Abstract
Intraspecific trait variation is a critical determinant of ecosystem processes, especially in agroecosystems where single species or genotypes exist in very high abundance. Yet to date, only a small number of studies have evaluated if, how, or why traits forming the Leaf Economics Spectrum (LES) vary within crops, despite such studies informing our understanding of: (1) the environmental factors that drive crop LES trait variation and (2) how domestication has altered LES traits in crops vs. wild plants. We assess intragenotype variation in LES traits in ‘Chardonnay’ (Vitis vinifera)—one of the world’s most commercially important crops—across a soil compaction gradient: one of the most prominent characteristics of agricultural soils that may drive crop trait variation. Our early evidence indicates that ‘Chardonnay’ traits covary along an intragenotype LES in patterns that are qualitatively similar to those observed among wild plants: resource-acquiring vines expressed a combination of high mass-based photosynthesis (Amass), mass-based dark respiration (Rmass), and leaf nitrogen concentrations (N), coupled with low leaf mass per area (LMA); the opposite set of trait values defined the resource-conserving end of the ‘Chardonnay’ LES. Traits reflecting resource acquisition strategies (Amass, Rmass, and leaf N) declined with greater bulk density, while traits related to investment in leaf construction costs (LMA) increased with greater bulk density. Our findings contribute to an understanding of the domestication syndrome in grapevines and also provide information relevant for quantifying trait-based crop responses to environmental change and gradients.
1. Introduction
The Leaf Economics Spectrum (LES) is a framework for understanding the causes and consequences of differences in the comparative ecophysiology, morphology, and biochemistry of plants [1]. On one end of the LES are species expressing “resource-acquiring” trait syndromes that include high maximum leaf-level photosynthesis (A) and dark respiration (R) rates, high leaf nitrogen (N) concentrations, and low leaf mass per unit area (LMA). The other end of the LES is defined by plants expressing the opposite suite of trait values which represent “resource conserving” trait syndromes [1]. Variability in traits along the LES underpin differences in how plant species respond to environmental conditions and change [2] and are central in driving relationships between plant species composition and ecosystem functioning [3]. Correlations and trade-offs among LES traits detected across thousands of plant species, both within and across biomes [4,5,6], have also informed our understanding of the evolutionary and environmental factors that constrain leaf form and function [7,8,9].
The original formulation and early research on the LES focused on trait differences across plant species or communities [1,4]. However, more recently, meta-analyses have shown that variation within species constitutes a considerable proportion (e.g., ~29% of LMA and leaf N) of total LES trait variation within plant communities [10,11,12]. Extending from this work, studies have now begun focusing on evaluating how plants of the same species differ in their LES traits, with conspecific plants commonly differing from one another along an intraspecific LES [13,14,15]. Moreover, in unmanaged systems, the within-species variation that exists in certain LES traits has also been found to be a significant correlate of ecosystem structure, function, and responses to environmental change [16,17,18], see also [19] and references therein.
Research on LES trait variation and relationships within species also informs an understanding of how and why the functional ecology of crops varies in managed agroecosystems. Specifically, studies have shown that individuals of the same crop species or genotype express wide variation in their LES traits, often along an intraspecific or intragenotypic Leaf Economics Spectrum. This includes studies detecting within-species or genotype LESs that exist in several of the world’s most common crops including soy [13], rice [20], coffee [14,21], wheat [22], and maize [23]. Across these studies, intraspecific or intragenotypic LES trait variation in crops was a statistical correlate of agroecosystem functions including yield [13,21], photosynthetic N-use efficiency [20], tissue decomposition [24], N2-fixing structures [25], and soil microbial diversity [26].
Studies on crops have also helped elucidate the factors that cause plants to differentiate along a given intraspecific or intragenotypic LES, which to date include temperature and precipitation regimes [23], soil nutrient availability [27], plant ontogenetic stages [13] or size [28], or light [21]. Results differ across crops and spatial scales, though generally studies have found plants of the same crop move towards the resource-conserving end of a within-species- or -genotype LES (i.e., plants expressing low A, low leaf N, high LMA) under the following: (1) hot and dry environments [14]; (2) shaded conditions, such as those in agroforestry systems [21]; and (3) after reproductive onset [13,28]. While these studies are instructive, there remain important factors that may also lead to differences in crop traits along an intraspecific or intragenotypic LES that have yet to be explored.
Soil compaction is a major characteristic of land degradation worldwide and a primary contributor to reductions in agricultural productivity and sustainability [29,30,31]. In some instances, increased soil compaction results in higher rates of A, growth, and yield [32] though more often, growth and yield reductions in plants under compaction occur as the cumulative consequence of reductions in root growth, which, in turn, limit water and nutrient uptake. Compaction also triggers complex plant signaling pathways which ultimately reduce leaf-level A via stomatal and non-stomatal factors [31,32,33,34,35]. Existing literature, therefore, supports the untested hypothesis that soil compaction drives trait covariation and/or trade-offs along an intraspecific or intragenotypic LES. Specifically, when soil compaction gradients exist within a site, plants in high compaction should express resource-conserving LES traits (i.e., low A, leaf N, and R, along with high LMA), while those in low compaction areas should express the opposite suite of traits.
Existing work on crops has also focused only on a subset of the six traits included in the original LES formulation. Specifically, studies on coffee [21,25], soy [13], and rice [20] have largely analyzed how three LES traits—A, LMA or SLA, and leaf N—covary or trade off within crop species or genotypes. For instance, Martin et al. [14] found lower A for a given leaf N in coffee vs. wild plants and, based on this finding, hypothesized that either artificial selection for caffeine or luxury consumption of N-based compounds from soil amendments has altered LES trait relationships in that crop. Conversely, Xiong and Flexas [20] found that rice expressed a higher A for a given leaf N vs. wild rice plants, supporting the hypothesis that artificial selection has resulted in higher photosynthetic nitrogen-use efficiency in that crop. Other studies have found that while crops such as soy, wheat, and maize occupy the extreme resource-acquiring end of the LES [23,36], domestication has not necessarily altered the slope or strength of bivariate trait relationships among A, LMA, and leaf N [13].
