209–224. [Google Scholar] [CrossRef]
- Gualdi, S.; Somot, S.; Li, L.; Artale, V.; Adani, M.; Bellucci, A.; Braun, A.; Calmanti, S.; Carillo, A.; Dell’Aquila, A.; et al. The CIRCE Simulations: Regional Climate Change Projections with Realistic Representation of the Mediterranean Sea. Bull. Am. Meteorol. Soc. 2013, 94, 65–81. [Google Scholar] [CrossRef]
- Orlandi, F.; Avolio, E.; Bonofiglio, T.; Federico, S.; Romano, B.; Fornaciari, M. Potential Shifts in Olive Flowering According to Climate Variations in Southern Italy. Meteorol. Appl. 2012, 7, 497–503. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Metz 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Fraga, H.; Pinto, J.G.; Viola, F.; Santos, J.A. Climate Change Projections for Olive Yields in the Mediterranean Basin. Int. J. Climatol. 2020, 40, 769–781. [Google Scholar] [CrossRef] [Green Version]
- García-Inza, G.P.; Castro, D.N.; Hall, A.J.; Rousseaux, M.C. Opposite Oleic Acid Responses to Temperature in Oils from the Seed and Mesocarp of the Olive Fruit. Eur. J. Agron. 2016, 76, 138–147. [Google Scholar] [CrossRef]
- Wang, S. Genotypic Variation in Growth and Physiological Response to Drought Stress and Re-Watering Reveals the Critical Role of Recovery in Drought Adaptation in Maize Seedlings. Front. Plant Sci. 2016, 6, 15. [Google Scholar]
- Graziani, G.; Cirillo, A.; Giannini, P.; Conti, S.; El-Nakhel, C.; Rouphael, Y.; Ritieni, A.; Di Vaio, C. Biostimulants Improve Plant Growth and Bioactive Compounds of Young Olive Trees under Abiotic Stress Conditions. Agriculture 2022, 12, 227. [Google Scholar] [CrossRef]
- Mazeh, M.; Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Tucci, M.; Lodolini, E.M.; Rosati, A.; Famiani, F. Use of an Organic Fertilizer Also Having a Biostimulant Action to Promote the Growth of Young Olive Trees. Agriculture 2021, 11, 593. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Ritieni, A.; Cirillo, A.; Cice, D.; Di Vaio, C. Effects of Biostimulants on Annurca Fruit Quality and Potential Nutraceutical Compounds at Harvest and during Storage. Plants 2020, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Di Vaio, C.; Graziani, G.; Gaspari, A.; De Luca, L.; Aiello, A.; Cirillo, A.; Bruno, A.; Romano, R.; Ritieni, A. Drupe Characters, Fatty Acids, Polyphenolic and Aromatic Profile of Olive Oil Obtained from “Oliva Bianca”, Minor Autochthonous Cultivar of Campania. Plants 2021, 10, 1119. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in Agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [Green Version]
