Properties of Recycled Nanomaterials and Their Effect on Biological Activity and Yield of Canola in Degraded Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Area
2.2. Nanobiochar (nB)
2.3. Nanowater Treatment Residue (nWTR)
2.4. Pot Experiment
2.5. Analysis of Soil Samples and Nanobiochar
2.6. Spectroscopic Analysis
2.7. Zeta Potential
2.8. Catalase Activity
2.9. Dehydrogenase Activity (DHA)
2.10. Microbial Biomass Carbon
2.11. Statistical Analysis
3. Results
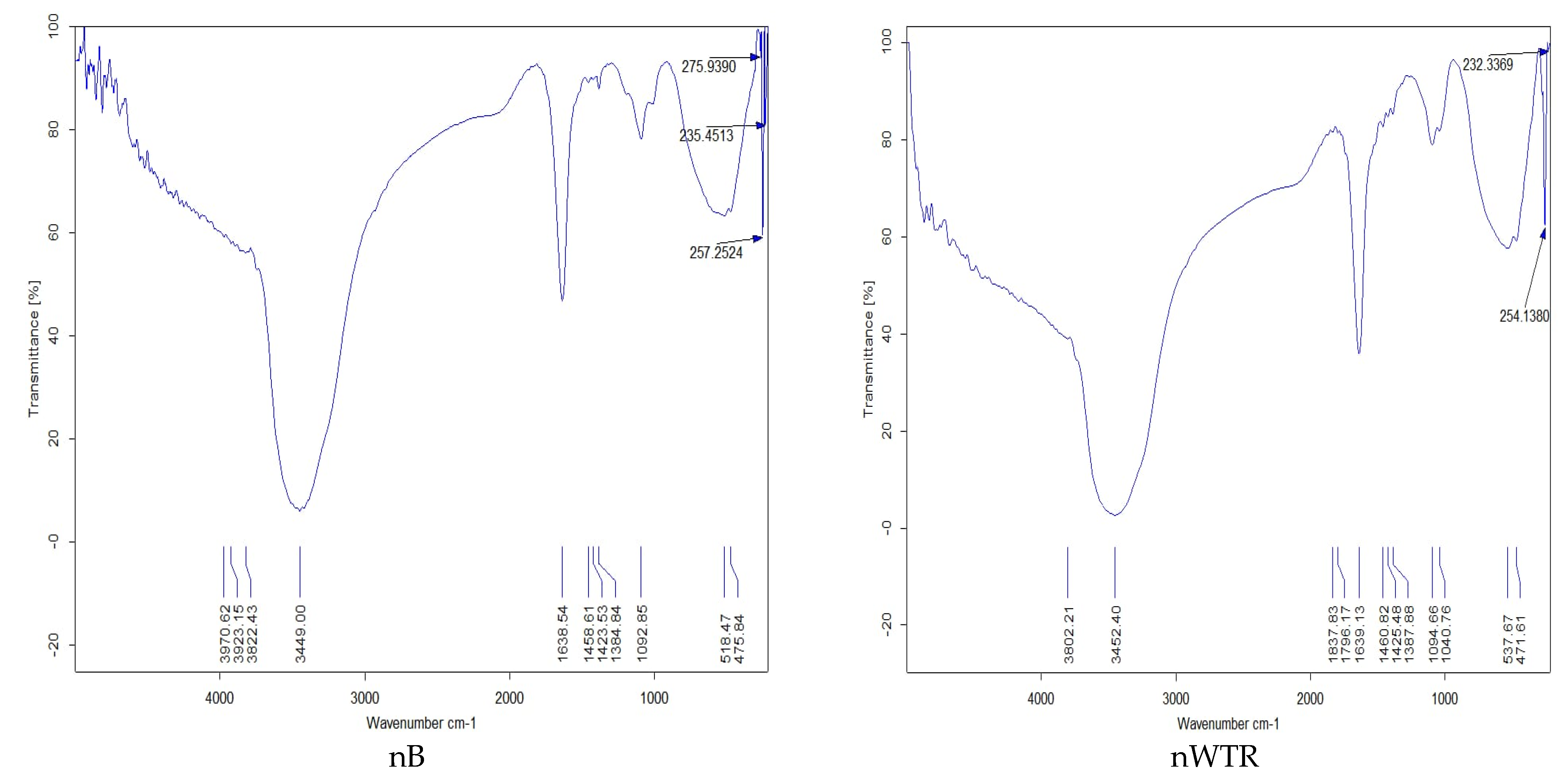
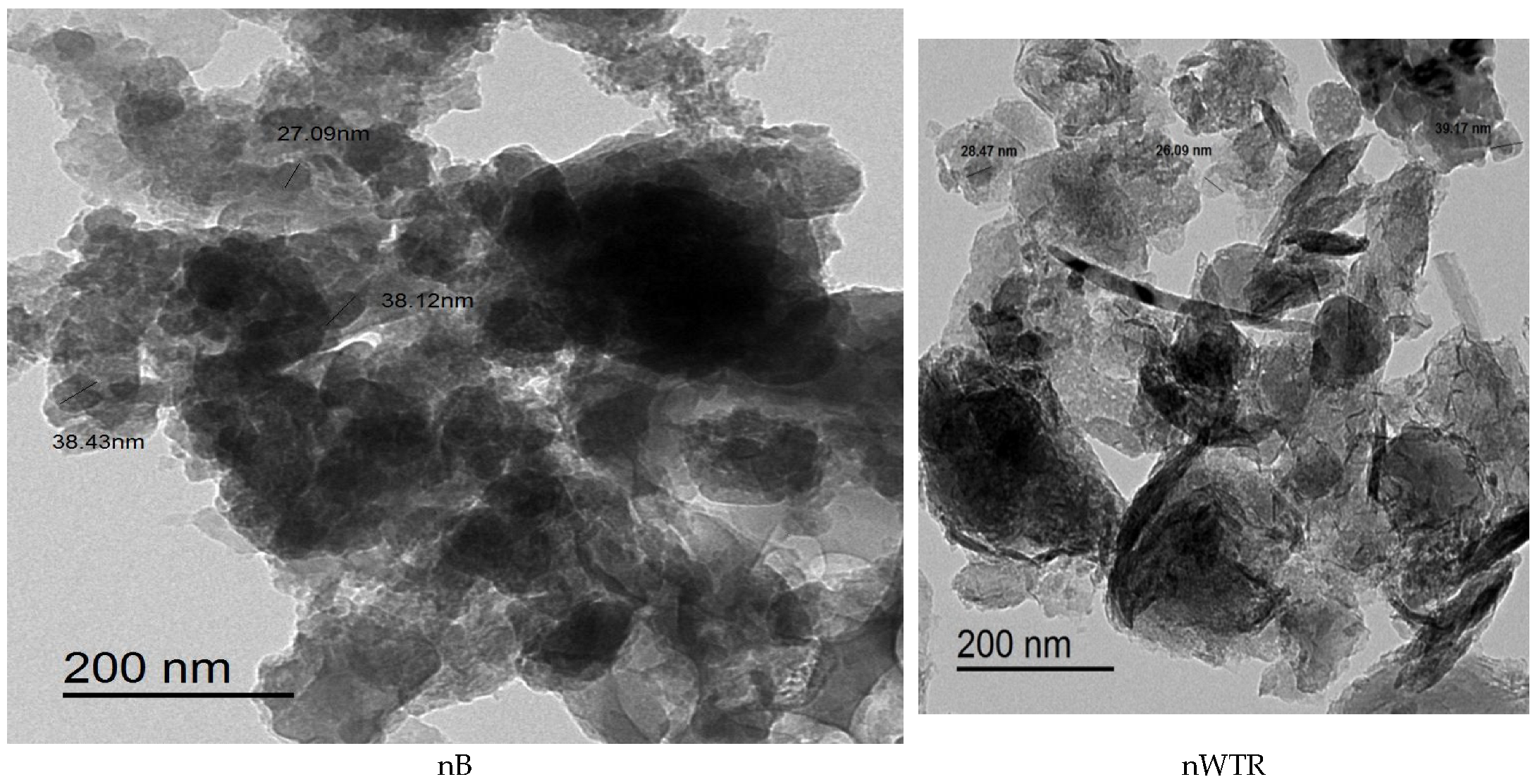
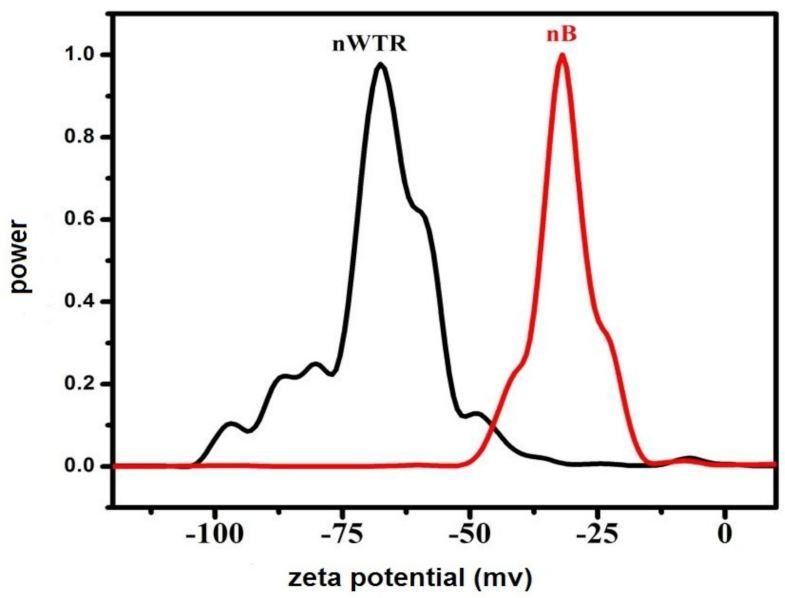
3.1. Properties of Recycled Nanomaterials
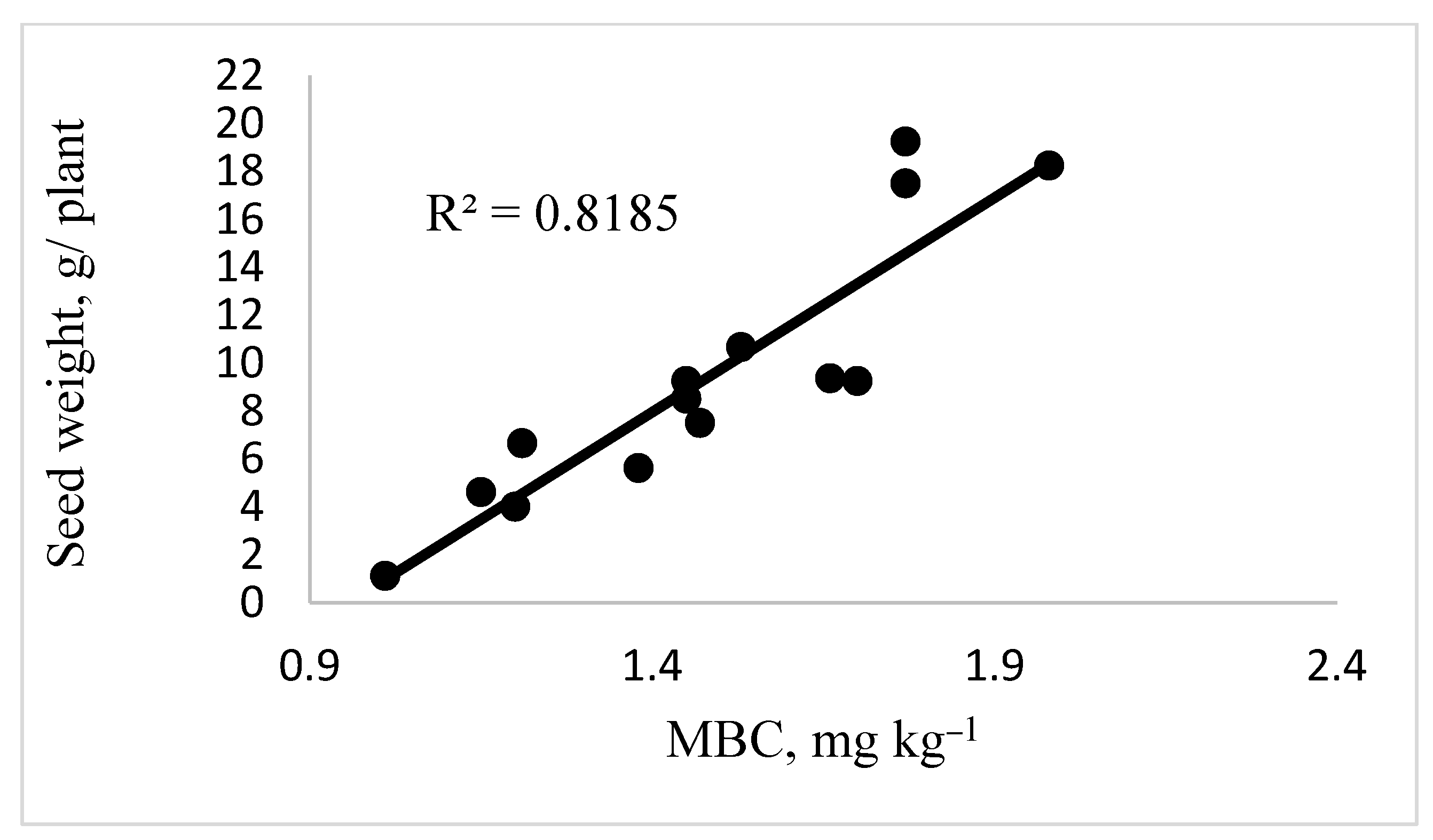
3.2. Effect of Recycled Nanomaterials on Soil Biological Activity
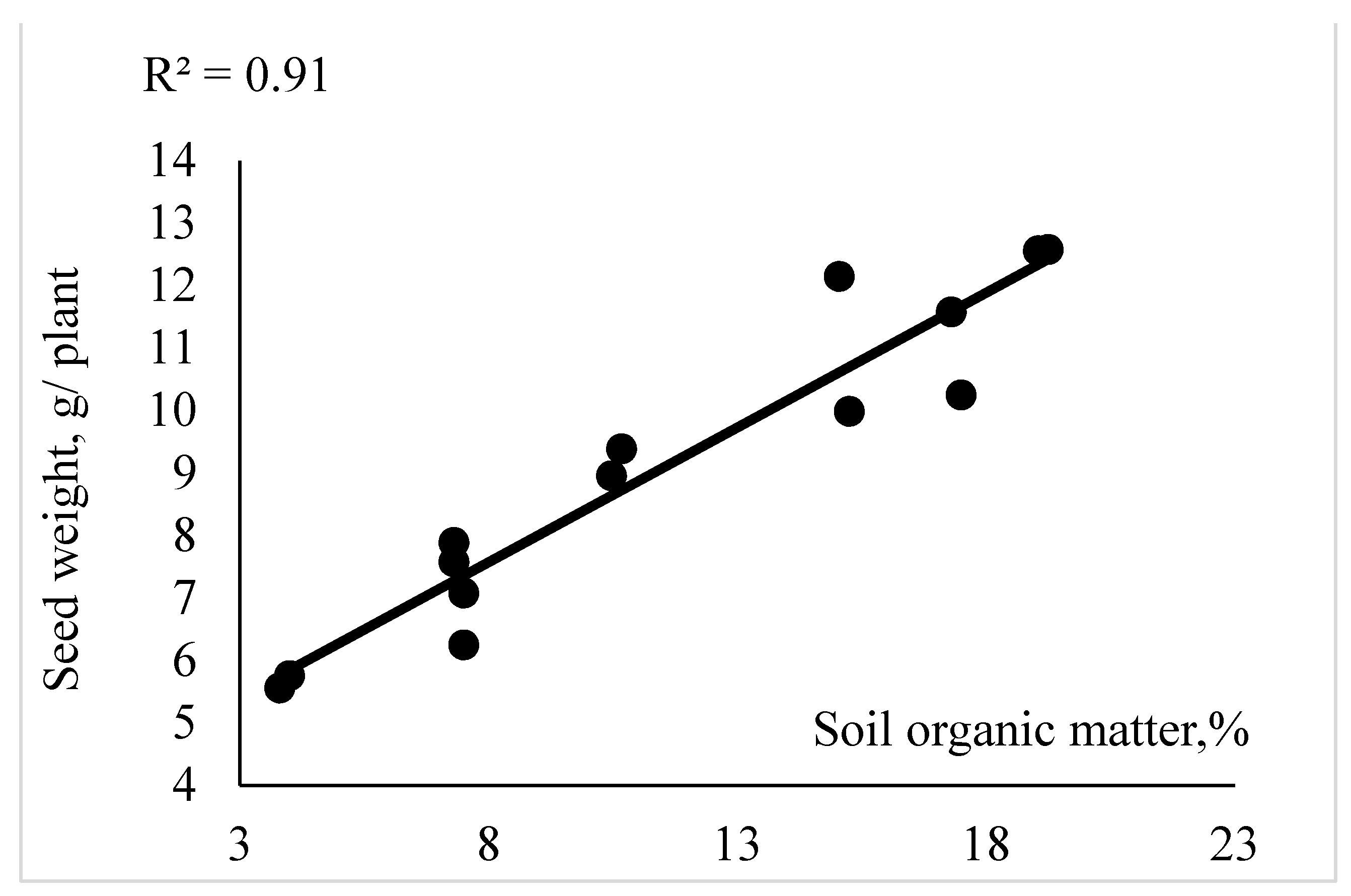
3.3. Effect of Recycled Nanomaterials on Canola Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmoud, E.; El Baroudy, A.; Ali, N.; Sleem, M. Soil amendment with nanoresidues from water treatment increases P adsorption in saline soils. Environ. Chem. Lett. 2020, 18, 171–179. [Google Scholar] [CrossRef]
- El-Shall, R.M. Studies on the Pollution by Heavy Metals in Some Adjacent Soils to Factories at EL-Gharbia Governorate, Egypt. Ph.D. Thesis, Tanta University, Tanta, Egypt, 2010. [Google Scholar]
- Mahmoud, E.; Ibrahim, M.; Robin, P.; Akkal-Corfini, N.; El-Saka, M. Rice straw composting and its effect on soil properties. Compos. Sci. Util. 2009, 17, 146–150. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.R.; Tabassum, H.E.; Ahmad, A.S.; Mabood, A.B.; Ahmad, A.D.; Ahmad, I.Z. Role of nanoparticles in growth and development of plant. Int. J. Pharm. Bio Sci. 2016, 7, 22–37. [Google Scholar] [CrossRef]
- Husein, A.; Siddiqi, K.S. Carbon and fullerene nanomaterials in plant system. J. Nanobiotechnol. 2014, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratap, T.; Patel, M.; Pittman, C.U.; Nguyen, T.A.; Mohan, D. Chapter 23—Nanobiochar: A Sustainable Solution for Agricultural and Environmental Applications. In Nanomaterials for Soil Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 501–519. ISBN 9780128228913. [Google Scholar] [CrossRef]
- Oleszczu, P.; Ćwikła-Bundyra, W.; Bogusz, A.; Skwarek, E.; Ok, Y.S. Characterization of nanoparticles of biochars from different biomass. J. Anal. Appl. Pyrolysis 2016, 121, 165–172. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Y.; Qiu, W.; Sun, Z.; Liu, Z.; Song, Z. Adsorption properties of nano-MnO2–biochar composites for copper in aqueous solution. Molecules 2017, 22, 173. [Google Scholar] [CrossRef] [Green Version]