While these and other findings have informed our understanding of how artificial selection influences plant trait syndromes, certain LES traits—namely leaf R—have largely been omitted from these and other analyses on crop trait syndromes. Leaf R is among the six core traits forming the LES which exists among plant species globally, being significantly correlated (r2 = 0.34–0.60) to all other LES traits [1]. The relationship between R and other traits along the global LES reflect evolved physiological, biochemical, and structural trade-offs in plants: the physiological cost of R, in terms of plant carbon (C) metabolism, increases with greater leaf N and A, and declines with increasing LMA [1,37,38]. The incorporation of R into any LES is, therefore, central as it reflects a quantifiable physiological cost of resource acquisition.
In crops, reducing R while maintaining plant growth and yield is one of several goals of selection programs, with research on tomato [39], canola [40], cucumber [41], and rye grass [42] showing that reductions in plant C losses via R, due to artificial selection, were related to higher yields. Therefore, one might expect that artificial selection has altered the shape (i.e., the intercept and slope) and strength of the relationship between leaf R and other LES traits in crops vs. wild plants. Moreover, changes in crop leaf R have been evaluated in responses to soil nutrient amendments, irrigation, and growing temperatures, though relationships between leaf R and soil compaction are less commonly assessed [43]. Since (1) croplands now cover at least ~12.2–17.1 million km2 of Earth’s ice-free land [44] and (2) compaction is a central feature on an estimated 68 million ha of soils on the world’s arable lands [29,31], then (3) understanding how R, and its relationship to other LES traits in crops, is influenced by compaction is particularly important for refining Earth System models [45].
Here, we explore how LES traits vary in ‘Chardonnay’ (Vitis vinifera var. ‘Chardonnay’), one of the world’s most commercially important, widespread, and rapidly expanding wine grape varieties [46]. We evaluated LES and related traits on individual ‘Chardonnay’ vines that exist across a soil compaction gradient to address the following questions: (1) Is intra-genotype variation in Chardonnay LES traits related to soil compaction? If so, then (2) does soil compaction lead to ‘Chardonnay’ leaves and vines differentiating from one another along an intragenotype LES? Finally, we assess (3) whether the shape of a potential intra-genotype LES in Chardonnay differs from the LES detected across plants globally?
2. Materials and Methods
2.1. Study Site and Design
Our study was situated at the Niagara College Teaching Vineyard, a 16.2 ha operational vineyard situated at the Daniel J. Patterson Campus in Niagara-on-the-Lake, Ontario, Canada (43.1522° N, 79.1652° W), which was established in 1996. The vineyard is situated in the Niagara-on-the-Lake Regional Appellation, which, in turn, is nested within the Niagara Peninsula Appellation (Figure S1). Soils at the site are classified as imperfectly drained silty clays (to 40–100 cm depth) over clay loam till mixed with poorly drained lacustrine heavy clay. The farm is under commonly employed vineyard management systems, which include applications of calcium nitrate and/or muriate of potash and/or sulphate of potash magnesium (K-Mag; 22-10.8-22) applied uniformly across the farm in mid-June. Liquid calcium (8-0-0-10) is also applied as a foliar spray early in each growing season. In mid-June of each year, cover crops are planted in every second row with 65% annual rye, 20% crimson clover, and 15% eco-till radish through deep ripping, discing, and harrowing passes. At the site there is 7.26 cm diameter tile drainage installed in every other row, the site is not irrigated, and vines are trained using a 2-arm flat vertical shoot position system.
Our study was conducted over a 1-week period between 1–7 July 2020 when vines were in the fruit setting/berry development phenological stage [47]. We selected a total of 15 individual ‘Chardonnay’ vines (all of which were “Dijon Clone 76” grafted onto SO4 rootstock) for functional trait analyses, which were distributed evenly across five different planting rows spaced 15–20 m apart (corresponding to an even 10 interceding planting rows). These sampling rows run parallel to one another and broadly follow a soil compaction gradient that runs along a northwest to southeast orientation in the vineyard. This gradient is related to the vineyard’s imperfectly drained soils and hydrology. Generally, northwest areas and planting rows are well drained by mid- to late-May. By comparison, areas and planting rows in the southeast remain poorly drained for roughly an additional month, drying by mid- to late-June. Since farm machinery is required for foliar applications and cover crop plantings across the entire vineyard in mid-June, southeast areas of the vineyard, therefore, experience enhanced mechanical compaction every year in early late spring/early summer.
Soil bulk density was collected for each sampled vine (described below) using a 1 cm diameter core borer. Following collection, soil samples were transported to the University of Toronto Scarborough where they were oven-dried to constant mass at 105 °C and then weighed. Bulk density was then calculated as the fresh volume divided by dry mass. At our site, bulk density varied significantly across rows (Analysis of Variance (ANOVA) F4, 10 = 5.84, p < 0.001), with rows 1 through 5 expressing bulk density values of 1.36 ± 0.12 (S.D.) g cm−3, 1.58 ± 0.08 g cm−3, 1.67 ± 0.09 g cm−3, 1.64 ± 0.1 g cm−3, and 1.72 ± 0.1 g cm−3, respectively (Figure S1).
Within each sampling row, three individual vines situated 13–15 m away from one another were chosen for leaf trait measurements. Each of the 15 vines chosen for our study was 1.5–2 cm in resprout diameter and free of any pest, pathogen, or mechanical damage. On each plant, we selected three recently developed and fully expanded leaves that were free of any damage or disease and situated on the upper-most cane in full-sun conditions. This nested study design, therefore, resulted in leaf traits being measured on 45 leaves from 15 individual vines that were of the same size, age, rootstock, and pruning regimes, which were, in turn, situated within five distinct sample rows.