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the Growth, Yield and Volatile Oil Content of Pelargonium graveolens L. Herit by Foliar Application with Moringa Leaf Extract through Motivating Physiological and Biochemical Parameters. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Fleming, T.R.; Fleming, C.C.; Levy, C.C.B.; Repiso, C.; Hennequart, F.; Nolasco, J.B.; Liu, F. Biostimulants Enhance Growth and Drought Tolerance in Arabidopsis Thaliana and Exhibit Chemical Priming Action. Ann. Appl. Biol. 2019, 174, 153–165. [Google Scholar] [CrossRef]
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A Biostimulant Based on Protein Hydrolysates Promotes the Growth of Young Olive Trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]
- Cirillo, A.; Conti, S.; Graziani, G.; El-Nakhel, C.; Rouphael, Y.; Ritieni, A.; Di Vaio, C. Mitigation of High-Temperature Damage by Application of Kaolin and Pinolene on Young Olive Trees (Olea europaea L.): A Preliminary Experiment to Assess Biometric, Eco-Physiological and Nutraceutical Parameters. Agronomy 2021, 11, 1884. [Google Scholar] [CrossRef]
- Chouliaras, V.; Therios, I.; Chatzissavvidis, C.; Kouropalatis, T.; Μantzoutsos, Ι. Organic farming fertilization treatments on productivity, skin carotenoids and nutritional status of ‘kalamon’ table olives. Olivebioteq 2011, 2, 465–470. [Google Scholar]
- Franzoni, G.; Trivellini, A.; Bulgari, R.; Cocetta, G.; Ferrante, A. Bioactive Molecul es as Regulatory Signals in Plant Responses to Abiotic Stresses. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–182. ISBN 978-0-12-816451-8. [Google Scholar]
- Briante, R.; Febbraio, F.; Nucci, R. Antioxidant Properties of Low Molecular Weight Phenols Present in the Mediterranean Diet. J. Agric. Food Chem. 2003, 51, 6975–6981. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.-X.; Mayeux, R.; Luchsinger, J.A. Mediterranean Diet and Risk for Alzheimer’s Disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Braga, C.; Vecchia, C.L.; Franceschi, S.; Negri, E.; Parpinel, M.; Decarli, A.; Giacosa, A.; Trichopoulos, D. Olive Oil, Other Seasoning Fats, and the Risk of Colorectal Carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1998, 82, 448–453. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Carillo, P.; Colla, G.; Graziani, G.; Di Mola, I.; Mori, M.; Kyriacou, M.C.; Rouphael, Y.; Soteriou, G.A.; et al. Plant-Derived Biostimulants Differentially Modulate Primary and Secondary Metabolites and Improve the Yield Potential of Red and Green Lettuce Cultivars. Agronomy 2022, 12, 1361. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Dini, I.; Pascale, M.; Staropoli, A.; Marra, R.; Vinale, F. Effect of Selected Trichoderma Strains and Metabolites on Olive Drupes. Appl. Sci. 2021, 11, 8710. [Google Scholar] [CrossRef]
