Fruit Yield in Sweet Orange Trees under Huanglongbing (HLB) Conditions Is Influenced by Reproductive Phenological Characteristics of the Scion-Rootstock Combination
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Material and Fruiting Evaluation
2.2. Fruit Detachment Force (FDF) Measurement
2.3. In Vitro Treatments
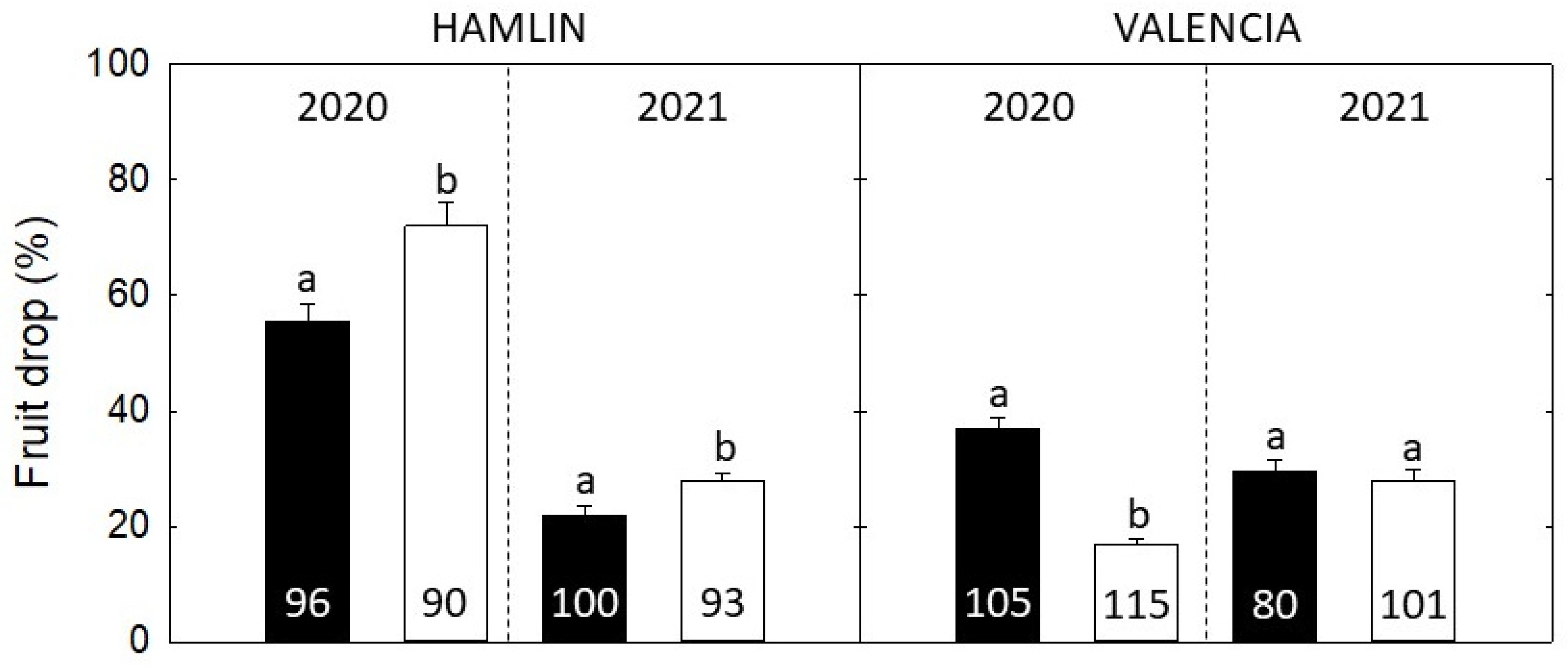
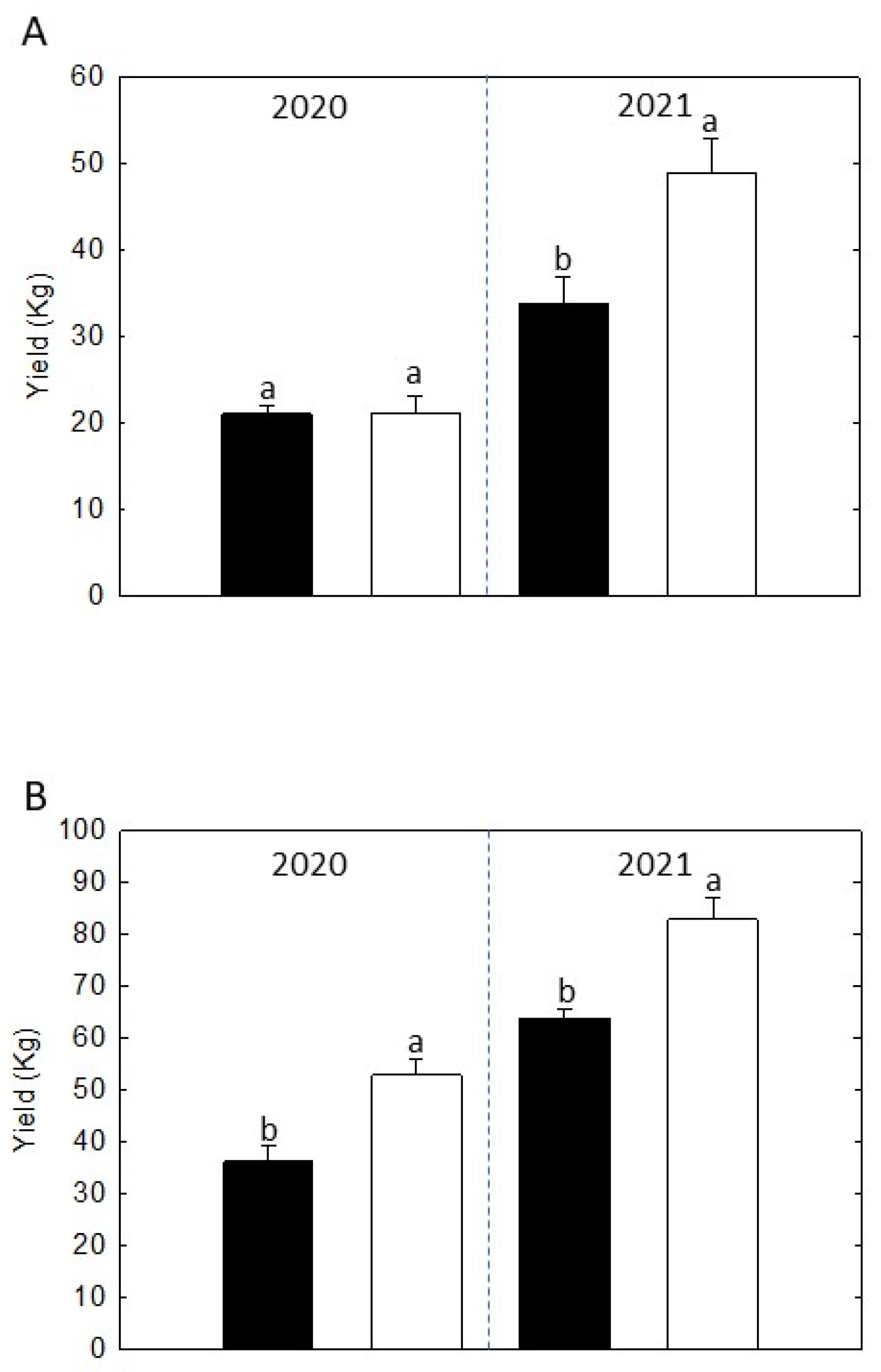
2.4. Pre-Harvest Fruit Drop (PHFD) and Yield Assessment
2.5. Statistical Analysis
3. Results
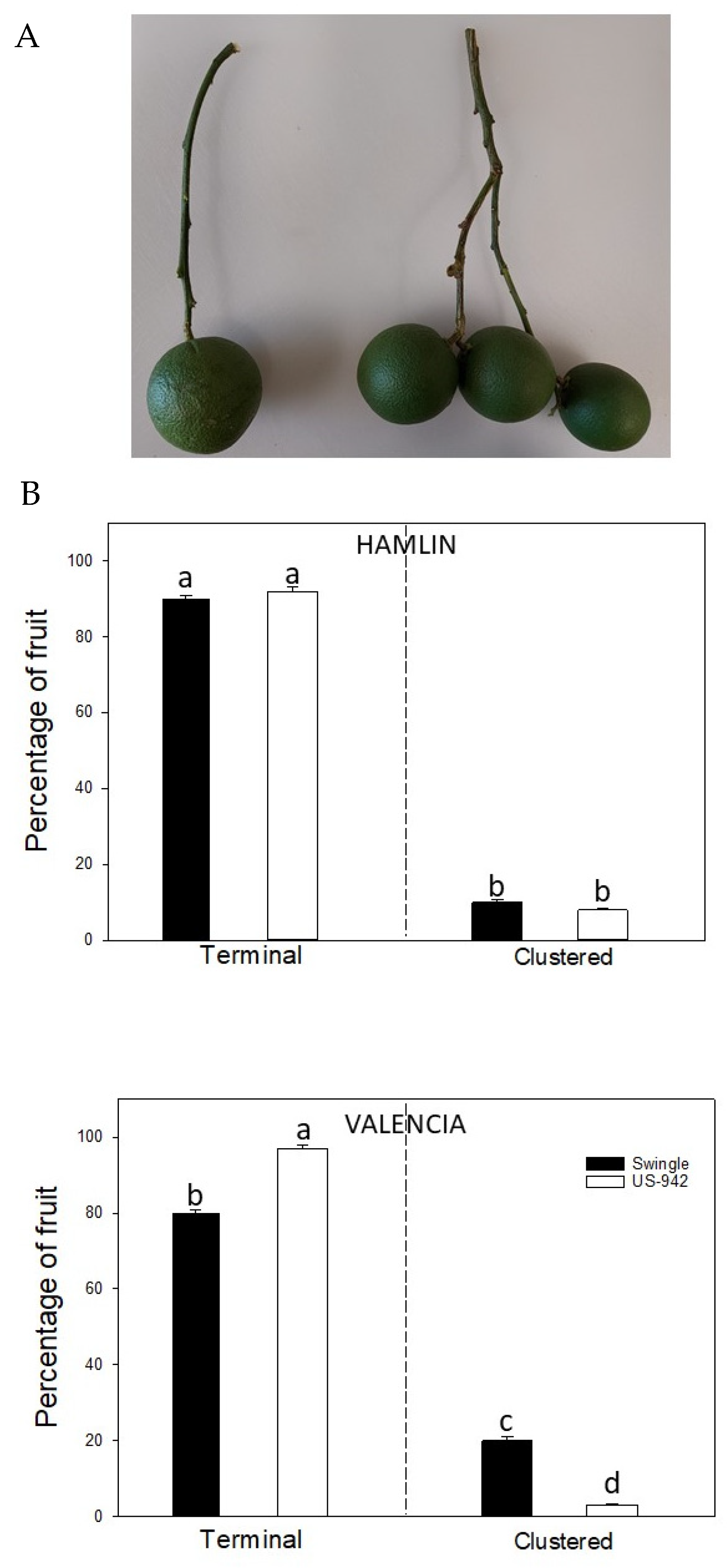
3.1. Fruit Type Distribution