2.2. Functional Trait Measurements
We quantified 11 physiological, morphological, and chemical traits for each individual leaf. To do so, we executed 14-point photosynthetic light–response curves on each leaf using a LI-6800 Portable Photosynthesis System (Licor Bioscience, Lincoln, Nebraska, USA), to measure area-based photosynthetic rates (Aarea; μmol CO2 m−2 s−1) across a range of photosynthetic photo flux density (I) levels (i.e., 2000, 1500, 1200, 1000, 800, 600, 400, 200, 100, 80, 60, 40, 20, and 0 μmol of photosynthetically active radiation (PAR) m−2 s−1). All photosynthetic rates were allowed to stabilize at each level of I for at least 120 s prior to data acquisition [48,49], with each light–response curve, therefore, taking a minimum of 30 min to complete. All Aarea measurements were made 8:00–12:00 a.m. to avoid mid-day stomatal closure, with leaf chamber conditions maintained at CO2 concentrations of 400 ppm, relative humidity at 53.1–73.5%, leaf vapour pressure deficits of 1.2–1.7 KPa, and leaf temperatures 24.3–31.6 °C.
Physiological traits were calculated for each leaf by fitting non-rectangular hyperbola to each of the 45 light–response curves as:
where Rarea represents area-based leaf R rate (μmol CO2 m−2 s−1), Φ is the apparent quantum yield of photosynthesis (mol CO2 mol PPFD−1), Amax is the light-saturated maximum area-based photosynthetic rate (μmol CO2 m−2 s−1), and θ represents a curvature parameter. From these models, we also derived leaf-level light compensation points (LLCP, μmol PAR m−2 s−1), calculated as I, where Aarea = 0. Light–response curves (presented in Figure S2) were fitted using the ‘nls’ function in R v.3.3.3 statistical software (R Foundations for Statistical Computing, Vienna, Austria).
After light–response curves were completed in the field, each leaf was immediately collected and transported to the University of Toronto Scarborough for morphological and chemical trait determinations. First, leaf area (cm2) was measured using a LI-3100C leaf area meter (Licor Bioscience, Lincoln, NE, USA), and all leaves were then dried at 65 °C to constant mass and weighed (g). We then calculated LMA (g m−2) as leaf mass/leaf area and used these LMA values to calculate maximum mass-based photosynthetic (Amass) and mass-based dark respiration rates (Rmass), as Amax or Rarea/LMA, respectively. Lastly, dried leaf tissue was ground into a fine powder using a MM400 Retsch ball mill (Retsch Ltd., Hann, Germany), and ~0.1 g of tissue was weighed and analyzed for C and N concentrations (both on a % mass basis) on a LECO CN 628 elemental analyzer (LECO Instruments, Mississauga, ON, Canada).
2.3. Analysis of Leaf Trait Variation
We first evaluated whether traits were either normally or log-normally distributed using the ‘fitdist’ function in the ‘fitdistrplus’ R package [50], with the highest log-likelihood scores indicating the best-fit data distribution. For traits that were normally distributed, we calculated descriptive statistics as means and standard deviations (SD), while medians, median, and SD values were calculated for log-normal traits. We also calculated and present trait ranges and coefficients of variation (CV) for each trait. In addition, we tested for differences in mean trait values as a function of planting rows, using analysis of variance (ANOVA). These descriptive statistics and ANOVAs were complemented by variance-partitioning analyses which were used to identify the primary sources of trait variation in our dataset. These analyses entailed fitting linear mixed models (using the ‘lme’ function in the ‘nlme’ R package [51]) to each trait individually (where n = 45 in all cases), where an intercept was the only fixed effect, and plant identity within sample row identity were included as nested random effects. The proportion of trait variation explained by each nested random effect, as well as the proportion of variation unexplained by the factors considered here, was then estimated using the ‘varcomp’ function in the ‘ape’ R package [52].
We then assessed the influence of soil compaction on both individual traits and multivariate trait syndromes. First, we fit separate mixed models for each trait individually, where trait values were predicted as a function of soil bulk density (included as a fixed effect), while accounting for plant identity nested within sampling row (included as nested random effects). We then used a Principal Component Analysis (PCA) to test whether multivariate leaf trait syndromes of ‘Chardonnay’ varied as a function of planting row/compaction or vine identity. Our PCA included four LES traits (i.e., Amass, Rmass, leaf N, and LMA), two traits derived from light–response curves (i.e., Φ and LLCP), and one trait related to light interception (i.e., leaf area). The relationship between individual traits and each PCA axis was evaluated using the ‘dimdesc’ function in the ‘FactoMineR’ R package [53]. We also visualized our PCA biplot with 95% confidence ellipses surrounding the data points within each sample row. Then, we used a permutational multivariate analysis of variance (PerMANOVA) based on Euclidean distances and 10,000 permutations to test whether multivariate trait syndromes vary as a function of planting row (reflecting the soil bulk density gradient), plant identity, and a row-by-plant interaction term. All multivariate analyses were implemented in the ‘vegan’ R package [54].
Finally, we evaluated bivariate trait relationships among ‘Chardonnay’ leaves using Pearson correlation tests. However, one of the main goals of our analysis was to test for the presence of an intragenotype LES in Chardonnay and to evaluate whether these relationships differed from the global LES defined by Wright et al. [1]. For this, we used standardized major axis (SMA) regressions to evaluate the slope of the bivariate trait relationships that exist among four LES traits measured in our study vines, including Amass, Rmass, LMA, and leaf N. All of these SMA regressions had a sample size of n = 45, and were implemented with the ‘sma’ function in the ‘smatr’ R package [55]. Then, we evaluated whether these SMA slopes of the intragenotype ‘Chardonnay’ LES differed from the trait relationships found across a functionally and phylogenetically diverse set of plants species globally. This analysis entailed merging our ‘Chardonnay’ data with the GLOPNET dataset (i.e., the dataset used in the original LES analysis of Wright et al. [1]) and testing for statistical differences in the SMA slope of each trait–trait relationship between the ‘Chardonnay’ vs. GLOPNET, using the ‘slope.test’ function of the ‘smatr’ R package [55].