- Sanz-Cortes, F.; Martinez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llacer, G.; Meier, U. Phenological Growth Stages of Olive Trees (Olea europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- Aiello, A.; Pizzolongo, F.; Scognamiglio, G.; Romano, A.; Masi, P.; Romano, R. Effects of Supercritical and Liquid Carbon Dioxide Extraction on Hemp (Cannabis Sativa L.) Seed Oil. Int. J. Food Sci. Technol. 2020, 55, 2472–2480. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Francis, F.J.; Markakis, P.C. Food Colorants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Silva, E.; Brito, C.; Martins, S.; Pinto, L.; Dinis, L.; Luzio, A.; Martins-Gomes, C.; Fernandes-Silva, A.; Ribeiro, C.; et al. Olive Tree Physiology and Chemical Composition of Fruits Are Modulated by Different Deficit Irrigation Strategies. J. Sci. Food Agric. 2020, 100, 682–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Dini, I.; Graziani, G.; Fedele, F.L.; Sicari, A.; Vinale, F.; Castaldo, L.; Ritieni, A. Effects of Trichoderma Biostimulation on the Phenolic Profile of Extra-Virgin Olive Oil and Olive Oil By-Products. Antioxidants 2020, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; De Luca, L.; Paduano, A.; Sacchi, R. Influence of Olive Oil Phenolic Compounds on Headspace Aroma Release by Interaction with Whey Proteins. J. Agric. Food Chem. 2015, 63, 3838–3850. [Google Scholar] [CrossRef]
- Romano, R.; De Luca, L.; Aiello, A.; Rossi, D.; Pizzolongo, F.; Masi, P. Bioactive Compounds Extracted by Liquid and Supercritical Carbon Dioxide from Citrus Peels. Int. J. Food Sci. Technol. 2022, 57, 3826–3837. [Google Scholar] [CrossRef]
- Bajoub, A.; Hurtado-Fernández, E.; Ajal, E.A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A.; Ouazzani, N. Quality and Chemical Profiles of Monovarietal North Moroccan Olive Oils from “Picholine marocaine” Cultivar: Registration Database Development and Geographical Discrimination. Food Chem. 2015, 179, 127–136. [Google Scholar] [CrossRef]
- Bouaziz, M.; Jemai, H.; Khabou, W.; Sayadi, S. Oil Content, Phenolic Profiling and Antioxidant Potential of Tunisian Olive Drupes. J. Sci. Food Agric. 2010, 90, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Di-Vaio, C.; Cirillo, A.; Cice, D.; El-Nakhel, C.; Rouphael, Y. Biostimulant Application Improves Yield Parameters and Accentuates Fruit Color of Annurca Apples. Agronomy 2021, 11, 715. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Harman, G.E. Myths and Dogmas of Biocontrol Changes in Perceptions Derived from Research on Trichoderma harzinum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef] [Green Version]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Shafiq, S.; Akram, N.A.; Ashraf, M.; García-Caparrós, P.; Ali, O.M.; Latef, A.A.H.A. Influence of Glycine Betaine (Natural and Synthetic) on Growth, Metabolism and Yield Production of Drought-Stressed Maize (Zea mays L.) Plants. Plants 2021, 10, 2540. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Pannico, A.; Giordano, M.; Sifola, M.; Kyriacou, M.; et al. Biostimulant Application with a Tropical Plant Extract Enhances Corchorus Olitorius Adaptation to Sub-Optimal Nutrient Regimens by Improving Physiological Parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in Mitigating NaCl Stress in Indian Mustard (Brassica juncea L.) through Antioxidative Defense System. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [Green Version]