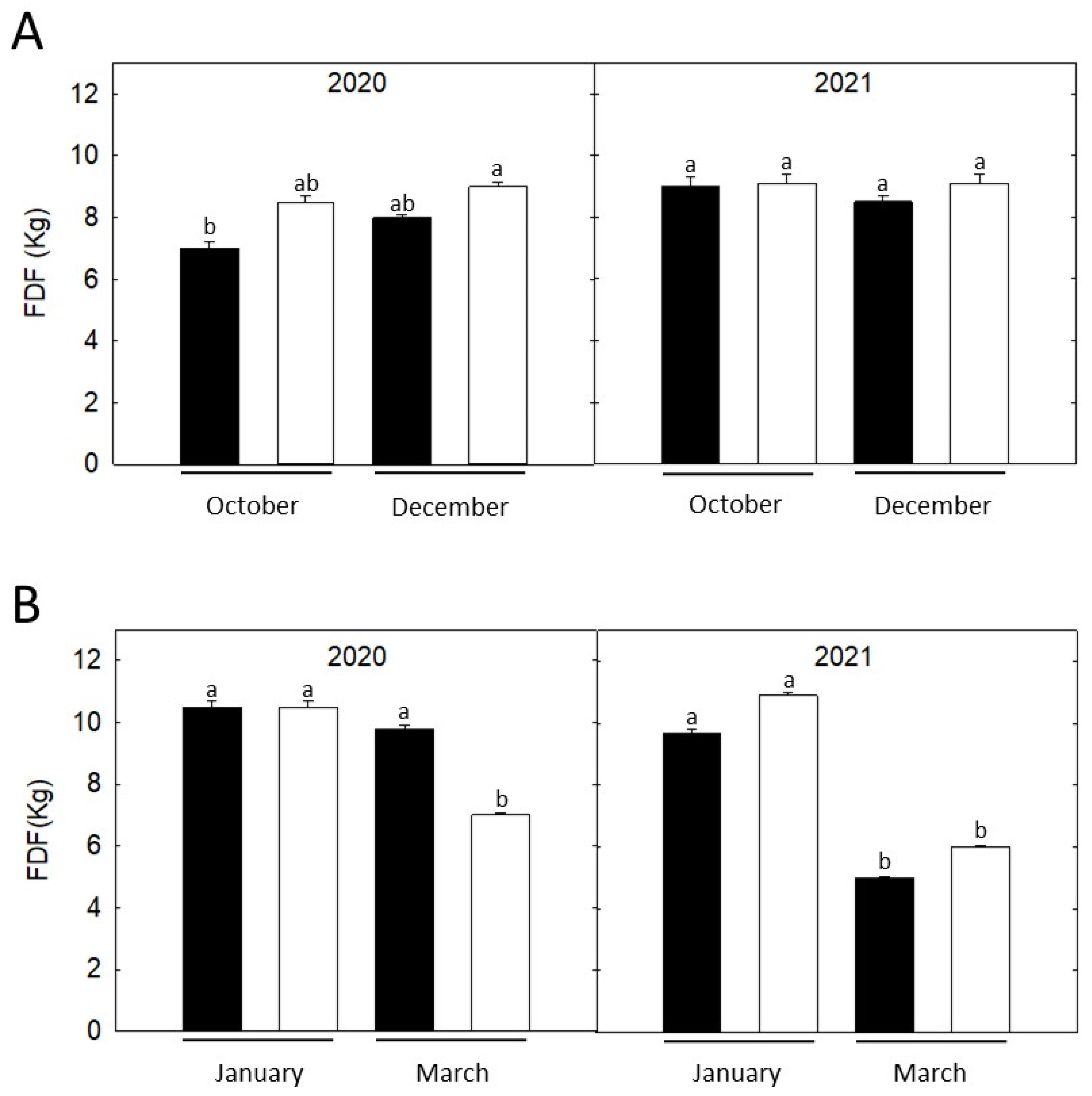
3.2. Fruit Detachment Force
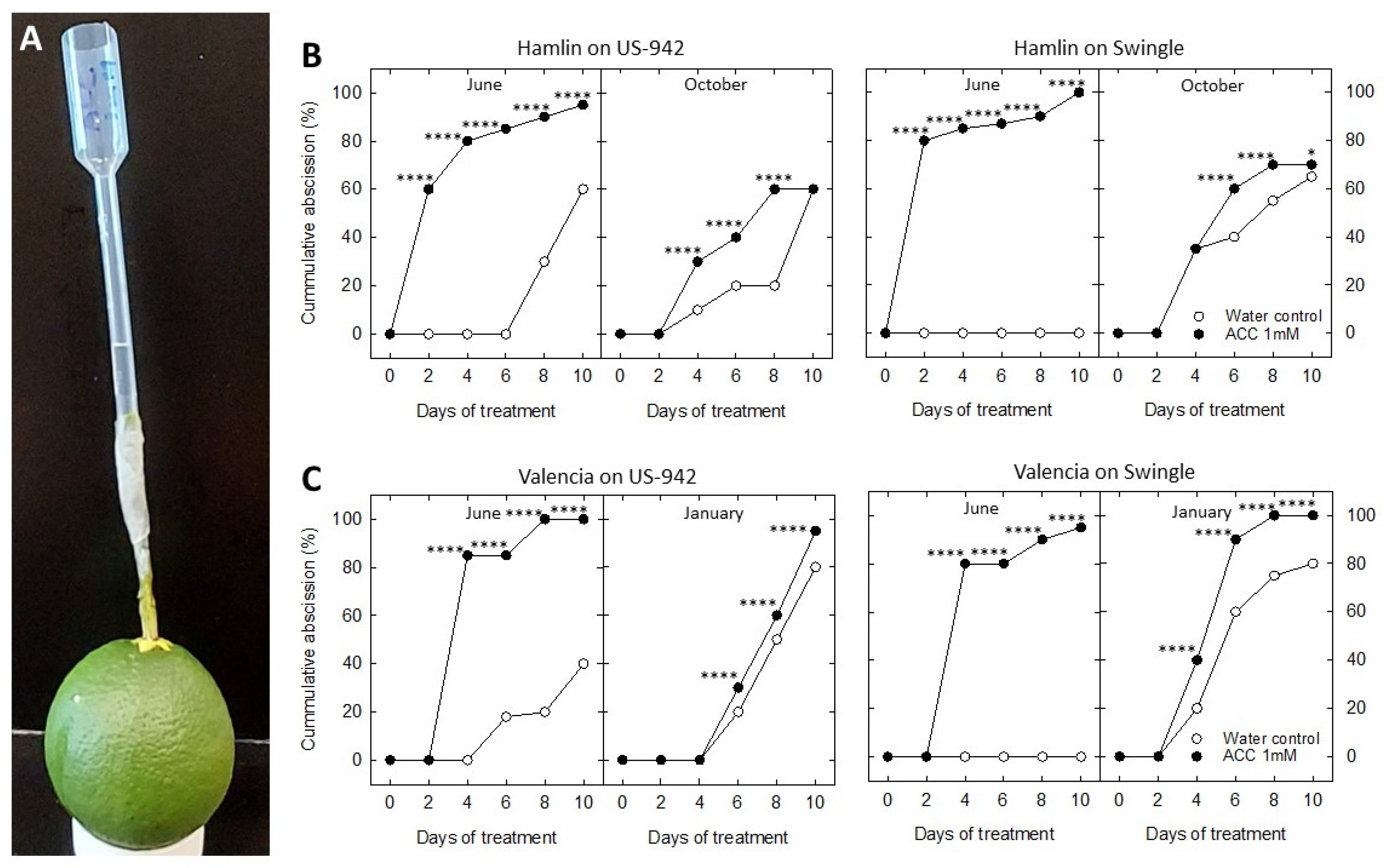
3.3. In Vitro Analysis of Fruit Abscission
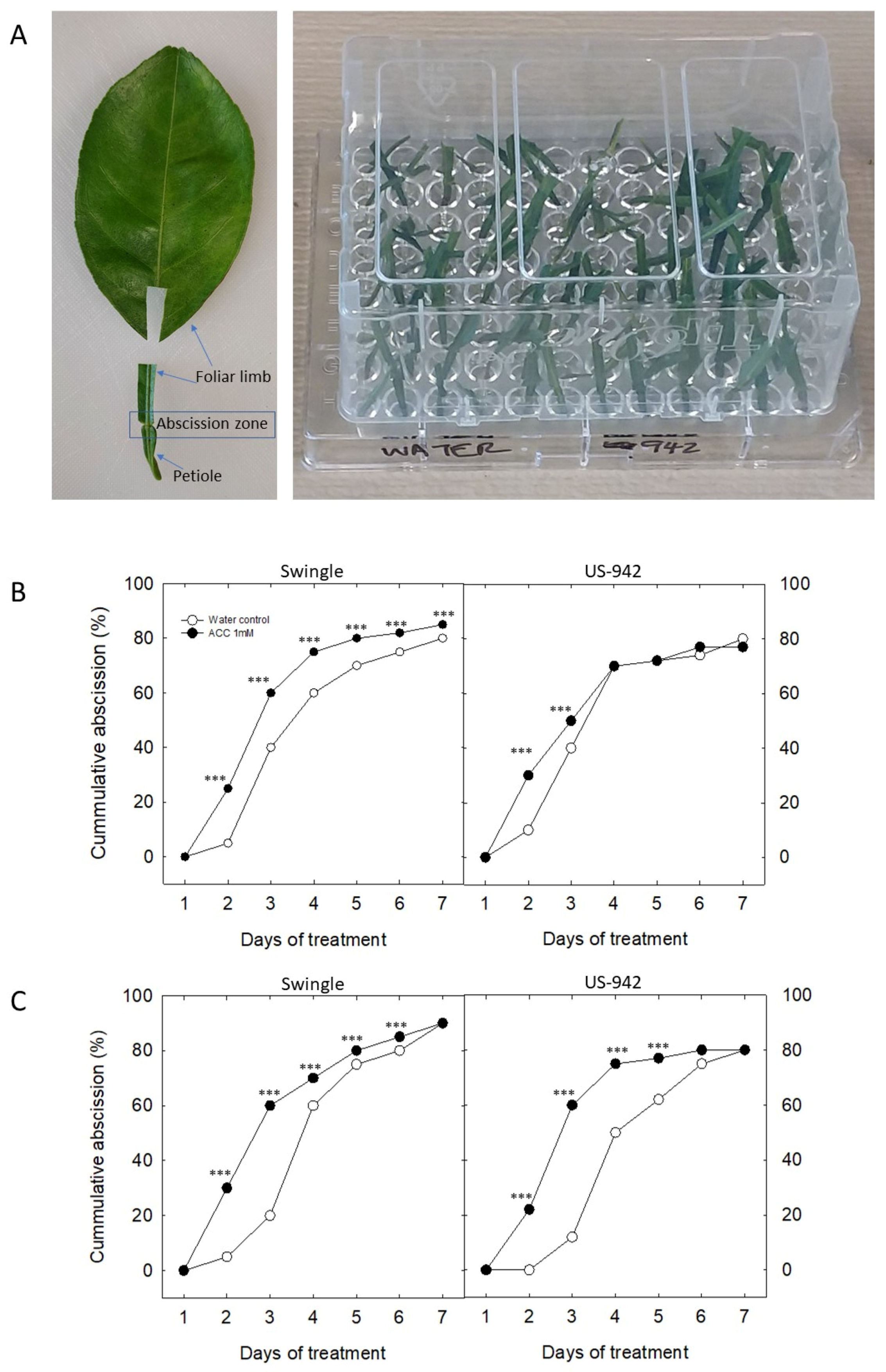
3.4. In Vitro Analysis of Leaf Abscission
3.5. Fruit Drop and Fruit Size Assessment
3.6. Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, J.; Morgan, K. Why bicarbonates matter for HLB management. Citrus. Ind. 2017, 98, 16–21. [Google Scholar]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Albrecht, U.; Bowman, D.K. Gene expression in Citrus sinensis (L.) Osbeck following