3. Results
3.1. ‘Chardonnay’ Functional Trait Variation in Relation to Soil Bulk Density
All leaf traits measured here, with the exception of LLCP, varied significantly across planting rows (Table S1). Photosynthesis and Φ varied most widely across the ‘Chardonnay’ vines and leaves evaluated here (CV = 25.6–43.2), and all of these traits related to C assimilation rates declined significantly with soil compaction. Specifically, sampling row identity explained 45.4% and 64.8% of the variation in Amax and Amass, respectively (Table 1). In turn, both declined significantly with higher bulk density (p < 0.01 in the mixed model slope term), with soil compaction explaining 31.3% and 40.3% of the variation in Amax and Amass, respectively (Figure 1A,B, Table S2). Similarly, 42.9% of the variation in leaf Φ was explained by sampling row identity (Table 1), and this trait was negatively correlated with soil bulk density (mixed model slope term p = 0.01, marginal r2 = 0.243; Figure 1F, Table S2). Although 19.3% and 26.0% of the variability in Rarea and Rmass, respectively, was attributable to sampling row identity (Table 1), and these traits differed significantly across rows (Table S1), neither of these traits was statistically correlated with soil bulk density when individual plant identity was accounted for in our mixed models (Figure 1D,E, Table S2). Across our dataset, LLCP was also not correlated with soil bulk density (marginal r2 = 0.03), and sampling row explained <1% of the variation in this trait (Figure 1C).

Table 1.
Descriptive statistics for 11 leaf functional traits from 45 ‘Chardonnay’ leaves, measured in 2020 on 15 individual vines growing at a single site, across a soil compaction gradient.
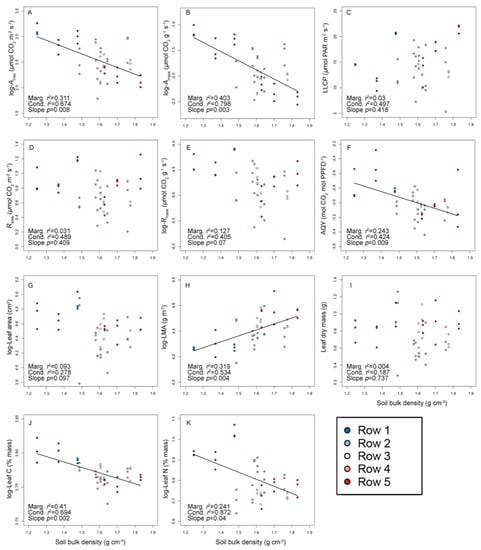
Figure 1.
Relationships between 11 ‘Chardonnay’ leaf traits and soil bulk density. Relationships are presented for: light-saturated maximum photosynthetic rate on a per leaf area basis (Amax, Panel A); light-saturated maximum photosynthetic rate on a per leaf mass basis (Amass, Panel B); leaf-level light compensation point (LLCP, Panel C); leaf respiration rate on a per leaf area basis (Rarea, Panel D); leaf respiration rate on a per leaf mass basis (Rmass, Panel E); apparent quantum yield (AQY, Panel F); leaf area (Panel G); leaf mass per unit area (LMA, Panel H); lead dry mass (Panel I), leaf carbon (C) concentration (Panel J); and leaf nitrogen (N) concentration (Panel K). Trend lines correspond to statistically significant relationships (where p ≤ 0.05 for the slope parameter) between each trait and bulk density, based on linear mixed effects models predicting traits as a function of soil bulk density (as a fixed factor) while accounting for plant identity (as a random factor; see Table S2 for full model diagnostics and fits). Also presented are marginal r2 values (“Marg. r2”) for each relationship, which represents the proportion of variation in a given trait explained by fixed factors alone (i.e., bulk density and model intercept), and condition r2 values (“Cond. r2”), which represent the proportion of trait variation explained by fixed and random factors. Sample sizes for all models were 45 leaves, measured across 15 individual vines. Log-transformed trait values were used in models according to results presented in Table 1, and trait acronyms are presented in Table 1.
Soil bulk density had a statistically significant influence on LMA and leaf area, with planting row identity explaining 62.9% and 30.3% of the variation in these traits, respectively (Table 1). Both traits were significantly correlated to bulk density (mixed model slope p ≤ 0.01, Figure 1, Table S2). Generally, ‘Chardonnay’ leaves were smaller in terms of leaf area and expressed a higher LMA in areas of higher soil compaction: leaf area varied by a factor of three across our dataset (CV = 25.3), ranging from 44.0 to 153.9 cm2 and declining significantly as bulk density increased, while LMA varied nearly 2-fold (range 63.5–111.2 g m−2, CV = 12.2) and increased significantly with higher bulk density (Figure 1G,H, Table 1 and Table 2). Leaf dry mass did vary from 0.28 to 1.26 g across our dataset (CV = 28.1), though this variation was weakly explained by planting row (15.5% variation explained) and was not related to soil bulk density (Figure 1I, Table S2). Therefore, statistically significant increases in LMA across a bulk density gradient were attributable to declines in leaf area and not increases in leaf mass.

Table 2.
Leaf Economics Spectrum (LES) trait relationships in ‘Chardonnay’ and wild plant species.
Compared with other suites of traits, leaf chemical traits, including C and N concentrations, were less variable across ‘Chardonnay’ leaves (CV = 1.8 and 10.4, respectively). However, consistent with declines in photosynthesis and leaf Φ that occurred in relation to bulk density, leaf N concentrations also declined significantly as bulk density increased (mixed model slope p = 0.04, marginal r2 = 0.241; Figure 1K, Table S2). Across all leaves, N concentrations ranged 1.9–2.9% with sampling row explaining over half of the variation in this trait (Table 1). Leaf C also declined significantly with greater bulk density (mixed model slope p ≤ 0.04, marginal r2 = 0.41, respectively; Figure 1J, Table S2). Sampling row explained 54 and 56% of the variability in leaf N and C, respectively (Table 1).