- Fourati, R.; Scopa, A.; Ben Ahmed, C.; Ben Abdallah, F.; Terzano, R.; Gattullo, C.E.; Allegretta, I.; Galgano, F.; Caruso, M.C.; Sofo, A. Leaf Biochemical Responses and Fruit Oil Quality Parameters in Olive Plants Subjected to Airborne Metal Pollution. Chemosphere 2017, 168, 514–522. [Google Scholar] [CrossRef] [PubMed]
- do Rêgo Meneses, F.J.; de Oliveira Lopes, Á.L.; Setubal, I.S.; da Costa Neto, V.P.; Bonifácio, A. Inoculation of Trichoderma asperelloides Ameliorates Aluminum Stress-Induced Damages by Improving Growth, Photosynthetic Pigments and Organic Solutes in Maize. 3 Biotech 2022, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Motilva, M.-J.; Romero, M.-P. The Effect of the Ripening Process of the Olive Fruit on the Chlorophyll and Carotenoid Fractions of Drupes and Virgin Oils. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 59–68. ISBN 978-0-12-374420-3. [Google Scholar]
- Yorulmaz, A.; Erinc, H.; Tekin, A. Changes in Olive and Olive Oil Characteristics During Maturation. J. Am. Oil Chem. Soc. 2013, 90, 647–658. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Mbarki, N.; Cheheb, H.; Hammami, M.; Attia, F. Arbuscular Mycorrhizal Fungus Rhizophagus irregularis Influences Key Physiological Parameters of Olive Trees (Olea europaea L.) and Mineral Nutrient Profile. Photosynthetica 2017, 55, 308–316. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Zaheer Akbar, M.; Shakoor, M.B.; Mahmood, A.; Ishaque, W.; Hussain, A. Foliar Application of Aspartic Acid Lowers Cadmium Uptake and Cd-Induced Oxidative Stress in Rice under Cd Stress. Environ. Sci. Pollut. Res. 2017, 24, 21938–21947. [Google Scholar] [CrossRef] [PubMed]
- Zouari, I.; Mechri, B.; Tekaya, M.; Dabbaghi, O.; Cheraief, I.; Mguidiche, A.; Annabi, K.; Laabidi, F.; Attia, F.; Hammami, M.; et al. Olive Oil Quality Influenced by Biostimulant Foliar Fertilizers. Braz. J. Biol. Sci. 2020, 7, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Carillo, P.; Cristofano, F.; Cardarelli, M.; Colla, G. Effects of Vegetal- versus Animal-Derived Protein Hydrolysate on Sweet Basil Morpho-Physiological and Metabolic Traits. Sci. Hortic. 2021, 284, 110123. [Google Scholar] [CrossRef]
- Massenti, R.; Ciaccio, V.; Bianco, R.L. Foliar applications with sunred® biostimulant advance and uniform fruit ripening in orange and olive. Int. J. Plant Anim. Environ. Sci. 2015, 5, 227–232. [Google Scholar]