- Yue, L.; Lian, F.; Han, Y.; Bao, Q.; Wang, Z.; Xing, B. The effect of biochar nanoparticles on rice plant growth and the uptake of heavy metals: Implications for agronomic benefits and potential risk. Sci. Total Environ. 2019, 656, 9–18. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Zhang, W.; Gardea-Torresdey, J.L.; White, J.C.; Ji, R. Silver nanoparticles alter soil microbial community compositions and metabolite profiles in unplanted and cucumber-planted soils. Environ. Sci. Technol. 2020, 54, 3334–3342. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Mao, J.; Chen, B. Effects of biochar nanoparticles on seed germination and seedling growth. Environ. Pollut. 2020, 256, 113409. [Google Scholar] [CrossRef]
- Beheshti, M.; Etesami, H.; Alikhani, H.A. Effect of different biochars amendment on soil biological indicators in a calcareous soil. Environ. Sci. Pollut. Res. 2018, 25, 14752–14761. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Zhu, T.; Ye, M. Effects of concentration and adsorption product on the adsorption of SO2 and NO on activated carbon. Energy Fuels 2013, 27, 360–366. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, B.; Hu, Z.; Lin, H. The effects of nano-biochar on maize growth in northern Shaanxi Province on the Loess Plateau. Appl. Ecol. Environ. Res. 2020, 18, 2863–2877. [Google Scholar] [CrossRef]
- Yanli, L.; Beibei, Z.; Quanjiu, W. Effects of nano-carbon on water movement and solute transport in loessial soil. J. Soil Water Conserv. 2015, 29, 21–25. [Google Scholar]
- Zhang, P.; Huang, P.; Xu, X.; Sun, H.; Jiang, B.; Liao, Y. Spectroscopic and molecular characterization of biochar-derived dissolved organic matter and the associations with soil microbial responses. Sci. Total Environ. 2019, 708, 134619. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Yu, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S. Activation of persulfate by modifed drinking water treatment residuals for sulfamethoxazole degradation. Chem. Eng. 2018, 353, 490–498. [Google Scholar] [CrossRef]
- Fouad, M.; Razek, T.M.; Elgendy, A.S. Utilization of drinking water treatment slurry to produce aluminum sulfate coagulant. Water Environ. Res. 2017, 89, 186–191. [Google Scholar] [CrossRef]
- Elkhatib, E.A.; Mahdy, A.M.; Sherif, F.K.; Salama, K.A. Water treatment residual nanoparticles: A novel sorbent for enhanced phosphorus removal from aqueous medium. Curr. Nanosci. 2015, 11, 655–668. [Google Scholar] [CrossRef]
