Pomegranate Quality Evaluation Using Non-Destructive Approaches: A Review
Abstract
:1. Introduction
2. Quality Attributes of Pomegranate Fruit
2.1. External Quality Attributes of Pomegranate Fruit
| Quality Attributes | Cultivar | Intact Fruit | Typical Values | References | |||
|---|---|---|---|---|---|---|---|
| Fresh Aril | Dried Aril | Seed Oil | Juice | ||||
| Weight (g) | Bhagwa, Ruby | 250.0–509.8 | [39,49] | ||||
| Shape index | Bhagwa, Ruby | 0.91–1.10 | [39] | ||||
| Volume (cm3) | 220–300 | [39] | |||||
| Sphericity | 1.02–1.08 | [39] | |||||
| Aril yield (%) | 46.76–58.82 | [39] | |||||
| CIE colour coordinates (L*) | Oman, Bhagwa, Ruby | 44.15–46.51 | 25.00–30.88 | 20.54–33.62 | [10,15,39,49] | ||
| (a*) | 40.33–43.13 | 16.06–23.07 | 12.26–24.44 | 3.37–4.73 | |||
| (b*) | – | 6.63–7.77 | 0.15–0.52 | ||||
| (C*) | 48.35–53.39 | 15.75–17.82 | 12.84–29.83 | 3.38–4.77 | |||
| (h°) | 30.61–33.07 | 23.85–25.81 | 12.00–27.10 | 3.12–3.30 | |||
| Moisture (%) | Oman, Bhagwa, Ruby | 66.00–75.58 | |||||
| Total soluble solids (%) | Ruby, Wonderful | 28.9 | 17.5–22.2 | 50.1–77.3 | [49,50] | ||
| TSS (°Brix) | 13.70–15.21 | 1.14–3.15 | 5.8–14.27 | ||||
| TA (%CA) | 3.29–3.93 | ||||||
| pH | Bhagwa, Ruby, Wonderful | 3.60–3.87 | [15,49] | ||||
| Anthocyanins (mg/100 g) | Wonderful | 9.73 | [49] | ||||
| TPC (mg/100 g) | 7.03 ± 0.19 | ||||||
| TSS/TA | [49] | ||||||
| PV (meqO2/kg) | Wonderful, Herskawitz, Acco | 0.04–0.35 | [51] | ||||
| RI | 1.5215–1.5218 | ||||||
| AV | 43.39–125.26 | 2.00–14.22 | |||||
| TOTOX | 105.9 | 2.53–14.30 | |||||
| TCC (mg β-carotene/100 g) | Wonderful, Herskawitz, Acco | 19.25–22.26 | [52] | ||||
| TPC (mg GAE/g) | 1.91–3.45 | [50,52] | |||||
| YI (25 °C) | 75.53–83.76 | 65.47–91.52 | |||||
| Firmness (N) | Shavel, Bhagwa, Ruby | 101.33–154.63 | 67.44–99.20 | [45,46] | |||
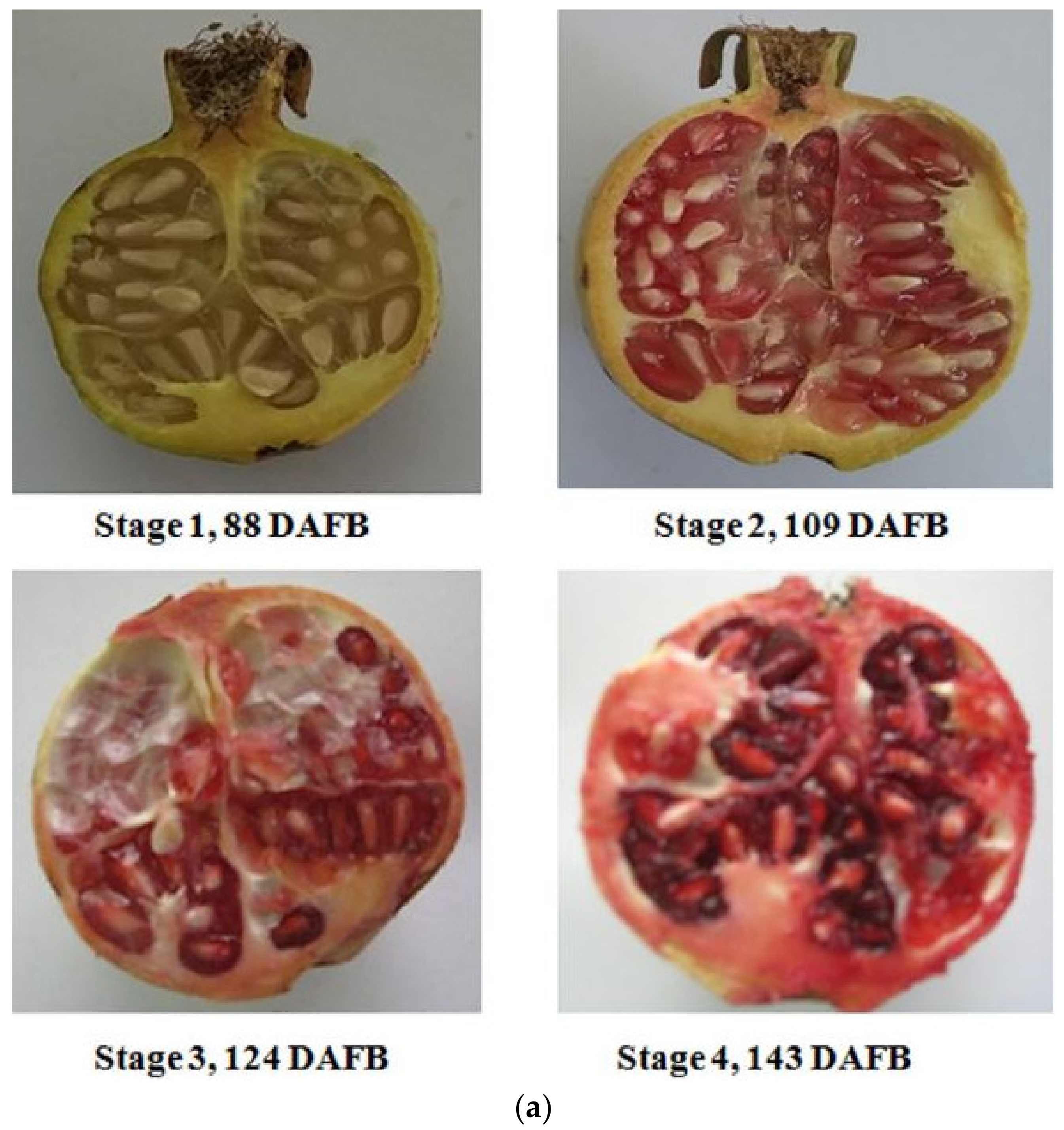
2.2. Internal Quality Attributes of Pomegranate Fruit
2.3. Quality Attributes of Pomegranate Products
3. Non-Destructive Methods for Quality Evaluation of Intact Pomegranate Fruit
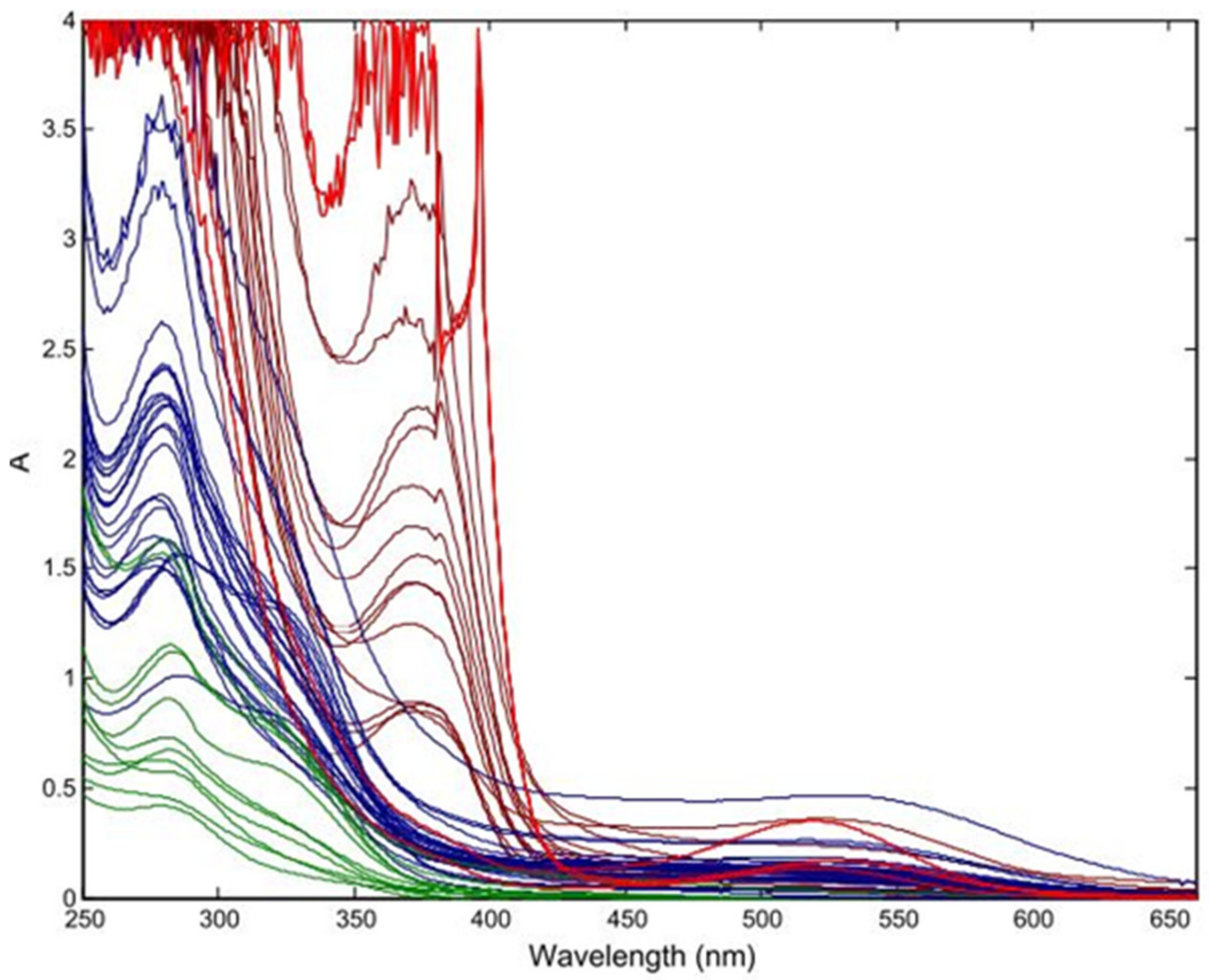
3.1. Infrared (IR) Spectroscopy
3.1.1. Application on Intact Fruit
3.1.2. For Internal Quality Parameters
3.1.3. Application on Processed Products
| Quality Attributes | Prediction Statistics | Data Analysis | References |
|---|---|---|---|
| TSS TA pH | R2 = 0.960, RMSEP = 0.092 °Brix R2 = 0.920, RMSEP = 0.19% R2 = 0.920, RMSEP = 0.089 | PLS, PCA | [70] |
| TSS TA pH | R2 = 0.920, RMSEP = 0.23 °Brix R2 = 0.930, RMSEP = 0.26% R2 = 0.800, RMSEP = 0.064 | PLS, PCA | [22] |
| TSS TA pH TAC TPC Brim A TSS/TA Hue angle Vitamin C Chroma a* Firmness (N) Hue Fruit Weight | R2 = 0.781, RMSEP = 0.28% R2 = 0.768, RMSEP = 0.13% R2 = 0.849, RMSEP = 0.06 R2 = 0.626, RMSEP = 0.09 g/L R2 = 0.889, RMSEP = 0.11 g/L R2 = 0.762, RMSEP = 0.39 R2 = 0.868, RMSEP = 0.74 R2 = 0.466, RMSEP = 1.67 R2 = 0.762, RMSEP = 0.09 g/L R2 = 0.830, RMSEP = 2.15 R2 = 0.909, RMSEP = 1.61 R2 = 0.830, RMSEP = 7.45 R2 = 0.839, RMSEP = 1.67 R2 = 0.621, RMSEP = 0.013 | PLS, PCA | [75] |
| TSS Firmness (N) pH | R2 = 0.940, RMSEP = 0.21 °Brix R2 = 0.940, RMSEP = 0.68 R2 = 0.860, RMSEP = 0.069 | PLS, PCA | [74] |
| Ectomyelois ceratoniae infestation | CA = 97.9% | PCA-DA | [72] |
| Presence of husk scald | CA ≥ 92.6% | OPLS-DA | [71] |
| Carob moth infestation detection | CA ≥ 86% | PLS-DA | [73] |
3.2. Raman Spectroscopy
4. Imaging-Based Non-Destructive Techniques for Evaluating Pomegranate Quality
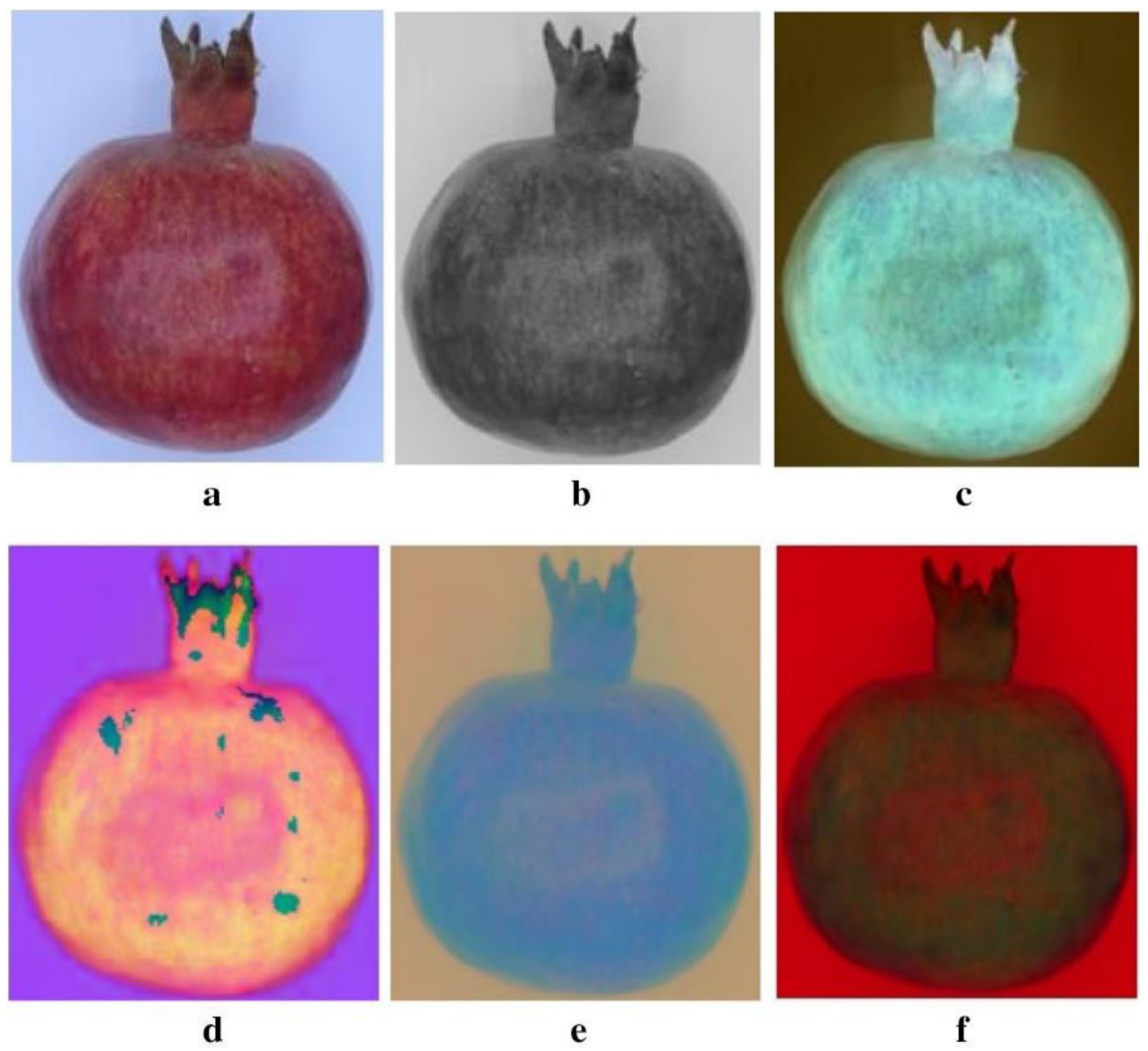
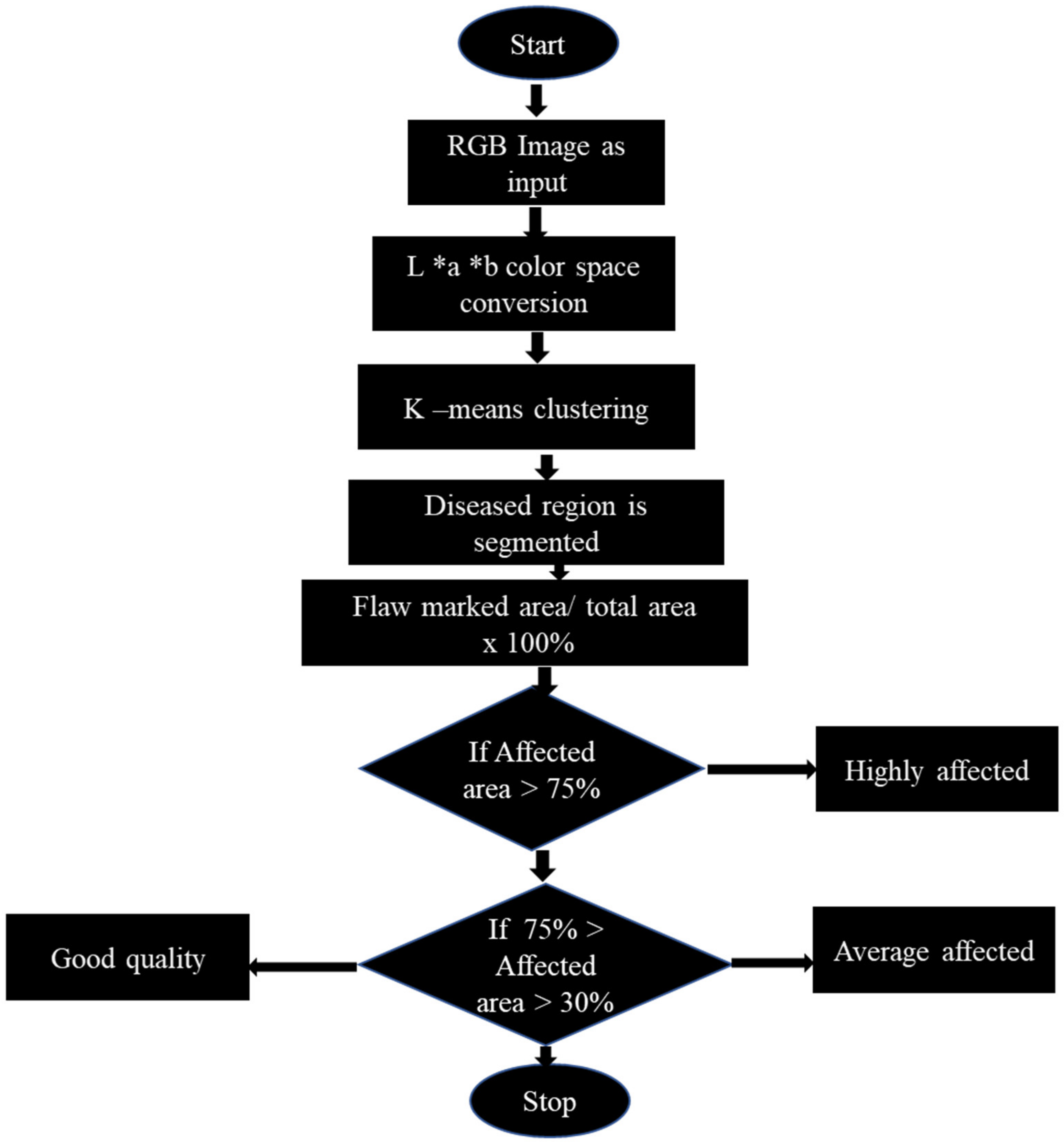
4.1. Machine Vision Systems (MVS)
4.1.1. Application on Intact Fruit
4.1.2. Application on Processed Products
| Technique | Application | Data Analysis | Accuracy | References |
|---|---|---|---|---|
| X-ray | Volume estimation | STA | [99] | |
| MVS | Grading | 2DLDA, FLDA, F2DLDA, FF2DLDA | 97% | [91] |
| MVS | Grading | ANN | 97.83% | [25] |
| MVS | Grading | ANN | 77.46–91.3% | [89] |
| NMR | Black heart | PLS-DA | 92% | [100] |
| E-nose system | Fungal disease | LDA, BPNN and SVM | 100% | [101] |
| MVS | Disease | - | 79.73% | [1] |
| Raman spectroscopy | Tannin changes | PLS | R2 = 0.9603 | [74] |
| MVS | Aril color and size | ANN, ANFIS, RSM | 75.5–98% | [94] |
| E-nose | Fruit ripening | PCA, LDA | 95.20% | [102] |
| MVS | pH | ANFIS, RSM, ANN | R2 = 0.984, MSE = 0.202 | [90] |