- Del Buono, D.; Regni, L.; Del Pino, A.M.; Bartucca, M.L.; Palmerini, C.A.; Proietti, P. Effects of Megafol on the Olive Cultivar ‘Arbequina’ Grown Under Severe Saline Stress in Terms of Physiological Traits, Oxidative Stress, Antioxidant Defenses, and Cytosolic Ca2+. Front. Plant Sci. 2021, 11, 603576. [Google Scholar] [CrossRef]
- Bendini, A. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [Green Version]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and Sensory Properties of Virgin Olive Oil Hydrophilic Phenols: Agronomic and Technological Aspects of Production That Affect Their Occurrence in the Oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Noorali, M.; Barzegar, M.; Sahari, M.A. Sterol and Fatty Acid Compositions of Olive Oil as an Indicator of Cultivar and Growing Area. J. Am. Oil Chem. Soc. 2014, 91, 1571–1581. [Google Scholar] [CrossRef]
- Marra, F.P.; Caruso, T.; Costa, F.; Di Vaio, C.; Mafrica, R.; Marchese, A. Genetic Relationships, Structure and Parentage Simulation among the Olive Tree (Olea europaea L. Subsp. europaea) Cultivated in Southern Italy Revealed by SSR Markers. Tree Genet. Genomes 2013, 9, 961–973. [Google Scholar] [CrossRef]
- Hernández-Hernandez, G.; Salazar, D.M.; Martínez-Tomé, J.; López-Cortés, I. The Use of Biostimulants in High-Density Olive Growing: Quality and Production. AJAAR 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jukić Špika, M.; Perica, S.; Žanetić, M.; Škevin, D. Virgin Olive Oil Phenols, Fatty Acid Composition and Sensory Profile: Can Cultivar Overpower Environmental and Ripening Effect? Antioxidants 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Bengana, M.; Bakhouche, A.; Lozano-Sánchez, J.; Amir, Y.; Youyou, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Influence of Olive Ripeness on Chemical Properties and Phenolic Composition of Chemlal Extra-Virgin Olive Oil. Food Res. Int. 2013, 54, 1868–1875. [Google Scholar] [CrossRef]
- El Qarnifa, S.; El Antari, A.; Hafidi, A. Effect of Maturity and Environmental Conditions on Chemical Composition of Olive Oils of Introduced Cultivars in Morocco. J. Food Qual. 2019, 2019, 1854539. [Google Scholar] [CrossRef] [Green Version]
- Pinelli, P.; Galardi, C.; Mulinacci, N.; Vincieri, F.F.; Cimato, A.; Romani, A. Minor Polar Compound and Fatty Acid Analyses in Monocultivar Virgin Olive Oils from Tuscany. Food Chem. 2003, 80, 331–336. [Google Scholar] [CrossRef]
- Torić, J.; Brozovic, A.; Baus Lončar, M.; Jakobušić Brala, C.; Karković Marković, A.; Benčić, Đ.; Barbarić, M. Biological Activity of Phenolic Compounds in Extra Virgin Olive Oils through Their Phenolic Profile and Their Combination with Anticancer Drugs Observed in Human Cervical Carcinoma and Colon Adenocarcinoma Cells. Antioxidants 2020, 9, 453. [Google Scholar] [CrossRef]