- Mahmoud, E.; El Baroudy, A.; Ali, N.; Sleem, M. Spectroscopic studies on the phosphorus adsorption in salt-affected soils with or without nano-biochar additions. Environ. Res. 2020, 184, 1092. [Google Scholar] [CrossRef]
- Lee, C.I.; Yang, W.F.; Chiou, C.S. Utilization of water clarifier sludge for copper removal in a liquid fluidized-bed reactor. J. Hazard. Mater. 2006, 129, 58–63. [Google Scholar] [CrossRef]
- Kheir, A.; Kamara, M. Effects of sugar beet factory lime, vinasse, and compost mixed with vinasse application on sandy soil properties and canola productivity. J. Soil Sci. Agric. Eng. 2019, 10, 69–77. [Google Scholar] [CrossRef]
- Kazemeini, S.A.; Hamzehzarghani, H.; Edalat, M. The impact of nitrogen and organic matter on winter canola seed yield and yield components. Aust. J. Crop Sci. 2010, 4, 335–342. [Google Scholar]
- US EPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; Office of Solid Waste and Emergency Response: Washington, DC, USA, 2002. Available online: http://www.epa.gov/superfund/health/conmedia/soil/index.htm (accessed on 2 June 2014).
- Mahmoud, E.; El-Beshbeshy, T.; El-Kader, N.; El Shall, R.; Khalafallah, N. Impacts of biochar application on soil fertility, plant nutrients uptake and maize (Zea mays L.) yield in saline sodic soil. Arab. J. Geosci. 2019, 12, 719. [Google Scholar] [CrossRef]
- Mahmoud, E.; Ghoneim, A.; El Baroudy, A.; Abd El-Kader, N.; Aldhumri, S.; Othman, S.; El Khamisy, R. Effects of phosphogypsum and water treatment residual application on key chemical and biological properties of clay soil and maize yield. Soil Use Manag. 2021, 37, 494–503. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Methods of Soil Analysis. In Total Carbon, Organic Carbon and Organic Matter; Page, A.L., Miller, R.H., Kenny, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Methods of Soil Analysis. Part 2. In Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; Volume 9. [Google Scholar]
- Graber, E.R.; Singh, B.; Lehmann, K.H. Determination of Cation Exchange Capacity in Biochar. In Biochar: A Guide to Analytical Methods; Singh, B., Camps-Arbestain, M., Lehmann, J., Eds.; Csiro Publishing: Clayton, Australia, 2017; pp. 74–84. [Google Scholar]
- Yukselen, Y. A Study of Zeta Potential of Clay Minerals in the Presence of Various Chemical Solutions. Master’s Thesis, Dokuz Eylul University, Konak, Turkey, 2001; 82p. Submitted to the Graduate School of Natural and Applied Sciences. [Google Scholar]
- Johnson, J.I.; Temple, K.L. Some variables affecting the measurement of catalase activity in soil. Soil Sci. Soc. Am. Proc. 1964, 28, 207–216. [Google Scholar] [CrossRef]