| Raman | Maturity indexing | PLS-DA, SIMCA and PCA | 95% | [85] |
| X-Ray | Disease detection | - | t value = 0.469 with a 95% loss | [103] |
| MVS | Industrial grading of fresh aril | LDA, threshold on the R/G ratio | 83.3–100% | [93] |
| MVS | Preharvest yield estimation | adaptive threshold algorithm | ER = 91% | [96] |
| MVS | On-tree fruit recognition | RGB, HSV and YCC colour space analysis | 100% | [97] |
| MVS | Yield estimation | CHT, K-Means Clustering | R2 = 0.7652 | [98] |
| MSV | Physicochemical attributes | PCA, PLS-R | [23] | |
| HSI | Maturity indexing | PLS-DA | 95.0%, | [23] |
| MSI | TSS, TA and pH | PLS, MLR | R2 ≥ 0.88, RPD ≥ 5.01 | [22] |
4.2. Nuclear Magnetic Resonance (NMR) and Magnetic Resonance Imaging (MRI)
4.3. X-ray Computed Tomography
4.4. Hyperspectral and Multispectral Imaging
5. Electronic Nose (E-Nose)
6. Challenges of Non-Destructive Measurement for Pomegranate Fruit
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, C.; Sethy, P.K.; Biswas, P.; Behera, S.K.; Khan, M.R. Quality evaluation of pomegranate fruit using image processing techniques. In Proceedings of the 2020 International Conference on Communication and Signal Processing (ICCSP), Chennai, India, 28–30 July 2020; p. 19914067. [Google Scholar] [CrossRef]
- Opara, U.L.; Pathare, P.B. Bruise damage measurement and analysis of fresh horticultural produce—A review. Postharvest Biol. Technol. 2014, 91, 9–24. [Google Scholar] [CrossRef]
- Spielmanns, R.; Spielmanns, J.; Damerow, L.; Blanke, M.M. Non-destructive determination of surface features of pomegranate fruit. Acta Hortic. 2016, 1137, 247–250. [Google Scholar] [CrossRef]
- Khoshroo, A.; Keyhani, A.; Rafiee, S.; Zoroofi, R.A.; Zamani, Z. Pomegranate quality evaluation using machine vision. Acta Hortic. 2009, 818, 347–352. [Google Scholar] [CrossRef]
- Wang, H.; Peng, J.; Xie, C.; Bao, Y.; He, Y. Fruit quality evaluation using spectroscopy technology: A review. Sensors 2015, 15, 11889–11927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czieczor, L.; Bentkamp, C.; Damerow, L.; Blanke, M. Non-invasive determination of the quality of pomegranate fruit. Postharvest Biol. Technol. 2018, 136, 74–79. [Google Scholar] [CrossRef]
- Elmasry, G.; Kamruzzaman, M.; Sun, D.-W.; Allen, P. Principles and applications of hyperspectral imaging in quality evaluation of agro-food products: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 8398. [Google Scholar] [CrossRef]
- Matityahu, I.; Marciano, P.; Holland, D.; Ben-Arie, R.; Amir, R. Differential effects of regular and controlled atmosphere storage on the quality of three cultivars of pomegranate (Punica granatum L.). Postharvest Biol. Technol. 2016, 115, 132–141. [Google Scholar] [CrossRef]
- Khoshroo, A.; Keyhani, A.; Zoroofi, R.A.; Rafiee, S.; Zamani, Z.; Alsharif, M.R. Classification of pomegranate fruit using texture analysis of MR images. Agric. Eng. Int. CIGR J. 2009, 11, 1182. [Google Scholar]
- Okere, E.E. Non-Invasive Measurement of Quality Attributes of Processed Pomegranate Products. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. [Google Scholar]
- Pareek, S.; Valero, D.; Serrano, M. Postharvest biology and technology of pomegranate. J. Sci. Food Agric. 2015, 95, 2360–2379. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Sadeghi, R.; Kokini, J. Pomegranate as a promising opportunity in medicine and nanotechnology. Trends Food Sci. Technol. 2017, 69, 59–73. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Developmental changes in maturity indices of pomegranate fruit: A descriptive review. Sci. Hortic. 2013, 159, 152–161. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Opara, L.U.; Al-Ani, M.R.; Al-Shuaibi, Y.S. Physico-chemical properties, vitamin c content, and antimicrobial properties of pomegranate fruit (Punica granatum L.). Food Bioprocess Technol. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Holland, D.; Hatib, K.; Barya, I. Pomegranate: Botany, horticulture, breeding. In Horticultural Reviews; John Wiley Sons, Inc.: Hoboken, NJ, USA, 2009; Volume 35, pp. 127–192. [Google Scholar]
- Dhinesh, K.; Ramasamy, D. Pomegranate processing and value addition: Review. J. Food Process. Technol. 2016, 7, 565. [Google Scholar] [CrossRef] [Green Version]