3.2. Multivariate Trait Syndromes in ‘Chardonnay’ in Relation to Soil Bulk Density
The seven traits incorporated into our multivariate analysis covaried along two primary PCA axes, which accounted for 46.4% and 21.5% of variation in ‘Chardonnay’ physiological (Amass, Rmass, Φ, LLCP), morphological (leaf area, LMA), and chemical (leaf N) traits (Figure 2). The first PCA axis represented ‘Chardonnay’ leaf trait covariation and trade-offs consistent with an intragenotype LES. Specifically, PCA axis 1 was most strongly and positively related to Amass, leaf N, leaf Φ, and Rmass (r = 0.644–0.888 and p < 0.001 in all cases), all of which traded off with LMA (r = −0.615, p < 0.001; Figure 2, Table S3). Therefore, ‘Chardonnay’ leaves expressing a higher PCA axis 1 score were associated with “resource-acquiring” LES trait syndromes, while “resource-conserving” LES trait syndromes characterized ‘Chardonnay’ leaves with lower PCA axis 1 scores (Figure 2). The second PCA axis reflected the covariation of traits associated with resource investment in light interception, including leaf area (r = 0.431, p < 0.001), LMA (r = 0.69, p < 0.001), and leaf-level light requirements (LLCP, r = 0.767, p < 0.001; Figure 2, Table S3).

Figure 2.
Principal Component Analysis (PCA) for seven ‘Chardonnay’ wine grape leaf traits measured in 2020 across a soil compaction gradient. Data point colours correspond to vine sampling rows, which are situated along a gradient of bulk density values, and dotted black lines represent 95% confidence ellipses for leaves across different rows. Trait acronyms are presented in Table 1.
Planting row identity explained 39.6% of the variation in multivariate leaf trait syndromes in ‘Chardonnay’ (PerMANOVA p < 0.001, Table S4), with the rows of lowest bulk density being concentrated and differentiated (in terms of their 95% confidence ellipses) at the resource-acquiring end of PCA 1 axis (Figure 2). Planting rows with the highest mean bulk density (i.e., Row 5) did tend to be concentrated at the resource-conserving end PCA axis 1 (Figure 2). However, differentiation of leaves sampled in planting rows 2–5, where bulk density did increase, albeit not significantly, was weaker along PCA axis 1. Soil bulk density, as represented categorically by planting row identity, did not influence ‘Chardonnay’ leaf position along PCA axis 2 with rows showing overlapping 95% confidence ellipses along this axis (Figure 2). Neither individual plant identity nor its interaction with planting row influenced the position of ‘Chardonnay’ leaves in multivariate trait space (PerMANOVA p = 0.788 and 0.301, respectively, Table S4).
3.3. An Intragenotype LES in Chardonnay Driven by Soil Compaction
The four leaf traits included in the original interspecific LES [1] including Amass, Rmass, leaf N, and LMA, were correlated with one another in patterns that were consistent with an intragenotype LES in ‘Chardonnay’ (Figure 3, see also Table S5 for complete trait correlation matrix). Specifically, Amass, Rmass, and leaf N all covaried positively across leaves (SMA r2 range = 0.332–0.354, p < 0.001 in all three relationships; Figure 3D–F), while LMA traded off negatively against all three of these traits (SMA r2 range = 0.146–0.397, p ≤ 0.01 in all three relationships; Figure 3A–C). Largely consistent with relationships found between each of these four traits and bulk density (Figure 1), as well as our PCA (Figure 2), ‘Chardonnay’ leaves generally differentiated from one along the intragenotype LES in relation to soil bulk density.

Figure 3.
Relationships across four Leaf Economics Spectrum traits in ‘Chardonnay’ wine grapes. Trait acronyms are light-saturated maximum photosynthetic rate on a per leaf mass basis (Amass; Panels B,E,F); leaf respiration rate on a per leaf mass basis (Rmass; Panels C–E); leaf mass per unit area (LMA; Panels A–C); and leaf nitrogen (N) concentration (Panels A,D,F). Colours correspond to sampling rows reflecting a soil compaction gradient: black solid trend lines correspond to the standardized major axis (SMA) regression model of a given bivariate trait relationship in ‘Chardonnay’, and dashed black trend lines represent convex hull models that encapsulate the two-dimensional trait space occupied by ‘Chardonnay’ leaves. Also shown are the data and SMA models for the same LES trait relationships observed among wild plants in the GLOPNET dataset (grey dashed trend lines and points). Details on all SMA models shown here are presented in full in Table 2.
Although this differentiation was imperfect and entailed some overlap, generally (A) leaves from vines grown in the lowest bulk density (sampling row 1) defined the resource-acquiring end of the Chardonnay LES; (B) those in the highest bulk density rows (sampling row 5) defined the resource conserving end of the Chardonnay LES; and (C) those in intermediary sampling rows were interspersed between these endpoints along the intragenotype LES bivariate trait space (Figure 3). In all cases, LES trait relationships across the intragenotype LES in ‘Chardonnay’ were statistically different from those observed among wild plants in the GLOPNET dataset (test for differences in SMA slopes in ‘Chardonnay’ vs. wild plants p ≤ 0.03 in all six bivariate relationships; Figure 3, Table 2).
4. Discussion
In finding that ‘Chardonnay’ leaf traits covary and trade off along an intragenotype LES, our research contributes evidence that LES traits covary in one of the world’s most commercially important and widespread crop species. This information adds to the few existing studies that have quantified within-species or -genotype Leaf Economics Spectra in crops [13,14,21,28] and complements the extensive literature documenting the causes and consequences of ecophysiological variation in grapevines [56]. Moreover, our work here contributes to an understanding of how soil compaction—a critical feature of agricultural systems globally—is a managed environmental factor correlated with crop differentiation along multivariate functional trait spectra.
4.1. Intraspecific Trait Variation and Soil Compaction
Differences in ‘Chardonnay’ leaf traits along the intragenotype LES found here, which were correlated with soil compaction, were largely attributable to variability in Amass. This trait expressed the highest CV (43.2%), was the most strongly and negatively correlated to soil bulk density (marginal r2 = 0.403), and, in turn, centrally defined multivariate trait differences (i.e., r = 0.831 along Axis 1 in our PCA) and bivariate LES trait relationships in ‘Chardonnay’. Soil compaction may reduce photosynthesis through both stomatal limitations and N limitations [32], both of which, therefore, likely play a role in structuring the compaction-induced intragenotype LES in ‘Chardonnay’ observed here.