- Thalmann, A. Zur methodik der bestimmung der dehydrogenase aktivität in boden mittels triphenyltetrazoliumchlorid (TTC) methods of dehydrogenase activity determination with triph enyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Wu, J.; Joergensen, R.G.; Pommerening, B. Measurement of soil microbial biomass C by fumigation-extraction an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Hu, X.; Cao, C.; Zhiping, A. Size and activity of the soil microbial biomass and chemical and biological properties. Commun. Soil Sci. Plant Anal. 2007, 40, 2072–2086. [Google Scholar]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Wartelle, L.H. Contaminant immobilization and nutrient release by biochar soil amendment: Roles of natural organic matter. Chemosphere 2010, 80, 935–940. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Ahmad, M.; Mohan, D.; Chang, S.X.; Ok, Y.S. Pyrolysis condition affected sulfamethazine sorption by tea waste biochars. Bioresour. Technol. 2014, 166, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Ellerbrock, R.H. Functional characterization of soil organic matter fractions different in solubility originating from a long-term field experiment. Geoderma 2005, 127, 196–206. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Wang, H.; Yu, H.; Gao, B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. 2013, 20, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yang, X.; Zhang, J.; Zhu, L.; Lv, Y. Adsorption mechanisms of dodecylbenzene sulfonic acid by corn straw and poplar leaf biochars. Materials 2017, 10, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, L.; Chen, B. Dual role of biochars as adsorbents for aluminum: The effects of oxygen-containing organic components and the scattering of silicate particles. Environ. Sci. Technol. 2013, 47, 8759–8768. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Yu, L.; Sun, T. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived bio-chars: Impact of structural properties of biochars. J. Hazard. Mater. 2013, 244, 217–224. [Google Scholar] [CrossRef]
- Tsai, W.T.; Liu, S.C.; Chen, H.R.; Chang, Y.M.; Tsai, Y.L. Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 2012, 89, 198–203. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Scheckel, K.G.; Barbarick, K.A. Selenium adsorption to aluminum-based water treatment residuals. J. Colloid Interface Sci. 2009, 338, 48–55. [Google Scholar] [CrossRef]