- Akuru, E.A.; Oyeagu, C.E.; Mpendulo, T.C.; Rautenbach, F.; Oguntibeju, O.O. Effect of pomegranate (Punica granatum L.) peel powder meal dietary supplementation on antioxidant status and quality of breast meat in broilers. Heliyon 2020, 6, e05709. [Google Scholar] [CrossRef]
- Akuru, E.A.; Mpendulo, C.T.; Oyeagu, C.E.; Nantapo, C.W.T. Pomegranate (Punica granatum L.) peel powder meal supplementation in broilers: Effect on growth performance, digestibility, carcase and organ weights, serum and some meat antioxidant enzyme biomarkers. Ital. J. Anim. Sci. 2021, 20, 119–131. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; la Grange, C.; Stander, M.A.; Fawole, O.A.; Opara, U.L. Blanching pre-treatment promotes high yields, bioactive compounds, antioxidants, enzyme inactivation and antibacterial activity of ‘wonderful’ pomegranate peel extracts at three different harvest maturities. Antioxidants 2021, 10, 1119. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Stander, M.A.; Opara, U.L. Antioxidant, antimicrobial, and metabolomic characterization of blanched pomegranate peel extracts: Effect of cultivar. Molecules 2022, 27, 2979. [Google Scholar] [CrossRef]
- Khodabakhshian, R.; Emadi, B.; Khojastehpour, M.; Golzarian, M.R.; Sazgarnia, A. Development of a multispectral imaging system for online quality assessment of pomegranate fruit. Int. J. Food Prop. 2017, 20, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Munera, S.; Hernández, F.; Aleixos, N.; Cubero, S.; Blasco, J. Maturity monitoring of intact fruit and arils of pomegranate cv. ‘Mollar de Elche’ using machine vision and chemometrics. Postharvest Biol. Technol. 2019, 156, 110936. [Google Scholar] [CrossRef]
- Zhang, L.; McCarthy, M.J. Assessment of pomegranate postharvest quality using nuclear magnetic resonance. Postharvest Biol. Technol. 2013, 77, 59–66. [Google Scholar] [CrossRef]
- Kumar, A.; Rajpurohit, V.S.; Bidari, K.Y. Multi class grading and quality assessment of pomegranate fruits based on physical and visual parameters. Int. J. Fruit Sci. 2019, 19, 372–396. [Google Scholar] [CrossRef]
- Arendse, E.; Amos, O.; Samukelo, L.; Linus, U. Non-destructive prediction of internal and external quality attributes of fruit with thick rind: A review. J. Food Eng. 2018, 217, 11–23. [Google Scholar] [CrossRef]
- Nturambirwe, J.F.I.; Opara, U.L. Machine learning applications to non-destructive defect detection in horticultural products. Biosyst. Eng. 2020, 189, 60–83. [Google Scholar] [CrossRef]
- Okere, E.E.; Arendse, E.; Nieuwoudt, H.; Fawole, O.A.; Perold, W.J.; Opara, U.L. Non-invasive methods for predicting the quality of processed horticultural food products, with emphasis on dried powders, juices and oils: A review. Foods 2021, 10, 3061. [Google Scholar] [CrossRef]
- Su, W.-H.; Sun, D.-W. Fourier transform infrared and Raman and hyperspectral imaging techniques for quality determinations of powdery foods: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 104–122. [Google Scholar] [CrossRef]
- Su, W.-H.; He, H.-J.; Sun, D.-W. Non-destructive and rapid evaluation of staple foods quality by using spectroscopic techniques: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1039–1051. [Google Scholar] [CrossRef]
- Zhen, O.P.; Hashim, N.; Maringgal, B. Quality evaluation of mango using non-destructive approaches: A review. J. Agric. Food Eng. 2020, 1, 0003. [Google Scholar] [CrossRef]
- Arendse, E.; Nieuwoudt, H.; Magwaza, L.S.; Fredric, J.; Nturambirwe, I.; Fawole, O.A.; Opara, U.L. Recent advancements on vibrational spectroscopic techniques for the detection of authenticity and adulteration in horticultural products with a specific focus on oils, juices and powders. Food Bioprocess Technol. 2020, 14, 1–22. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.J.R.; Saeys, W. NIR spectroscopy applications for internal and external quality analysis of citrus fruit—A review. Food Bioprocess Technol. 2012, 5, 425–444. [Google Scholar] [CrossRef]
- Sun, T.; Huang, K.; Xu, H.; Ying, Y. Research advances in nondestructive determination of internal quality in watermelon/melon: A review. J. Food Eng. 2010, 100, 569–577. [Google Scholar] [CrossRef]
- Seeram, N.P.; Zhang, Y.; Reed, J.D.; Krueger, C.G.; Vaya, J. Pomegranates. In Ancient Roots to Modern Medicine; Medicinal and Aromatic Plants—Industrial Profiles; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–263. [Google Scholar]