First, in our dataset, at saturating irradiance (where PPFD = 2000 μmol m−2 s−1), log-transformed stomatal conductance (gs, mol H2O m−2 s−1) predicts 83.6% of the variation in log-Amax (simple linear regression p < 0.001, n = 45), and declines significantly with bulk density (mixed model slope=−0.46 ± 0.12 (s.e.), p < 0.001, marginal r2 = 0.407). This would indicate that stomatal limitations are at least partially driving differences in ‘Chardonnay’ leaves and plants along our intragenotype LES. Research has shown that when water is limited, cavitation in the petioles of grape leaves prevents embolisms from propagating to other parts of the plant, which, in turn, acts as a signal for reduced gs via stomatal closure [57]. Thus, in our study, reductions in Amax and gs in relation to increased bulk density, and, in turn, differentiation of leaves along our intragenotype LES, are likely partially related to reduced ability to access soil water.
Second, we also found (1) a statistically significant positive correlation between leaf N and both Amax and Amass (Table S5) and (2) a statistically significant decline in leaf N as a function of soil bulk density (Figure 1). This would indicate that differences in plant N availability and assimilation—i.e., conversion of inorganic nitrate (NO3−) and ammonium (NO4+) into amino acids and proteins—across our site also drives intragenotype LES trait variation. Across a soil compaction gradient, N uptake is often reduced as plant roots are less able to forage N via root elongation [31]. At our site, where fertilizers are applied uniformly, reduced ability of roots to penetrate areas of high soil N likely contributes to differences in leaf N across planting rows (Table S1) and in relation to soil bulk density (Figure 1).
These processes, though, are unlikely to be independent, and ultimately our intragenotype LES in ‘Chardonnay’—particularly the strong relationships between Amass and leaf N in bivariate and multivariate trait space—likely is owing to complex covariation, feedbacks, and pathways among soil N availability, N and C assimilation, and translocation of photosynthates (i.e., sucrose and starch). In short, N uptake and assimilation is often reduced when grapevine C status declines since both are energy-dependent processes that require a supply of C through the Krebs cycle [56]. Thus, stomatal limitations to photosynthesis may also contribute to reduced N assimilation and leaf N concentrations. Path analyses would help uncover the causal pathways structuring LES trait covariation in ‘Chardonnay’ [57]. Yet ultimately, the literature suggests the intragenotype LES in ‘Chardonnay’ found here has likely arisen as a function of plant-, leaf-, and/or root-scale responses to micro-site variation in water or inorganic soil N availability, both of which are, in turn, influenced by soil compaction. Notably though, previous studies on intraspecific or intragenotypic variation in crops have not uncovered strong relationships between LES traits and soil N or moisture content, likely due to limitations of static point sampling these environmental variables [25,58].
4.2. Leaf Economics Traits in Relation to Crop Domestication Syndromes
Studies reporting differences in the LES traits relationships in crops vs. wild plants have supported inferences and hypotheses surrounding the unintended consequences of artificial selection [22,36]. Perhaps most consistent with hypotheses related to artificial selection is our find that in comparison with wild plants, ‘Chardonnay’ expressed a steeper increase in Amass and Rmass per unit increase in leaf N. Specifically, based on our SMA models fits (Table S2), across the range of leaf N values in ‘Chardonnay’ observed here (1.9–2.9%), predicted Amass increased by ~84.3% (from 0.06 to0.38 μmol CO2 g−1 s−1) and Rmass increased by ~71.8% (from 0.008 to 0.028 μmol CO2 g−1 s−1). Comparatively, in wild plants from the GLOPNET dataset, this same increase in leaf N from 1.9 to 2.9% corresponds to only a 47.0% predicted increase in Amass (from 0.138 to 0.264 μmol CO2 g−1 s−1) and a 42.2% increase in Rmass (from 0.028 to 0.048 μmol CO2 g−1 s−1). Higher photosynthetic rates for a given value or increase in leaf N concentrations have been similarly detected in rice [20] and may reflect conscious or unconscious artificial selection for more rapid growth responses to N availability in crops vs. wild plants. However, this is not universal among crops. Certain crops, namely coffee, show significantly lower increases in Amass with greater leaf N [14,28], while others including soy express Amass–leaf N relationships that are statistically indistinguishable from those in wild plants [13]. In sum, the growing literature to which we contribute with our findings here, indicates that LES trait relationships are a unique and idiosyncratic feature of crop domestication syndromes.
In this regard, a novel contribution of our early findings here is the integration of R into studies evaluating intraspecific or intragenotypic LES in crops. Specifically, previous studies evaluating crop trait (co-)variation in comparison with non-domesticated wild plants have not included R in their analyses [13,14,20,22,59], despite this trait representing a key trade-off along the LES [1,38]. The LES trait relationships in ‘Chardonnay’ which included Rmass were qualitatively unique in that none of these bivariate datasets and SMA models intersected the global LES defined by wild plants (Figure 2C–E). Instead, at a given value of Amass, LMA, or leaf N, in nearly all of the leaves measured here (i.e., 43 or 45 leaves), ‘Chardonnay’ Rmass was consistently lower than average vs. Rmass in wild plants. This indicates that domestication has favoured vines that express leaves with a low rate of C loss at a given rate of structural or chemical investment in C assimilation.
These results have two possible explanations: (1) even the lowest bulk density/compaction values at our study site still restrict physiological functioning and/or (2) lower Rmass for a given value of Amass, leaf N, or LMA is a signature of domestication in Vitis vinifera varieties. Since the primary targets of grape domestication are related to yield, quality, growth form, and harvestability [56], our findings point to an unintended consequence of domestication related to plant C economy. Expanding our work across a wider range of ‘Chardonnay’ growing sites (particularly where bulk density is lower) and grape varieties is therefore central in testing either proposed explanation.
One unexpected finding in our analysis here were patterns of leaf C variation. Although not a primary focus of our analysis—since it is not considered a primary trait forming the LES [1]—we found that this trait covaried in an unexpected pattern along the intragenotype LES in ‘Chardonnay’. Specifically, we detected a statistically significant positive relationship between leaf C and Amass, Rmass, and leaf N, and a significant negative relationship between leaf C and LMA (Table S5). Furthermore, when incorporated into an additional PCA, leaf C covaried across the first PCA axis positively with Amass and leaf N but negatively with LMA (Table S6). Therefore, in our dataset, leaf C covaries along LES traits such that higher leaf C values reflect a resource acquisitive trait syndrome. This finding is counter to studies of certain other domesticated plants where leaf C is by and large positively related to leaf construction costs, leaf dry matter content, and LMA [14,21]. In ‘Chardonnay’, coordination of leaf C along an intragenotype LES likely is owing to the selection for C loading in leaves and plants in the form of sugars and starches [56].