- Li, F.; Shen, K.; Long, X.; Wen, J.; Xie, X.; Zeng, X. Preparation and characterization of biochars from Eichorniacrassipes for cadmium removal in aqueous solutions. PLoS ONE 2016, 11, e0148132. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, J.A.; Barbarick, K.A.; Elliott, H.A. Drinking water treatment residuals: A review of recent uses. J. Environ. Qual. 2011, 40, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.; Ahmad, K.; Alam, M. Characterization and constructive utilization of sludge produced in clari- flocculation unit of water treatment plant. Mater. Res. Express 2018, 5, 035511. [Google Scholar] [CrossRef]
- Ocinski, D.; Jacukowicz-Sobala, I.; Mazur, P.; Raczyk, J.; Kociolek-Balawejder, E. Water treatment residuals containing iron and manganese oxides for arsenic removal from water—Characterization of physicochemical properties and adsorption studies. Chem. Eng. 2016, 294, 210–221. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, A.Y.; Deng, H.; Ye, C.H.; Wu, Y.Q.; Linmu, Y.D.; Hang, H.L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W.; Wang, Q.; Yang, F. Endogenous minerals have influences on surface electrochemistry and ion exchange properties of biochar. Chemosphere 2015, 136, 133–139. [Google Scholar] [CrossRef]
- Hotta, Y.; Banno, T.; Nomura, Y.; Sano, S.; Oda, K. Factors affecting the plasticity of Georgia Kaolin Green Body. J. Ceram. Soc. 1999, 107, 868–871. [Google Scholar] [CrossRef] [Green Version]
- Méndez, J.P.; García, F.P.; González, N.T.; Santillán, Y.M.; Sandoval, O.A. Zeta potential and electrophoretic mobility for the recovery of a saline soil with organic amendments. Ciênc. Agrotecnol. 2018, 42, 420–430. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyro 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Yang, W.; Shang, J.; Li, B.; Flury, M. Surface and colloid properties of biochar and implications for transport in porous media. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2484–2522. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Caporale, A.G.; Punamiya, P.; Pigna, M.; Violante, A.; Sarkar, D. Effect of particle size of drinking-water treatment residuals on the sorption of arsenic in the presence of competing ions. J. Hazard. Mater. 2013, 260, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Ying, G.G.; Kookana, R.S. Sorption and desorption behaviors of diuron in soils amended with charcoal. J. Agric. Food Chem. 2006, 54, 8545–8550. [Google Scholar] [CrossRef]