- Palla, M.; Digiacomo, M.; Cristani, C.; Bertini, S.; Giovannetti, M.; Macchia, M.; Manera, C.; Agnolucci, M. Composition of Health-Promoting Phenolic Compounds in Two Extra Virgin Olive Oils and Diversity of Associated Yeasts. J. Food Compos. Anal. 2018, 74, 27–33. [Google Scholar] [CrossRef]
- Tovar, M.J.; Motilva, M.J.; Romero, M.P. Changes in the Phenolic Composition of Virgin Olive Oil from Young Trees (Olea europaea L. cv. Arbequina) Grown under Linear Irrigation Strategies. J. Agric. Food Chem. 2001, 49, 5502–5508. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Alonso, S.; Salvador, M.D.; Fregapane, G. Phenolic Compounds Profile of Cornicabra Virgin Olive Oil. J. Agric. Food Chem. 2002, 50, 6812–6817. [Google Scholar] [CrossRef] [PubMed]
- El Riachy, M.; Priego-Capote, F.; León, L.; Luque de Castro, M.D.; Rallo, L. Virgin Olive Oil Phenolic Profile and Variability in Progenies from Olive Crosses. J. Sci. Food Agric. 2012, 92, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [Green Version]
- Mafrica, R.; Piscopo, A.; De Bruno, A.; Poiana, M. Effects of Climate on Fruit Growth and Development on Olive Oil Quality in Cultivar Carolea. Agriculture 2021, 11, 147. [Google Scholar] [CrossRef]
- Dini, I.; Graziani, G.; Gaspari, A.; Fedele, F.L.; Sicari, A.; Vinale, F.; Cavallo, P.; Lorito, M.; Ritieni, A. New Strategies in the Cultivation of Olive Trees and Repercussions on the Nutritional Value of the Extra Virgin Olive Oil. Molecules 2020, 25, 2345. [Google Scholar] [CrossRef]
- Leogrande, R.; El Chami, D.; Fumarola, G.; Di Carolo, M.; Piegari, G.; Elefante, M.; Perrelli, D.; Dongiovanni, C. Biostimulants for Resilient Agriculture: A Preliminary Assessment in Italy. Sustainability 2022, 14, 6816. [Google Scholar] [CrossRef]
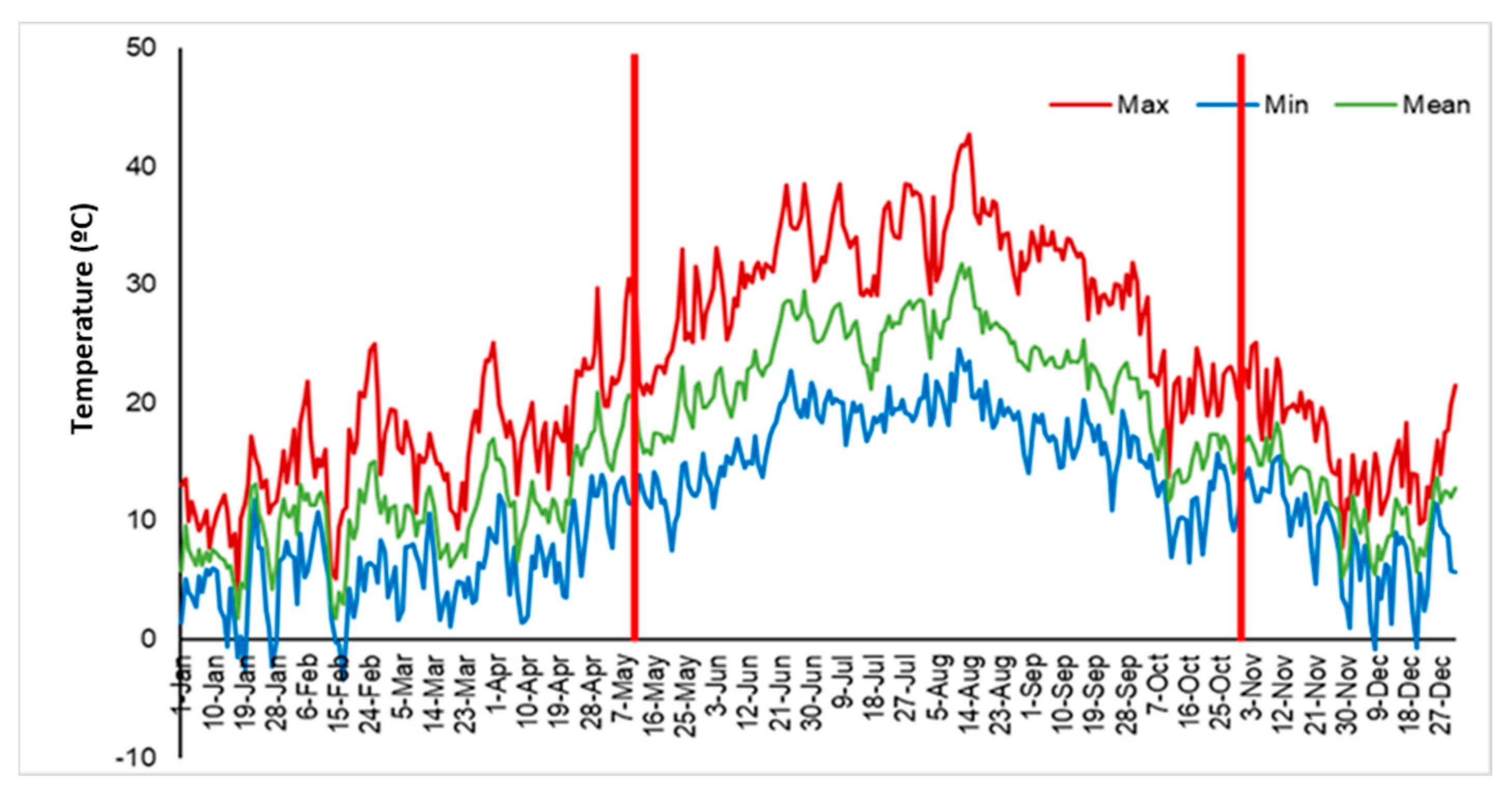
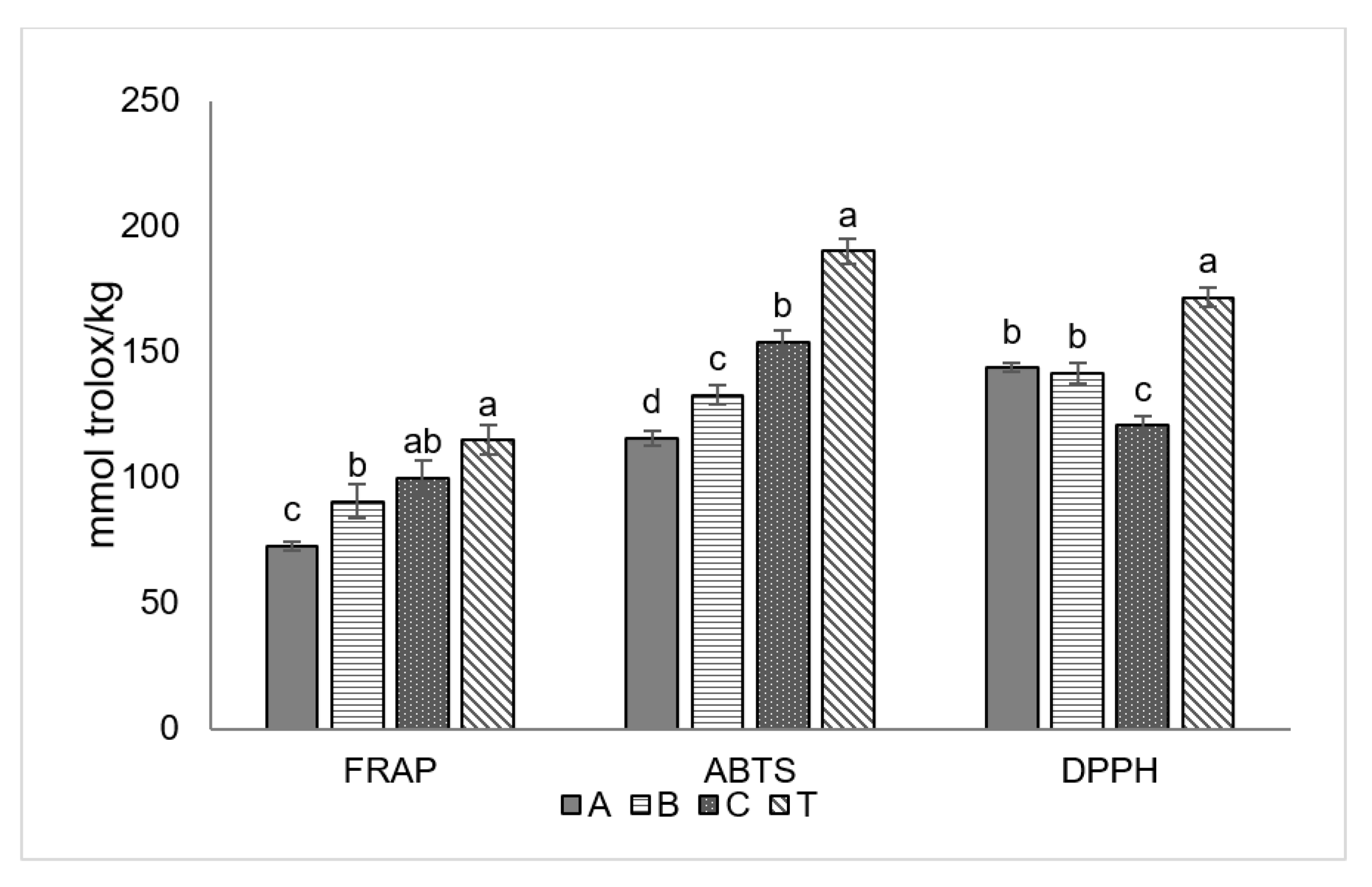

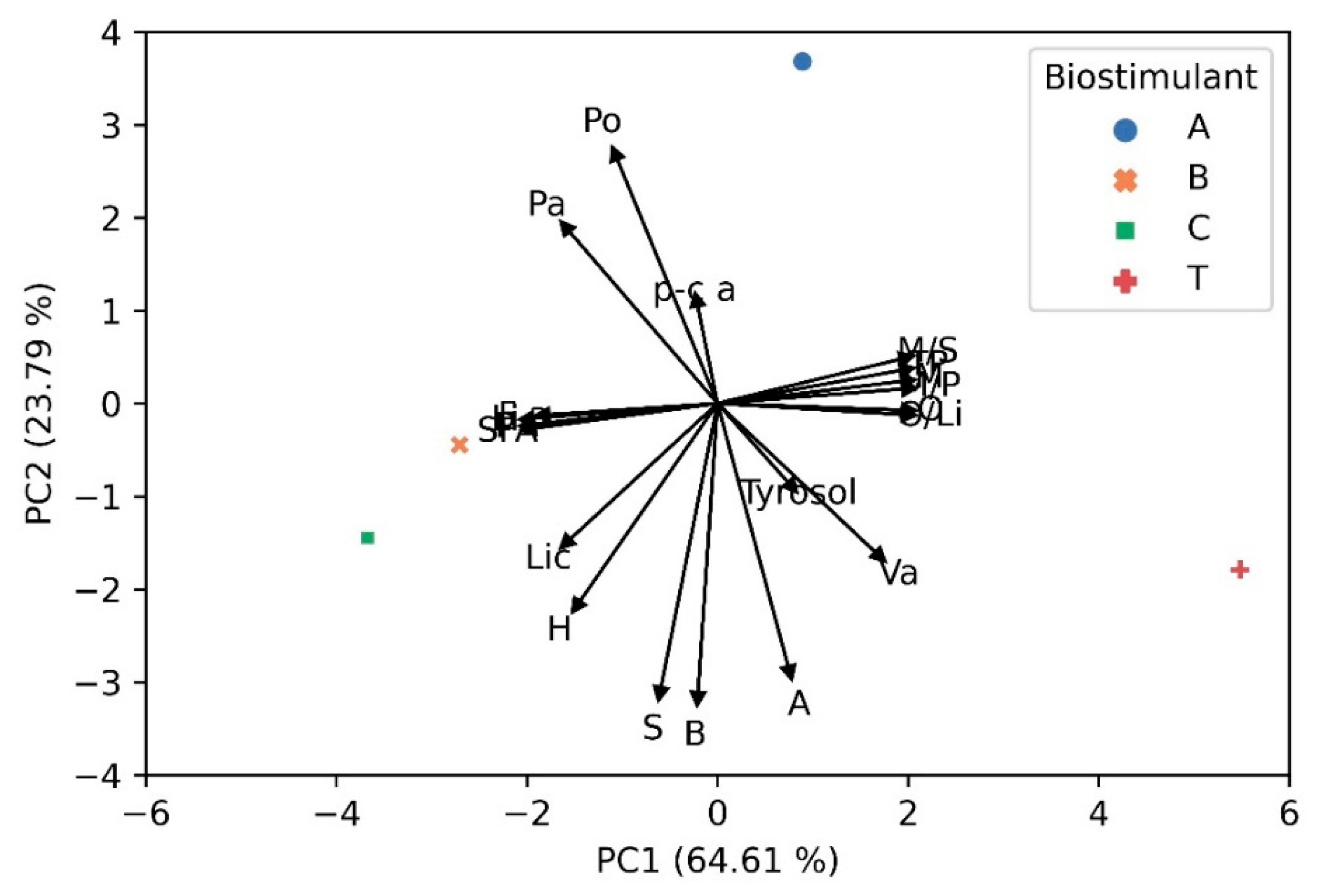
| A | B | C | T | Significance | |
|---|---|---|---|---|---|
| Jaen index | 2.99 | 2.89 | 2.87 | 2.9 | |
| Production plant−1 (kg) | 34.44 ± 2.51 b | 32.00 ± 1.96 b | 25.00 ± 0.95 c | 42.73 ± 2.43 a | *** |
| Oil content drupe (%) | 14.3 ± 0.10 b | 14.1 ± 0.07 b | 14.3 ± 0.14 b | 14.7 ± 0.10 a | * |
| Anthocyanins (mgCGE/kg) | 451.16 ± 1.35 a | 445.65 ± 2.47 a | 428.03 ± 4.57 b | 407.96 ± 2.72 c | *** |
| Carotenoids (mg/kg) | 5.97 ± 0.09 c | 5.42 ± 0.14 d | 6.78 ± 0.08 b | 7.57 ± 0.16 a | *** |
| A | B | C | T | Significance | |
|---|---|---|---|---|---|
| Hydroxytyrosol glucoside | 17.10 ± 2.29 a | 12.42 ± 1.31 b | 4.85 ± 0.47 c | 5.92 ± 0.96 c | *** |
| Hydroxytyrosol (3,4-DHPEA) | 119.88 ± 52.19 a | 116.97 ± 35.11 a | 73.18 ± 7.10 b | 148.30 ± 22.15 a | *** |
| Tyrosol (4-HPEA) | 13.63 ± 0.88 bc | 17.76 ± 1.39 b | 8.26 ± 1.12 c | 49.16 ± 3.95 a | *** |
| Vanillic acid | 17.68 ± 0.61 a | 18.21 ± 2.08 a | 7.52 ± 1.03 b | 11.82 ± 2.75 b | ** |