- Teixeira da Silva, J.A.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Fukuda, S. Emotion and innovation. In Emotional Engineering; Springer: Cham, Switzerland, 2013; Volume 2, pp. 11–21. [Google Scholar]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.A.; Opara, U.L. Effects of maturity status on biochemical content, polyphenol composition and antioxidant capacity of pomegranate fruit arils (cv. ‘Bhagwa’). S. Afr. J. Bot. 2013, 85, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Mphahlele, R.R.; Caleb, O.J.; Fawole, O.A.; Opara, U.L. Effects of different maturity stages and growing locations on changes in chemical, biochemical and aroma volatile composition of “Wonderful” pomegranate juice. J. Sci. Food Agric. 2016, 96, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Opara, I.K.; Fawole, O.A.; Kelly, C.; Opara, U.L. Quantification of on-farm pomegranate fruit postharvest losses and waste, and implications on sustainability indicators: South African case study. Sustainability 2021, 13, 5168. [Google Scholar] [CrossRef]
- Opara, I.K.; Fawole, O.A.; Opara, U.L. Postharvest losses of pomegranate fruit at the packhouse and implications for sustainability indicators. Sustainability 2021, 13, 5187. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Zele, A.J.; Feigl, B.; Adhikari, P.; Maynard, M.L.; Cao, D. Melanopsin photoreception contributes to human visual detection, temporal and colour processing. Sci. Rep. 2018, 8, 3842. [Google Scholar] [CrossRef] [Green Version]
- Al-Said, F.A.; Opara, L.U.; Al-Yahyai, R.A. Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman. J. Food Eng. 2009, 90, 129–134. [Google Scholar] [CrossRef]
- Ekrami-Rad, N.; Khazaei, J.; Khoshtaghaza, M.H. Selected mechanical properties of pomegranate peel and fruit. Int. J. Food Prop. 2011, 14, 570–582. [Google Scholar] [CrossRef]
- Rao, M.A.; Steffe, J.F. Viscoelastic Properties of Foods; Elsevier Applied Science: New York, NY, USA, 1992. [Google Scholar]
- Cavalcante, I.H.L.; Cavalcante, L.F.; de Sousa Miranda, J.M.; Martins, A.B.G. Physical and chemical characteristics of tropical and non-conventional fruits. In Food Industrial Processes—Methods and Equipment; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Fawole, O.A.; Opara, U.L. Changes in physical properties, chemical and elemental composition and antioxidant capacity of pomegranate (cv. Ruby) fruit at five maturity stages. Sci. Hortic. 2013, 150, 37–46. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of hot-air and freeze-drying on the quality attributes of dried pomegranate (Punica granatum L.) arils during long-term cold storage of whole fruit. Agriculture 2020, 10, 493. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Effect of microwave pretreatment of seeds on the quality and antioxidant capacity of pomegranate seed oil. Foods 2020, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Kaseke, T.; Fawole, O.A.; Mokwena, L.; Opara, U.L. Effect of cultivar and blanching of pomegranate seeds on physicochemical properties, nutritional qualities and antioxidant capacity of extracted oil. J. Food Meas. Charact. 2021, 15, 93–106. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-Lóaez, J.; Pérez-álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Parashar, A.; Gupta, S.K.; Kumar, A. Studies on separation techniques of pomegranate seeds and their effect on quality of Anardana. African J. Biochem. Res. 2009, 3, 340–343. [Google Scholar]
- More, V.; Kapse, B.; Kadam, A.; Pimpalpalle, L. Studies on drying of pomegranate arils for preparation of anardana. Int. J. Chem. Stud. 2017, 5, 724–728. [Google Scholar]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of blanching on enzyme inactivation, physicochemical attributes and antioxidant capacity of hot-air dried pomegranate (Punica granatum L.) arils (cv. wonderful). Processes 2021, 9, 25. [Google Scholar] [CrossRef]
- Bakshi, P.; Bhushan, B.; Wali, V.K.; Bakshi, M.; Sharma, A.; Bhat, D.J. Standardization of drying method and organoleptic evaluation of wild pomegranate (Anardana) seeds. World J. Agric. Sci. 2013, 9, 397–400. [Google Scholar]
- Singh, D.; Dayal, H. Drying of pomegranate seeds (Anardana) under different conditions. Acta Hortic. 2015, 890, 433–439. [Google Scholar] [CrossRef]
- Khoddami, A.; Man, Y.B.C.; Roberts, T.H. Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur. J. Lipid Sci. Technol. 2014, 116, 553–562. [Google Scholar] [CrossRef]