infection with the bacterial pathogen Candidatus Liberibacter asiaticus causing Huanglongbing in Florida. Plant. Sci. 2008, 175, 291–306. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Brlansky, R.H.; Gmitter, F.G., Jr.; Li, Z.-G. Changes in carbohydrate metabolism in Citrus sinensis infected with ‘Candidatus Liberibacter asiaticus’. Plant Pathol. 2010, 59, 1037–1043. [Google Scholar] [CrossRef]
- Kim, J.-S.; Sagaram, U.S.; Burns, J.K.; Li, J.-L.; Wang, N. Response of Sweet Orange (Citrus sinensis) to ‘Candidatus Liberibacter asiaticus’ Infection: Microscopy and Microarray Analyses. Phytopathology 2009, 99, 50–57. [Google Scholar] [CrossRef]
- Graham, J.H.G.; Johnson, E.G.; Gottwald, T.R.; Irey, M.S. Presymptomatic fibrous root decline in citrus trees caused by huanglongbing and potential interaction with Phytophthora spp. Plant Dis. 2013, 97, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Hamido, S.A.; Morgan, K.T.; Kadyampakeni, D. The Effect of Huanglongbing on Young Citrus Tree Water Use. Horttechnology 2017, 27, 659–665. [Google Scholar] [CrossRef]