| Rutin | 81.62 ± 32.27 a | 91.88 ± 33.00 a | 77.36 ± 15.82 a | 88.65 ± 23.75 a | ns |
| Elenolic acid | 25.69 ± 1.92 a | 19.33 ± 1.40 a | 20.25 ± 2.17 a | 22.17 ± 3.68 a | ns |
| Verbascoside | 8274.50 ± 708.05 ab | 8486.11 ± 468.73 ab | 7078.61 ± 618.05 b | 9420.64 ± 477.80 a | ns |
| 3,4-DHPEA-EDA | 518.22 ± 148.5 a | 308.79 ± 124.3 a | 391.49 ± 181.3 a | 615.32 ± 38.6 a | ns |
| Ligstroside | 13.31 ± 12.39 c | 20.54 ± 16.86 bc | 47.00 ± 23.97 ab | 66.00 ± 31.76 a | ** |
| Oleuropein | 473.92 ± 80.91 a | 610.51 ± 119.94 b | 582.78 ± 64.24 b | 532.67 ± 122.40 ab | * |
| p HPEA-EDA | 10.10 ± 0.95 b | 14.09 ± 0.73 a | 10.51 ± 1.35 b | 15.04 ± 4.83 a | ns |
| Hydroxy-Oleuropein-aglycon | 8.57 ± 2.45 ab | 9.73 ± 1.77 a | 5.52 ± 1.66 b | 8.50 ± 4.52 ab | ns |
| Luteolin | 20.49 ± 1.61 bc | 31.31 ± 10.39 b | 14.92 ± 2.23 c | 67.20 ± 22.79 a | *** |
| 3,4-DHPEA-AC | 56.59 ± 44.83 a | 42.92 ± 33.15 ab | 12.27 ± 1.84 b | 65.38 ± 33.09 a | * |
| DHPEA-EA | 412.82 ± 147.25 bc | 225.98 ± 94.42 ab | 193.58 ± 109.45 a | 478.94 ± 89.65 c | ** |
| p-HPEA-EA | 17.04 ± 3.16 a | 17.84 ± 5.95 a | 8.37 ± 0.95 b | 17.14 ± 1.57 a | * |
| Total polyphenols | 10,081.16 ± 812.41 ab | 10,044.36 ± 544.34 ab | 8535.46 ± 698.45 b | 11,612.85 ± 534.10 a | ** |
| Oil Quality Index | A | B | C | T |
|---|---|---|---|---|
| Acidity (% oleic acid 100 g−1 oil) | 0.31 ± 0.01 a | 0.31 ± 0.01 a | 0.28 ± 0.01 b | 0.29 ± 0.01 b |
| Peroxide value (meqO2 kg−1) | 9.08 ± 0.04 a | 7.4 ± 0.04 b | 5.34 ± 0.03 d | 7.01 ± 0.03 c |
| K270 | 0.17 ± 0.00 c | 0.17 ± 0.00 c | 0.20 ± 0.00 b | 0.25 ± 0.00 a |
| K232 | 1.61 ± 0.01 b | 1.30 ± 0.01 d | 1.46 ± 0.00 c | 1.76 ± 0.00 a |
| Delta K | −0.01 ± 0.00 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | −0.01 ± 0.00 b |
| Panel Test | A | B | C | T |
|---|---|---|---|---|
| Fruity | 6.6 | 5.4 | 7.2 | 3.6 |
| Bitterness | 6 | 5.4 | 3.6 | 5.8 |
| Pungency | 6.4 | 4 | 4.6 | 6.2 |
| Heating/Sludge | 0 | 0 | 0 | 0 |
| Winey/Acid/acidic/sour | 0 | 0 | 0 | 0 |
| Rancid | 0 | 0 | 0 | 0 |
| Mold/moisture/ground | 0 | 0 | 0 | 0 |
| Frozen olive | 0 | 0 | 0 | 0 |
| % Fatty Acids | A | B | C | T | Significance |
|---|---|---|---|---|---|
| Palimitic (C16) | 14.00 ± 0.04 a | 13.67 ± 0.03 b | 13.90 ± 0.06 a | 12.66 ± 0.02 c | *** |
| Palmitoleic (C16:1) | 1.43 ± 0.01 a | 1.28 ± 0.02 b | 1.10 ± 0.01 c | 0.82 ± 0.00 d | *** |
| Heptadecanoic (C17) | 0.06 ± 0.00 c | 0.19 ± 0.01 a | 0.20 ± 0.00 a | 0.11 ± 0.00 b | *** |
| Stearic (C18) | 2.14 ± 0.01 c | 2.77 ± 0.02 b | 2.90 ± 0.02 a | 2.72 ± 0.01 b | *** |
| Oleic (C18.1n9c) | 68.81 ± 0.04 b | 67.15 ± 0.01 c | 66.23 ± 0.00 d | 71.53 ± 0.03 a | *** |
| Linoleic (C18:2 Z 9, 12) | 12.15 ± 0.04 c | 13.34 ± 0.05 b | 14.06 ± 0.04 a | 10.63 ± 0.01 d | *** |
| Arachidic (C20) | 0.33 ± 0.01 d | 0.36 ± 0.01 c | 0.39 ± 0.00 b | 0.41 ± 0.00 a | *** |