- Venkata, C.; Prakash, S.; Prakash, I. Bioactive chemical constituents from pomegranate (Punica granatum) juice, seed and peel-A review. Int. J. Res. Chem. Environ. Int. J. Res. Chem. Environ. 2011, 1, 1–181. [Google Scholar]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Novel seeds pretreatment techniques: Effect on oil quality and antioxidant properties: A review. J. Food Sci. Technol. 2021, 58, 4451–4464. [Google Scholar] [CrossRef]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Mokwena, L.M.; Opara, U.L. Effect of extraction method on chemical, volatile composition and antioxidant properties of pomegranate juice. S. Afr. J. Bot. 2016, 103, 135–144. [Google Scholar] [CrossRef]
- Mphahlele, R.R. Impacts of Preharvest and Postharvest Handling and Processing on Bioactive Compounds and Functional Properties of Pomegranate Fruit Fractions and by-Products. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2016. [Google Scholar]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A Review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Nicolaï, B.; Terry, L.A. The use of Vis/NIRS and chemometric analysis to predict fruit defects and postharvest behaviour of ‘Nules Clementine’ mandarin fruit. Food Chem. 2014, 163, 267–274. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Bulens, I.; De Baerdemaker, J.; De Ketelaere, B.; Hertog, M.L.A.T.M.; Verboven, P.; Lammertyn, J. Non-destructive evaluation: Detection of external and internal attributes frequently associated with quality and damage. In Postharvest Handling, 3rd ed.; A Systems Approach; Academic Press: Cambridge, MA, USA, 2014; pp. 363–385. [Google Scholar]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Nieuwoudt, H.H.; Opara, U.L. Development of calibration models for the evaluation of pomegranate aril quality by Fourier-transform near infrared spectroscopy combined with chemometrics. Biosyst. Eng. 2017, 159, 22–32. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Nieuwoudt, H.H.; Opara, U.L. Fourier transform near infrared diff use reflectance spectroscopy and two spectral acquisition modes for evaluation of external and internal quality of intact pomegranate fruit. Postharvest Biol. Technol. 2018, 138, 91–98. [Google Scholar] [CrossRef]
- Khodabakhshian, R.; Emadi, B.; Khojastehpour, M.; Golzarian, M.R.; Sazgarnia, A. Quick quality evaluation of pomegranate arils using NIR spectroscopy. Iran. Res. Organ. Food Sci. Technol. 2015, 2, 103–114. [Google Scholar]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Nieuwoudt, H.; Opara, U.L. Evaluation of biochemical markers associated with the development of husk scald and the use of diffuse reflectance NIR spectroscopy to predict husk scald in pomegranate fruit. Sci. Hortic. 2018, 232, 240–249. [Google Scholar] [CrossRef]
- Jamshidi, B.; Mohajerani, E.; Farazmand, H.; Mahmoudi, A.; Hemmati, A. Pattern recognition-based optical technique for non-destructive detection of Ectomyelois ceratoniae infestation in pomegranates during hidden activity of the larvae. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2019, 206, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshian, R.; Emadi, B.; Khojastehpour, M.; Golzarian, R.; Sazgarnia, A. Non-destructive evaluation of maturity and quality parameters of pomegranate fruit by visible/near infrared spectroscopy. Int. J. Food Prop. 2016, 20, 41–52. [Google Scholar] [CrossRef]
- Khodabakhshian, R. Feasibility of using Raman spectroscopy for detection of tannin changes in pomegranate fruits during maturity. Sci. Hortic. 2019, 257, 108670. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Nieuwoudt, H.; Opara, U.L. Comparing the analytical performance of near and mid infrared spectrometers for evaluating pomegranate juice quality. Food Sci. Technol. 2018, 91, 180–190. [Google Scholar] [CrossRef]
- Vardin, H.; Tay, A.; Ozen, B.; Mauer, L. Authentication of pomegranate juice concentrate using FTIR spectroscopy and chemometrics. Food Chem. 2008, 108, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Boggia, R.; Casolino, M.C.; Hysenaj, V.; Oliveri, P.; Zunin, P. A screening method based on UV-Visible spectroscopy and multivariate analysis to assess addition of filler juices and water to pomegranate juices. Food Chem. 2013, 140, 735–741. [Google Scholar] [CrossRef]