- Quinones, K.D.; Hovsepyan, A.; Oppong-Anane, A.; Bonzongo, J.C. Insights into the mechanisms of mercury sorption onto aluminum based drinking water treatment residuals. J. Hazard. Mater. 2016, 307, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silber, A.; Levkovitch, I.; Graber, E.R. pH-dependent mineral release and surface properties of corn straw biochar: Agronomic implications. Environ. Sci. Technol. 2010, 44, 9318–9323. [Google Scholar] [CrossRef]
- Günal, H.; Bayram, Ö.; Günal, E.; Erdem, H. Characterization of soil amendment potential of 18 different biochar types produced by slow pyrolysis. Eur. J. Soil Sci. 2019, 8, 329–339. [Google Scholar] [CrossRef]
- Munera-Echeverri, J.L.; Martinsen, V.; Strand, L.T.; Zivanovic, V.; Cornelissen, G.; Mulder, J. Cation exchange capacity of biochar: An urgent method modification. Sci. Total Environ. 2018, 15, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Sposito, G. The Chemistry of Soils; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Pinna, S.; Pais, A.; Campus, P.; Sechi, N.; Ceccherelli, G. Habitat preferences of the sea urchin, Paracentrotus lividus. Mar. Ecol. Prog. Ser. 2012, 445, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Mierzwa-Hersztek, M.; Wolny-Koładka, K.; Gondek, K. Effect of coapplication of biochar and nutrients on microbiocenotic composition, dehydrogenase activity index and chemical properties of sandy soil. Waste Biomass Valoriz. 2020, 11, 3911–3923. [Google Scholar] [CrossRef] [Green Version]
- Brendecke, J.W.; Axelson, R.D.; Pepper, I.L. Soil microbial activity as an indicator of soil fertility: Long-term effects of municipal sewage sludge on an arid soil. Soil Biol. Biochem. 1993, 25, 751–758. [Google Scholar] [CrossRef]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.; De Neve, S. Interactions between biochar stability and soil organisms: Review and research needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2010, 43, 296–301. [Google Scholar] [CrossRef]
- Kyncl, M. Opportunities for water treatment sludge reuse. Geo Sci. Eng. 2008, 1, 11–22. [Google Scholar]
- Erich, M.S.; First, P. Analysis of Papermill Waste Water Treatment Residuals and Process Residues. In Modern Methods of Plant Analysis—Analysis of Plant Waste Materials; Linskens, H.-F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 20. [Google Scholar]
- Wang, Y.; Han, Z.; Zhang, Z. Biochar applications promote growth parameters of soybean and reduces the growth difference. Commun. Soil Sci. Plant Anal. 2016, 47, 1493–1502. [Google Scholar] [CrossRef]