- Johnson, E.G.; Wu, J.; Bright, D.B.; Graham, J.H. Association of ‘Candidatus Liberibacter asiaticus’ root infection, but not phloem plugging with root loss on huanglongbing-affected trees prior to appearance of foliar symptoms. Plant Pathol. 2014, 63, 290–298. [Google Scholar] [CrossRef]
- Baldwin, E.; Plotto, A.; Manthey, J.; McCollum, G.; Bai, J.; Irey, M.; Cameron, R.; Luzio, G. Effect of Liberibacter infection (Huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: Chemical and physical analyses. J. Agric. Food Chem. 2010, 58, 1247–1262. [Google Scholar] [CrossRef] [PubMed]
- Albrigo, L.G.; Stover, E.W. Effect of plant growth regulators and fungicides on huanglongbing-related preharvest fruit drop of citrus. HortTechnology 2015, 25, 785–790. [Google Scholar] [CrossRef]
- Tang, L.; Chhajed, S.; Vashisth, T. Preharvest Fruit Drop in Huanglongbing-Affected ‘Valencia’ Sweet Orange. J. Am. Soc. Hort. Sci. 2019, 144, 107–117. [Google Scholar] [CrossRef]
- Kunwar, S.; Grosser, J.; Gmitter, F.G., Jr.; Castle, S.W.; Albrecht, U. Field Performance of ‘Hamlin’ Orange Trees Grown on Various Rootstocks in Huanglongbing-endemic Conditions. HortScience 2021, 56, 244–253. [Google Scholar] [CrossRef]
- Castle, W.S. A career perspective on citrus rootstocks, their development, andcommercialization. HortScience 2010, 45, 11–15. [Google Scholar] [CrossRef]
- Bureau of Citrus Budwood Registration, Florida Dept of Agriculture and consumer services. Annual Reports 2021; Bureau of Citrus Budwood Registration, Florida Dept of Agriculture and consumer services: Tallahassee, FL, USA, 2021; pp. 1–33.
- Bowman, K.D. Notice to Fruit Growers and Nurserymen Relative to the Naming and Release of the US-942 Citrus Rootstock; USDA: Washington, DC, USA, 2010; pp. 1–9.