5. Conclusions
Understanding how grapevine physiology responds to global environmental change drivers is a critical avenue of research with implications for agricultural sustainability, as well as understanding how domestication has influenced the functional traits and trait relationships of crops. Our results contribute to both of these areas of research, but considerable opportunities remain. First, we show greater compaction leads to grapes expressing more resource-conservative trait syndromes. Extending this work to evaluate whether leaf trait values along an intra-genotype LES Chardonnay are correlated with grape growth, yield, and quality can aid in refining predictions of grapevine and vineyard responses to environmental conditions, at local- through to global scales [60,61]. Expanding our study to multiple grape varieties presents an opportunity to test hypotheses on whether LES trait relationships in multiple grape varieties are constrained along a single intra-specific LES, or whether different varieties express unique LES trait relationships. We acknowledge that our findings could be supported through studies that explore wine grape trait variation across multiple vineyards, times in the growing season, grape varieties, or other environmental factors. These lines of research, as informed by our findings here, present novel opportunities to explore how domestication histories influence crop ecophysiological strategies and responses to environmental change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture12101675/s1, Table S1. Mean ‘Chardonnay’ leaf trait values and associated standard deviations across 15 plants, growing across five planting rows in 2020, differing in soil bulk density. Table S2. Variation in 11 ‘Chardonnay’ leaf traits as a function of bulk density (as a fixed factor), while accounting for plant identity (as a random factor). Table S3. Contributions of leaf traits towards two primary axes in a principal component analysis (PCA) across 45 ‘Chardonnay’ wine grape leaves, measured in 2020 on 15 vines growing across a soil compaction gradient. Table S4. Results of a permutational multivariate analysis of variance (PerMANOVA) evaluating variation in seven Leaf Economics traits measured in 2020 across 45 leaves from 15 ‘Chardonnay’ vines, growing across a soil compaction gradient. Table S5. Correlations among 11 leaf functional traits measured in 2020 on 45 ‘Chardonnay’ leaves from 15 individual vines growing across a soil bulk density gradient, in Niagara-on-the-Lake, Ontario, Canada. Table S6. Contributions of leaf traits, including leaf C concentrations, towards two primary axes in a principal component analysis (PCA) across 45 ‘Chardonnay’ leaves measured in 2020 on 15 vines growing across a soil compaction gradient. Figure S1. Location of the Niagara College Teaching Vineyard in Niagara-on-the-Lake, Ontario, Canada, alongside a study design schematic, and soil bulk density values at sampling row locations. Figure S2. Leaf level photosynthesis rates as a function of photosynthetically active photon flux density across 45 ‘Chardonnay’ leaves measured in 2020 on 15 individual vines, growing across five sampling rows (denoted by different colors), which correspond to a bulk density gradient.
Author Contributions
Conceptualization, A.R.M.; data curation, R.O.M. and N.J.P.; formal analysis, A.R.M.; funding acquisition, K.A.C., G.R. and A.R.M.; investigation, A.R.M., R.O.M., and N.J.P.; methodology, A.R.M., R.O.M., N.J.P. and G.R.; project administration, K.A.C., G.R., M.D. and A.R.M.; resources, K.A.C., G.R., A.R.M. and M.D.; supervision, A.R.M.; visualization, A.R.M.; writing—original draft, A.R.M.; writing—review and editing, R.O.M., M.D., N.J.P., G.R. and K.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Discovery Grant to A.R.M. from the Natural Sciences and Engineering Research Council of Canada, and by the University of Toronto Scarborough’s (UTSC) Sustainable Food and Farming Future (SF3) Cluster under UTSC’s Clusters of Scholarly Prominence Program.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are not yet provided but will be archived in the TRY Functional Trait Database upon publication of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.; Falster, D.S.; Groom, P.K.; Hikosaka, K.; Lee, W.; Lusk, C.H.; Niinemets, Ü.; Oleksyn, J.; et al. Modulation of leaf economic traits and trait relationships by climate. Global Ecol. Biogeogr. 2005, 14, 411–421. [Google Scholar] [CrossRef]
- Reich, P.B.; Rich, R.L.; Lu, X.; Wang, Y.P.; Oleksyn, J. Biogeographic variation in evergreen conifer needle longevity and impacts on boreal forest carbon cycle projections. Proc. Natl. Acad. Sci. USA 2014, 111, 13703–13708. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.J.; Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Kattge, J.; Diaz, S.; Vellend, M.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; et al. Global plant trait relationships extend to the climatic extremes of the tundra biome. Nat. Commun. 2020, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B.; Vose, J.M.; Gresham, C.; Volin, J.C.; Bowman, W.D. Generality of leaf trait relationships: A test across six biomes. Ecology 1999, 80, 1955–1969. [Google Scholar] [CrossRef]
- Shipley, B.; Lechowicz, M.J.; Wright, I.; Reich, P.B. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 2006, 87, 535–541. [Google Scholar] [CrossRef]
- Donovan, L.A.; Maherali, H.; Caruso, C.M.; Huber, H.; de Kroon, H. The evolution of the worldwide leaf economics spectrum. Trends Ecol. Evol. 2011, 26, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Soudant, A.; Boucher, F.; Saccone, P.; Lavorel, S. Intraspecific functional variability: Extent, structure and sources of variation. J. Ecol. 2010, 98, 604–613. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V.; et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef]