- Fincheira, P.; Tortella, G.; Duran, N.; Seabra, A.B.; Rubilar, O. Current applications of nanotechnology to develop plant growth inducer agents as an innovation strategy. Crit. Rev. Biotechnol. 2020, 40, 15–30. [Google Scholar] [CrossRef]
- Achari, G.A.; Kowshik, M. Recent developments on nanotechnology in agriculture: Plant mineral nutrition, health, and interactions with soil microflora. J. Agric. Food Chem. 2018, 66, 8647–8661. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Mahmoud, E.K.; Ibrahim, M.; Khader, A. Phosphorus fertilisation and biochar Impacts on soil fertility and wheat (Triticum aestivum) productivity under semiarid conditions. Crop Pasture Sci. 2021. [Google Scholar] [CrossRef]
- Ali, H.M. The Effect of Biochar and Compost Application in Remediating the Contaminated Soil with Some Heavy Metals and on Canola Plant Growth. Master’s Thesis, Tanta University, Tanta, Egypt, 2018. [Google Scholar]
- Xiao, Q.; Zhu, L.; Zhang, H.; Li, X.; Shen, Y.; Li, S. Soil amendment with biochar increases maize yields in a semi-arid region by improving soil quality and root growth. Crop Pasture Sci. 2016, 67, 495–507. [Google Scholar] [CrossRef]
- Zhao, F.; Xin, X.; Cao, Y.; Su, D.; Ji, P.; Zhu, Z.; He, Z. Use of carbon nanoparticles to improve soil fertility, crop growth and nutrient uptake by corn (Zea mays L.). Nanomaterials 2021, 11, 2717. [Google Scholar] [CrossRef]


| Properties | Units | Soil | nB | nWTR |
|---|---|---|---|---|
| Particle size distribution | ||||
| Clay | % | 41 | - | 68.6 |
| Silt | 33 | - | - | |
| Sand | 26 | - | - | |
| Texture | clay loam | - | - | |
| pH | 7.95 | 8.24 | 7.49 | |
| EC | dSm−1 | 4.58 | 2.45 | 1.12 |
| Ca++ | cmol kg−1 | 9.60 | 55.11 | 5.56 |
| Mg++ | 5.50 | 24.30 | 5.50 | |
| K+ | 0.89 | - | - | |
| Na+ | 31.10 | - | - | |
| Cl− | 21.80 | - | - | |
| HCO3− | 5.00 | - | - | |
| SO4−− | 3.60 | - | - | |
| SAR | 11.33 | - | - | |
| OM | g kg−1 | 13.6 | 498.0 | 48.20 |
| CEC | cmol kg−1 | - | 31.30 | 38.85 |
| Total Al | % | - | - | 0.25 |
| Available P | % | - | 856.02 | 14.46 |
| Treatments | Soil Microbial Biomass Carbon mg kg−1 | Dehydrogenase Activity mg TPF/g Dry Soil | Catalase Activity (mL of 0.02 mol/L KMnO4 g−1) |
|---|---|---|---|
| C | 162.3 h ± 0.12 | 0.64 g ± 0.01 | 0.03 c ± 0.01 |
| B | 277.8 b ± 0.09 | 0.68 f ± 0.01 | 0.07 b,c ± 0.01 |
| nB50 | 164.5 g ± 0.12 | 0.82 b ± 0.01 | 0.1 b ± 0.00 |
| nB100 | 271.3 c ± 0.12 | 0.77 c ± 0.02 | 0.08 b,c ± 0.01 |
| nB250 | 277.8 b ± 0.06 | 0.76 c ± 0.01 | 0.11 b ± 0.01 |
| WTR | 219.2 e ± 0.06 | 0.74 d ± 0.09 | 0.08 b,c ± 0.01 |
| nWTR50 | 388.9 a ± 0.11 | 0.85 a ± 0.01 | 0.2 a ± (0.06 |