- Okere, E.E.; Nieuwoudt, H.; Perold, W.J.; Opara, U.L. Non-destructive evaluation of the quality characteristics of pomegranate kernel oil by fourier transform near-infrared and mid-infrared spectroscopy. Front. Plant Sci. 2022, 13, 867555. [Google Scholar] [CrossRef]
- Uncu, O.; Napiórkowska, A.; Szajna, T.K.; Ozen, B. Evaluation of three spectroscopic techniques in determination of adulteration of cold pressed pomegranate seed oils. Microchem. J. 2020, 158, 105128. [Google Scholar] [CrossRef]
- Adiani, V.; Gupta, S.; Ambolikar, R.; Variyar, P.S. Development of rapid method to assess microbial quality of minimally processed pomegranate arils using FTIR. Sensors Actuators B. 2018, 260, 800–807. [Google Scholar] [CrossRef]
- Yang, D.; Ying, Y. Applications of Raman spectroscopy in agricultural products and food analysis: A review. Appl. Spectrosc. Rev. 2011, 46, 539–560. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Xu, Y.; Zhong, P.; Jiang, A.; Shen, X.; Li, X.; Xu, Z.; Shen, Y.; Sun, Y.; Lei, H. Raman spectroscopy coupled with chemometrics for food authentication: A review. Trends Anal. Chem. 2020, 131, 116017. [Google Scholar] [CrossRef]
- Nikbakht, A.M.; Hashjin, T.T.; Malekfar, R.; Gobadian, B. Nondestructive determination of tomato fruit quality parameters using raman spectroscopy. J. Agr. Sci. Tech. 2011, 13, 517–526. [Google Scholar]
- Khodabakhshian, R.; Abbaspour-Fard, M.H. Pattern recognition-based Raman spectroscopy for non-destructive detection of pomegranates during maturity. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 231, 118127. [Google Scholar] [CrossRef]
- Bhargava, A.; Bansal, A. Fruits and vegetables quality evaluation using computer vision: A review. J. King Saud Univ.-Comput. Inf. Sci. 2021, 33, 243–257. [Google Scholar] [CrossRef]
- Penumuru, D.P.; Muthuswamy, S.; Karumbu, P. Identification and classification of materials using machine vision and machine learning in the context of industry 4.0. J. Intell. Manuf. 2020, 31, 1229–1241. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Maktedar, D.D. A role of computer vision in fruits and vegetables among various horticulture products of agriculture fields: A survey. Inf. Process. Agric. 2020, 7, 183–203. [Google Scholar] [CrossRef]
- Kumar, R.A.; Rajpurohit, V.S.; Nargund, V.B. A neural network assisted machine vision system for sorting pomegranate fruits. In Proceedings of the 2017 Second International Conference on Electrical, Computer and Communication Technologies (ICECCT), Coimbatore, India, 22–24 February 2017. [Google Scholar] [CrossRef]
- Fashi, M.; Naderloo, L.; Javadikia, H. Pomegranate grading based on pH using image processing and artificial intelligence. J. Food Meas. Charact. 2020, 14, 3112–3121. [Google Scholar] [CrossRef]
- Gurubelli, Y.; Ramanathan, M.; Ponnusamy, P. Fractional fuzzy 2DLDA approach for pomegranate fruit grade classification. Comput. Electron. Agric. 2019, 162, 95–105. [Google Scholar] [CrossRef]
- Blasco, J. Short communication. Automatic inspection of the pomegranate (Punica granatum L.) arils quality by means of computer vision. SJAR 2008, 6, 12–16. [Google Scholar]
- Blasco, J.; Cubero, S.; Gómez-Sanchís, J.; Mira, P.; Moltó, E. Development of a machine for the automatic sorting of pomegranate (Punica granatum) arils based on computer vision. J. Food Eng. 2009, 90, 27–34. [Google Scholar] [CrossRef]
- Fashi, M.; Naderloo, L.; Javadikia, H. The relationship between the appearance of pomegranate fruit and color and size of arils based on image processing. Postharvest Biol. Technol. 2019, 154, 52–57. [Google Scholar] [CrossRef]
- Srivastava, S.; Sadistap, S. Data processing approaches and strategies for non-destructive fruits quality inspection and authentication: A review. J. Food Meas. Charact. 2018, 12, 2758–2794. [Google Scholar] [CrossRef]
- Akin, C.; Kirci, M.; Gunes, E.O.; Cakir, Y. Detection of the pomegranate fruits on tree using image processing. In Proceedings of the 2012 First International Conference on Agro- Geoinformatics (Agro-Geoinformatics), Shanghai, China, 2–4 August 2012; Volume 2, pp. 632–636. [Google Scholar]
- Bakhshipour, A.; Jafari, A.; Hosseini, S.M. Recognition of pomegranate on tree and stereoscopic locating of the fruit. J. Agric. Environ. Sci 2012, 12, 1288–1294. [Google Scholar]