| Linolenic (C18:3n3) | 0.96 ± 0.02 b | 1.12 ± 0.02 a | 1.08 ± 0.00 a | 0.98 ± 0.01 b | *** |
| Behenic (C22) | 0.11 ± 0.00 a | 0.14 ± 0.00 a | 0.14 ± 0.00 a | 0.14 ± 0.01 a | ns |
| MUFA | 70.24 ± 0.03 b | 68.43 ± 0.03 c | 67.34 ± 0.01 d | 72.35 ± 0.03 a | *** |
| PUFA | 13.12 ± 0.02 c | 14.46 ± 0.03 b | 15.14 ± 0.04 a | 11.61 ± 0.01 d | *** |
| SFA | 16.65 ± 0.05 c | 17.11 ± 0.00 b | 17.53 ± 0.04 a | 16.04 ± 0.03 d | *** |
| MUFA/PUFA | 5.35 ± 0.01 b | 4.64 ± 0.01 c | 4.38 ± 0.01 d | 6.16 ± 0.00 a | *** |
| MUFA/SFA | 4.22 ± 0.01 b | 3.92 ± 0.00 c | 3.78 ± 0.01 d | 4.46 ± 0.01 a | *** |
| Oleic/linoleic | 5.66 ± 0.01 b | 5.03 ± 0.02 c | 4.71 ± 0.01 d | 6.73 ± 0.00 a | *** |
| Compounds (mg/kg) | A | B | C | T | Significance |
|---|---|---|---|---|---|
| Tyrosol | 8.52 ± 0.06 b | 9.43 ± 0.13 a | 8.17 ± 0.11 c | 9.25 ± 0.09 a | *** |
| p-coumaric acid | 4.53 ± 0.00 b | 4.70 ± 0.01 a | 4.31 ± 0.01 d | 4.43 ± 0.01 c | *** |
| Ferulic acid | 0.76 ± 0.01 c | 0.87 ± 0.01 a | 0.81 ± 0.01 b | 0.70 ± 0.00 d | *** |
| Vanillic acid | 0.40 ± 0.00 bc | 0.38 ± 0.00 c | 0.42 ± 0.01 b | 0.58 ± 0.00 a | *** |
| Oleuropein | <LOD | <LOD | <LOD | <LOD | |
| Total polyphenols | 529.81 ± 7.52 b | 476.64 ± 11.93 c | 442.31 ± 10.77 d | 584.69 ± 3.93 a | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, A.; De Luca, L.; Graziani, G.; Cepparulo, M.; El-Nakhel, C.; Giordano, M.; Rouphael, Y.; Ritieni, A.; Romano, R.; Di Vaio, C. Biostimulants Application on Olea europaea L. in Mediterranean Conditions Increase the Production and Bioactive Compounds of Drupes and Oil. Agriculture 2022, 12, 2173. https://doi.org/10.3390/agriculture12122173
Cirillo A, De Luca L, Graziani G, Cepparulo M, El-Nakhel C, Giordano M, Rouphael Y, Ritieni A, Romano R, Di Vaio C. Biostimulants Application on Olea europaea L. in Mediterranean Conditions Increase the Production and Bioactive Compounds of Drupes and Oil. Agriculture. 2022; 12(12):2173. https://doi.org/10.3390/agriculture12122173
Chicago/Turabian StyleCirillo, Aurora, Lucia De Luca, Giulia Graziani, Marco Cepparulo, Christophe El-Nakhel, Maria Giordano, Youssef Rouphael, Alberto Ritieni, Raffaele Romano, and Claudio Di Vaio. 2022. "Biostimulants Application on Olea europaea L. in Mediterranean Conditions Increase the Production and Bioactive Compounds of Drupes and Oil" Agriculture 12, no. 12: 2173. https://doi.org/10.3390/agriculture12122173
APA StyleCirillo, A., De Luca, L., Graziani, G., Cepparulo, M., El-Nakhel, C., Giordano, M., Rouphael, Y., Ritieni, A., Romano, R., & Di Vaio, C. (2022). Biostimulants Application on Olea europaea L. in Mediterranean Conditions Increase the Production and Bioactive Compounds of Drupes and Oil. Agriculture, 12(12), 2173. https://doi.org/10.3390/agriculture12122173