| nWTR100 | 222.2 d ± 0.13 | 0.71 e ± 0.02 | 0.07 b,c ± 0.01 |
| nWTR250 | 169.7 f ± 0.06 | 0.69 f ± 0.01 | 0.08 b,c ± 0.01 |
| F-test | ** | ** | ** |
| LSD(0.05) | 0.251 | 0.017 | 0.059 |
| LSD(0.01) | 0.344 | 0.023 | 0.081 |
| Treatments | Plant Height, cm | 1000 Seeds Weight, g | Pod Number per Plant | Seeds Weight, g per Plant |
|---|---|---|---|---|
| C | 91.66 f ± 0.88 | 3.97 f ± 0.04 | 94 g ± 0.58 | 14.77 d ± 0.27 |
| B | 107 b ± 0.58 | 4.19 b ± 0.02 | 105 e ± 0.58 | 23.45 c ± 0.65 |
| nB50 | 121.66 a ± 0.88 | 4.17 b,c ± 0.01 | 108 d ± 0.33 | 23.18 c ± 0.33 |
| nB100 | 119 a ± 0.58 | 4.02 e ± 0.02 | 116 b ± 0.88 | 25.04 c ± 0.78 |
| nB250 | 96.33 e ± 0.88 | 4.15 c ± 0.02 | 115 b ± 0.58 | 34.35 b ± 0.48 |
| WTR | 101 c,d ± 1.15 | 4.08 d ± 0.01 | 112 c ± 0.33 | 36.87 a ± 0.36 |
| nWTR50 | 110 b ± 0.58 | 4.32 a ± 0.02 | 119 a ± 0.88 | 37.02 a ± 0.91 |
| nWTR100 | 98.33 d,e ± 0.67 | 4.19 b ± 0.02 | 96 f,g ± 0.33 | 23.79 c ± 0.7 |
| nWTR250 | 102.33 c ± 1.2 | 3.98 f ± 0.01 | 98 f ± 0.058 | 16.97 d ± 0.33 |
| F-test | ** | ** | ** | ** |
| LSD(0.05) | 2.535 | 1.777 | 1.777 | 1.711 |
| LSD(0.01) | 3.474 | 2.435 | 2.435 | 2.344 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsawy, H.; El-shahawy, A.; Ibrahim, M.; El-Halim, A.E.-H.A.; Talha, N.; Sedky, A.; Alfwuaires, M.; Alabbad, H.; Almeri, N.; Mahmoud, E. Properties of Recycled Nanomaterials and Their Effect on Biological Activity and Yield of Canola in Degraded Soils. Agriculture 2022, 12, 2096. https://doi.org/10.3390/agriculture12122096
Elsawy H, El-shahawy A, Ibrahim M, El-Halim AE-HA, Talha N, Sedky A, Alfwuaires M, Alabbad H, Almeri N, Mahmoud E. Properties of Recycled Nanomaterials and Their Effect on Biological Activity and Yield of Canola in Degraded Soils. Agriculture. 2022; 12(12):2096. https://doi.org/10.3390/agriculture12122096
Chicago/Turabian StyleElsawy, Hany, Asmaa El-shahawy, Mahmoud Ibrahim, Abd El-Halim Abd El-Halim, Naser Talha, Azza Sedky, Manal Alfwuaires, Hebah Alabbad, Nawa Almeri, and Esawy Mahmoud. 2022. "Properties of Recycled Nanomaterials and Their Effect on Biological Activity and Yield of Canola in Degraded Soils" Agriculture 12, no. 12: 2096. https://doi.org/10.3390/agriculture12122096
APA StyleElsawy, H., El-shahawy, A., Ibrahim, M., El-Halim, A. E.-H. A., Talha, N., Sedky, A., Alfwuaires, M., Alabbad, H., Almeri, N., & Mahmoud, E. (2022). Properties of Recycled Nanomaterials and Their Effect on Biological Activity and Yield of Canola in Degraded Soils. Agriculture, 12(12), 2096. https://doi.org/10.3390/agriculture12122096