- Behera, S.K.; Pattnaik, A.; Rath, A.K.; Barpanda, N.K.; Sethy, P.K. Yield estimation of pomegranate using image processing techniques. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 798–803. [Google Scholar]
- Salmanizadeh, F.; Nassiri, S.M.; Jafari, A.; Bagheri, M.H. Volume estimation of two local pomegranate fruit (Punica granatum L.) cultivars and their components using non-destructive X-ray computed tomography technique. Int. J. Food Prop. 2015, 18, 439–455. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; McCarthy, M.J. Black heart characterization and detection in pomegranate using NMR relaxometry and MR imaging. Postharvest Biol. Technol. 2012, 67, 96–101. [Google Scholar] [CrossRef]
- Nouri, B.; Mohtasebi, S.S.; Rafiee, S. Quality detection of pomegranate fruit infected with fungal disease. Int. J. Food Prop. 2020, 23, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Sanaeifar, A.; Mohtasebi, S.S.; Ghasemi-varnamkhasti, M.; Shafie, M.M. Evaluation of an electronic nose system for characterization of pomegranate varieties. AgricEngInt CIGR J. 2016, 18, 317–323. [Google Scholar]
- Payel, G.; Sunil, E.C.K. Quality analysis of pomegranate by non-destructive soft X-ray method. J. Food Process. Technol. 2014, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Nturambirwe, J.F.I. Advances in Spectral Techniques for Fruit Quality Evaluation: Case of ULF-NMR and NIRS. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2017. [Google Scholar]
- Stepisnik, J. Spectroscopy: NMR down to Earth. Nature 2006, 439, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Eberle, K. Evaluation of near Infrared and Nuclear Magnetic Resonance Spectroscopy for Rapid Quality Control of South African Extra Virgin Olive Oils. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2005. [Google Scholar]
- Schoeman, L.; Williams, P.; du Plessis, A.; Manley, M. X-ray micro-computed tomography (μCT) for non-destructive characterisation of food microstructure. Trends Food Sci. Technol. 2016, 47, 10–24. [Google Scholar] [CrossRef]
- Du Plessis, A.; Broeckhoven, C.; Guelpa, A.; le Roux, S.G. Laboratory x-ray micro-computed tomography: A user guideline for biological samples. Gigascience 2017, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Arendse, E.; Fawole, O.A.; Opara, U.L. Discrimination of pomegranate fruit quality by instrumental and sensory measurements during storage at three temperature regimes. J. Food Process. Preserv. 2014, 39, 1745–4549. [Google Scholar] [CrossRef]
- Léonard, A.; Blacher, S.; Nimmol, C.; Devahastin, S. Effect of far-infrared radiation assisted drying on microstructure of banana slices: An illustrative use of X-ray microtomography in microstructural evaluation of a food product. J. Food Eng. 2008, 85, 154–162. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Investigating non-destructive quantification and characterization of pomegranate fruit internal structure using X-ray computed tomography. Postharvest Biol. Technol. 2014, 95, 1–6. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Estimation of the density of pomegranate fruit and their fractions using X-ray computed tomography calibrated with polymeric materials. Biosyst. Eng. 2016, 148, 148–156. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.; Opara, U.L. Non-destructive estimation of pomegranate juice content of intact fruit using X-ray computed tomography. Acta Hortic. 2018, 1201, 297–302. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Non-destructive characterization and volume estimation of pomegranate fruit external and internal morphological fractions using X-ray computed tomography. J. Food Eng. 2016, 186, 42–49. [Google Scholar] [CrossRef]
- Hussein, Z. Bruise Damage Susceptibility of Pomegranates. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2019. [Google Scholar]
- Kamruzzaman, M.; Barbin, D.; Elmasry, G.; Sun, D.W.; Allen, P. Potential of hyperspectral imaging and pattern recognition for categorization and authentication of red meat. Innov. Food Sci. Emerg. Technol. 2012, 16, 316–325. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Peri, G.; Romaniello, R. Application of hyperspectral imaging for prediction of physico-chemical and sensory characteristics of table grapes. Comput. Electron. Agric. 2012, 87, 142–151. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Sun, D.-W. Application of hyperspectral imaging in food safety inspection and control: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 1039–1058. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A.A.; Feng, Y.; Gaston, E.; Valdramidis, V. Recent applications of hyperspectral imaging in microbiology. Talanta 2015, 137, 43–54. [Google Scholar] [CrossRef]