- Bowman, K.D.; McCollum, G.; Albrecht, U. Performance of ‘Valencia’ orange (Citrus sinensis [L.] Osbeck) on 17 rootstocks in a trial severely affected by huanglongbing. Sci. Hort. 2016, 201, 355–361. [Google Scholar] [CrossRef]
- Gairhe, B.; Dittmar, P.; Kadyampakeni, D.; Batuman, O.; Alferez, F.; Kanissery, R. Effects of glyphosate application on preharvest fruit drop and yield in Valencia citrus. HortScience 2022, 57, 897–900. [Google Scholar] [CrossRef]
- de Carvalho, D.U.; Boakye, D.G.; Gast, T.; Pereira Leite Jr, R.; Alferez, F. Determining seed viability during fruit maturation to improve seed production and availability of new citrus rootstocks. Front. Plant Sci. 2021, 12, 777078. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cadenas, A.; Mehouachi, J.; Tadeo, F.R.; Primo-Millo, E.; Talón, M. Hormonal regulation of fruitlet abscission induced by carbohydrate shortage in citrus. Planta 2000, 210, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Goren, R. Anatomical, physiological, and hormonal aspects of abscission in citrus. Hort. Rev. 1993, 15, 145–182. [Google Scholar]
- Tudela, D.; Primo-Millo, E. 1-Aminocyclopropane-1-carboxylic acid transported from roots to shoots promotes leaf abscission in Cleopatra Mandarin (Citrus reshni Hort. exTan.) seedlings rehydrated after water stress. Plant Physiol. 1992, 100, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Vashisth, T. New insight in Huanglongbing-associated mature fruit drop in citrus and its link to oxidative stress. Sci. Hort. 2020, 265, 109246. [Google Scholar] [CrossRef]
- Pozo, L.; Malladi, A.; John-Karuppiah, K.J.; Lluch, Y.; Alferez, F.; Burns, J.K. Daily Fluctuation in Fruit Detachment Force of ‘Valencia’ Orange Is Related to Time of Day, Temperature, Relative Humidity, Fruit Weight, and Juice Percentage. Proc. Fla. State Hort. Soc. 2007, 120, 41–44. [Google Scholar]
- Talon, M.; Tadeo, F.R.; Ben-Cheikh, W.; Gomez-Cadenas, A.; Mehouachi, J.; Perez-Botella, J.; Primo-Millo, E. Hormonal regulation of fruit set and abscission in citrus: Classical concepts and new evidence. Acta Hort. 1997, 463, 209–218. [Google Scholar] [CrossRef]
- Tang, L.; Singh, S.; Vashisth, T. Association between Fruit Developmentand Mature Fruit Drop in Huanglongbing-affected Sweet Orange. HortScience 2020, 55, 851–857. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boakye, D.A.; Alferez, F. Fruit Yield in Sweet Orange Trees under Huanglongbing (HLB) Conditions Is Influenced by Reproductive Phenological Characteristics of the Scion-Rootstock Combination. Agriculture 2022, 12, 1750. https://doi.org/10.3390/agriculture12111750
Boakye DA, Alferez F. Fruit Yield in Sweet Orange Trees under Huanglongbing (HLB) Conditions Is Influenced by Reproductive Phenological Characteristics of the Scion-Rootstock Combination. Agriculture. 2022; 12(11):1750. https://doi.org/10.3390/agriculture12111750
Chicago/Turabian StyleBoakye, Daniel A., and Fernando Alferez. 2022. "Fruit Yield in Sweet Orange Trees under Huanglongbing (HLB) Conditions Is Influenced by Reproductive Phenological Characteristics of the Scion-Rootstock Combination" Agriculture 12, no. 11: 1750. https://doi.org/10.3390/agriculture12111750
APA StyleBoakye, D. A., & Alferez, F. (2022). Fruit Yield in Sweet Orange Trees under Huanglongbing (HLB) Conditions Is Influenced by Reproductive Phenological Characteristics of the Scion-Rootstock Combination. Agriculture, 12(11), 1750. https://doi.org/10.3390/agriculture12111750