- Su, W.-H.; Sun, D.-W. Potential of hyperspectral imaging for visual authentication of sliced organic potatoes from potato and sweet potato tubers and rapid grading of the tubers according to moisture proportion. Comput. Electron. Agric. 2016, 125, 113–124. [Google Scholar] [CrossRef]
- Su, W.-H.; Sun, D.-W. Multispectral imaging for plant food quality analysis and visualization. CRFSFS 2018, 17, 220–239. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.W.; Chia, K.S. A review on recent near infrared spectroscopic measurement setups and their challenges. Meas. J. Int. Meas. Confed. 2021, 171, 108732. [Google Scholar] [CrossRef]
- Che, W.; Sun, L.; Zhang, Q.; Tan, W.; Ye, D.; Zhang, D.; Liu, Y. Pixel based bruise region extraction of apple using Vis-NIR hyperspectral imaging. Comput. Electron. Agric. 2018, 146, 12–21. [Google Scholar] [CrossRef]
- Fu, X.; Wang, M. Detection of early bruises on pears using fluorescence hyperspectral imaging technique. Food Anal. Methods 2022, 15, 115–123. [Google Scholar] [CrossRef]



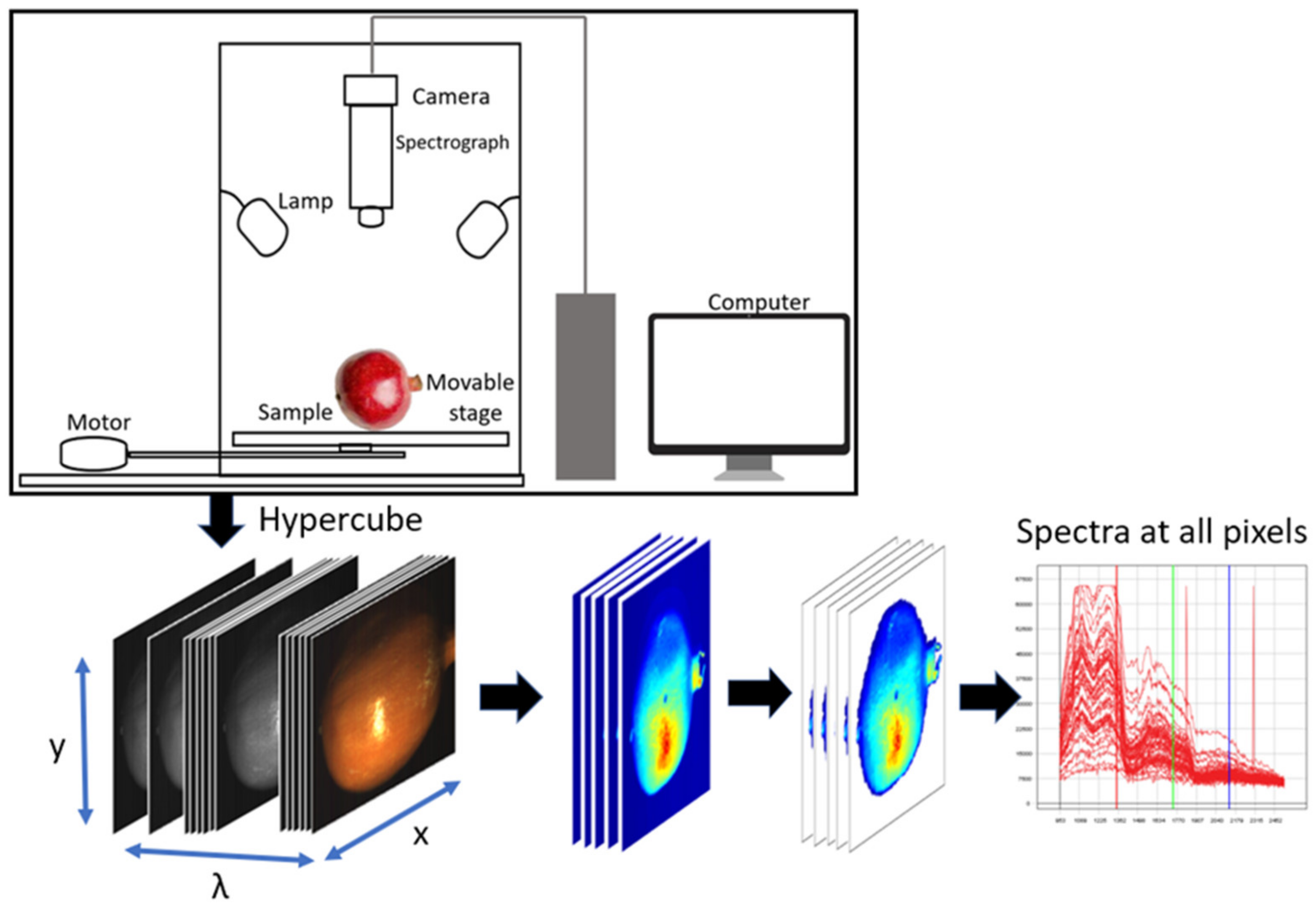
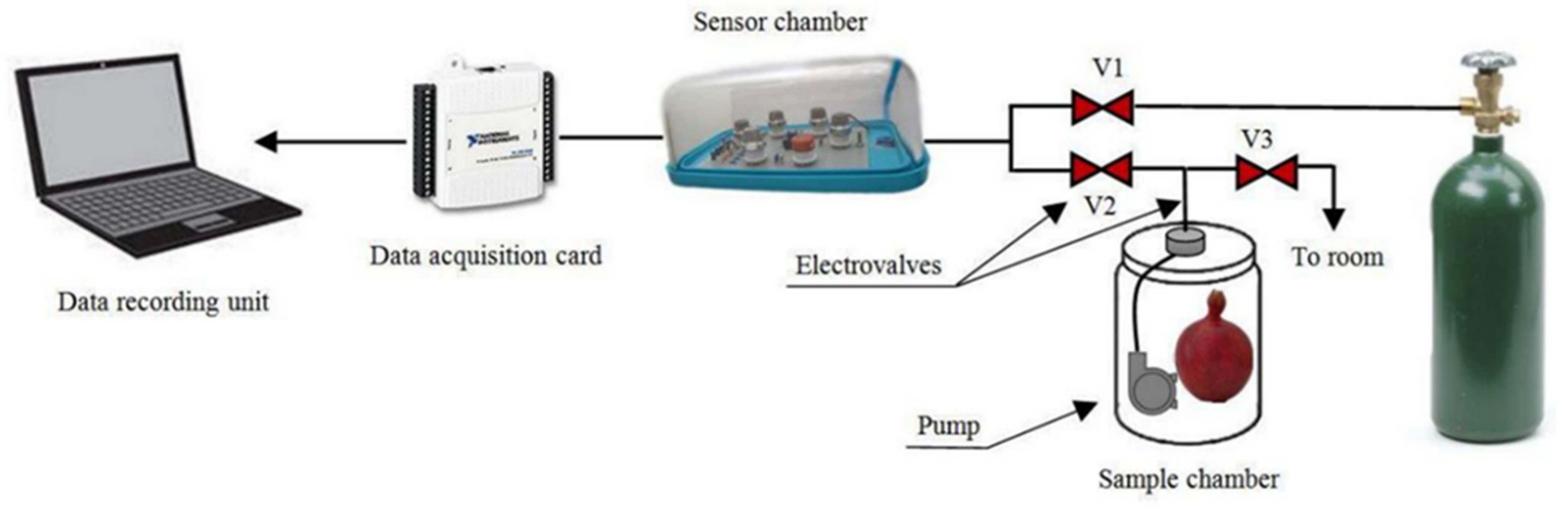
| Products | Quality Attributes | Prediction Statistics | Data Analysis | References |
|---|---|---|---|---|
| Fresh aril | TSS TA pH TAC TPC Brim A Firmness TSS/TA Hue angle Vitamin C Chroma a* | R2 = 0.875, RMSEP = 0.30% R2 = 0.855, RMSEP = 0.10% R2 = 0.851, RMSEP = 0.10 R2 = 0.705, RMSEP = 0.13 g/L R2 = 0.864, RMSEP = 0.11 g/L R2 = 0.834, RMSEP = 0.43 R2 = 0.684, RMSEP = 6.71 N R2 = 0.822, RMSEP = 1.03 R2 = 0.885, RMSEP = 4.19 R2 = 0.848, RMSEP = 0.09 g/L R2 = 0.783, RMSEP = 2.31 R2 = 0.735, RMSEP = 1.67 | PLS, PCA | [68] |
| Minimally processed aril | TVC Y&M | R2 = 0.909, SEP = 0.914 R2 = 0.929, SEP = 0.777 | ANN PLS-R | [80] |
| Dried aril | TA TSS/TA pH a* Chroma | R2 = 0.850, RMSEP = 0.041 R2 = 0.756, RMSEP = 1.951 R2 = 0.863, RMSEP = 0.131 R2 = 0.720, RMSEP = 1.815 R2 = 0.703, RMSEP = 1.986 | PLS, SVM | [10] |
| PJ | TSS TA pH TAC TPC Brim A TSS/TA Hue angle Vitamin C Chroma a* | R2 = 0.923, RMSEP = 0.31% R2 = 0.862, RMSEP = 0.11% R2 = 0.670, RMSEP = 0.17 R2 = 0.663, RMSEP = 0.19 g/L R2 = 0.591, RMSEP = 0.18 g/L R2 = 0.906, RMSEP = 0.40 R2 = 0.768, RMSEP = 1.00 R2 = 0.466, RMSEP = 1.67 R2 = 0.709, RMSEP = 0.11 g/L R2 = 0.832, RMSEP = 3.81 R2 = 0.816, RMSEP = 3.78 | PLS, PCA | [75] |
| Aril | TSS TA pH | R2= 0.92, RMSEP= 0.23 °Brix R2 = 0.93, RMSEP = 0.26% R2 = 0.85, RMSEP = 0.064 | PLS | [22] |
| PJ | Adulteration TA TSS | R2 = 0.975 R2 = 0.911 R2 = 0.991 | PCA, PLS | [76] |
| PSO | TCC PV RI | R2 = 0.8045 R2 = 0.620 R2 = 0.8092 | PLS-R | [10] |
| PSO | Adulteration detection | CA ≥ 88% | OPLS-DA | [79] |
| Feature | CMVS | Spectroscopy | HSI | MSI |
|---|---|---|---|---|
| Detect small sized sample | YES | NO | YES | YES |
| Flexibility of spectral extraction | NO | NO | YES | YES |
| Generation of quality attributes distribution | NO | NO | YES | Limited |
| Multi-constituent information | NO | YES | YES | Limited |
| Spectral information | NO | YES | YES | YES |
| Spatial information | YES | NO | YES | YES |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okere, E.E.; Arendse, E.; Ambaw Tsige, A.; Perold, W.J.; Opara, U.L. Pomegranate Quality Evaluation Using Non-Destructive Approaches: A Review. Agriculture 2022, 12, 2034. https://doi.org/10.3390/agriculture12122034
Okere EE, Arendse E, Ambaw Tsige A, Perold WJ, Opara UL. Pomegranate Quality Evaluation Using Non-Destructive Approaches: A Review. Agriculture. 2022; 12(12):2034. https://doi.org/10.3390/agriculture12122034
Chicago/Turabian StyleOkere, Emmanuel Ekene, Ebrahiema Arendse, Alemayehu Ambaw Tsige, Willem Jacobus Perold, and Umezuruike Linus Opara. 2022. "Pomegranate Quality Evaluation Using Non-Destructive Approaches: A Review" Agriculture 12, no. 12: 2034. https://doi.org/10.3390/agriculture12122034
APA StyleOkere, E. E., Arendse, E., Ambaw Tsige, A., Perold, W. J., & Opara, U. L. (2022). Pomegranate Quality Evaluation Using Non-Destructive Approaches: A Review. Agriculture, 12(12), 2034. https://doi.org/10.3390/agriculture12122034








_Ambaw.png)