- Fajardo, A.; Siefert, A. Intraspecific trait variation and the leaf economics spectrum across resource gradients and levels of organization. Ecology 2018, 99, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Hayes, F.J.; Buchanan, S.W.; Coleman, B.; Gordon, A.M.; Reich, P.B.; Thevathasan, N.V.; Wright, I.J.; Martin, A.R. Intraspecific variation in soy across the leaf economics spectrum. Ann. Bot. 2019, 123, 107–120. [Google Scholar] [CrossRef]
- Martin, A.R.; Rapidel, B.; Roupsard, O.; Van den Meersche, K.; de Melo Virginio Filho, E.; Barrios, M.; Isaac, M.E. Intraspecific trait variation across multiple scales: The leaf economics spectrum in coffee. Funct. Ecol. 2017, 31, 604–612. [Google Scholar] [CrossRef]
- Niinemets, Ü. Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol. 2015, 205, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Siefert, A.; Ritchie, M.E. Intraspecific trait variation drives functional responses of old-field plant communities to nutrient enrichment. Oecologia 2016, 181, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Laforest-Lapointe, I.; Martínez-Vilalta, J.; Retana, J. Intraspecific variability in functional traits matters: Case study of Scots pine. Oecologia 2014, 175, 1337–1348. [Google Scholar] [CrossRef]
- Mitchell, R.M.; Ames, G.M.; Wright, J.P. Intraspecific trait variability shapes leaf trait response to altered fire regimes. Ann. Bot. 2021, 127, 543–552. [Google Scholar] [CrossRef]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific trait variation in plants: A renewed focus on its role in ecological processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef]
- Xiong, D.; Flexas, J. Leaf economics spectrum in rice: Leaf anatomical, biochemical, and physiological trait trade-offs. J. Exp. Bot. 2018, 69, 5599–5609. [Google Scholar] [CrossRef]
- Gagliardi, S.; Martin, A.R.; Virginio Filho, E.D.M.; Rapidel, B.; Isaac, M.E. Intraspecific leaf economic trait variation partially explains coffee performance across agroforestry management regimes. Agric. Ecosyst. Environ. 2015, 200, 151–160. [Google Scholar] [CrossRef]
- Roucou, A.; Violle, C.; Fort, F.; Roumet, P.; Ecarnot, M.; Vile, D. Shifts in plant functional strategies over the course of wheat domestication. J. Appl. Ecol. 2018, 55, 25–37. [Google Scholar] [CrossRef]
- Martin, A.R.; Hale, C.E.; Cerabolini, B.E.; Cornelissen, J.H.; Craine, J.; Gough, W.A.; Kattge, J.; Tirona, C.K. Inter-and intraspecific variation in leaf economic traits in wheat and maize. AoB Plants 2018, 10, ply006. [Google Scholar] [CrossRef]
- Coleman, B.R.; Martin, A.R.; Thevathasan, N.V.; Gordon, A.M.; Isaac, M.E. Leaf trait variation and decomposition in short-rotation woody biomass crops under agroforestry management. Agric. Ecosyst. Environ. 2020, 298, 106971. [Google Scholar] [CrossRef]
- Martin, A.R.; Hayes, F.J.; Borden, K.A.; Buchanan, S.W.; Gordon, A.M.; Isaac, M.E.; Thevathasan, N.V. Integrating nitrogen fixing structures into above-and belowground functional trait spectra in soy (Glycine max). Plant Soil 2019, 440, 53–69. [Google Scholar] [CrossRef]
- Fulthorpe, R.R.; Martin, A.R.; Isaac, M.E. Root endophytes of coffee (Coffea arabica): Variation across climatic gradients and relationships with functional traits. Phytobiomes J. 2019, 4, 27–39. [Google Scholar] [CrossRef]
- Buchanan, S.; Isaac, M.E.; Van den Meersche, K.; Martin, A.R. Functional traits of coffee along a shade and fertility gradient in coffee agroforestry systems. Agrofor. Syst. 2019, 93, 1261–1273. [Google Scholar] [CrossRef]
- Martin, A.R.; Isaac, M.E. The leaf economics spectrum’s morning coffee: Plant size-dependent changes in leaf traits and reproductive onset in a perennial tree crop. Ann. Bot. 2021, 127, 483–493. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems: A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 82, 121–145. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrie, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef]
- Colombi, T.; Keller, T. Developing strategies to recover crop productivity after soil compaction—A plant eco-physiological perspective. Soil Tillage Res. 2019, 191, 156–161. [Google Scholar] [CrossRef]
- Morales, F.; Pavlovič, A.; Abadía, A.; Abadía, J. Photosynthesis in poor nutrient soils, in compacted soils, and under drought. In The Leaf: A Platform for Performing Photosynthesis; Adams, W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 371–399. [Google Scholar]
- Sadras, V.O.; O’Leary, G.J.; Roget, D.K. Crop responses to compacted soil: Capture and efficiency in the use of water and radiation. Field Crops Res. 2005, 91, 131–148. [Google Scholar] [CrossRef]
- Lipiec, J.; Stępniewski, W. Effects of soil compaction and tillage systems on uptake and losses of nutrients. Soil Tillage Res. 1995, 35, 37–52. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Soil compaction and growth of woody plants. Scand. J. For. Res. 1999, 14, 596–619. [Google Scholar] [CrossRef]
- Milla, R.; Osborne, C.P.; Turcotte, M.M.; Violle, C. Plant domestication through an ecological lens. Trends Ecol. Evol. 2015, 30, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Atkin, O.K.; Lusk, C.H.; Tjoelker, M.G.; Westoby, M. Irradiance, temperature and rainfall influence leaf dark respiration in woody plants: Evidence from comparisons across 20 sites. New Phytol. 2006, 169, 309–319. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S.; Vose, J.M.; Volin, J.C.; Gresham, C.; Bowman, W.D. Relationships of leaf dark respiration to leaf nitrogen, specific leaf area and leaf life-span: A test across biomes and functional groups. Oecologia 1998, 114, 471–482. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Carrari, F.; Lytovchenko, A.; Smith, A.M.; Loureiro, M.E.; Ratcliffe, R.G.; Sweetlove, L.J.; Fernie, A.R. Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol. 2005, 137, 611–622. [Google Scholar] [CrossRef]
- Hauben, M.; Haesendonckx, B.; Standaert, E.; Van Der Kelen, K.; Azmi, A.; Akpo, H.; Van Breusegem, F.; Guisez, Y.; Bots, M.; Lambert, B.; et al. Energy use efficiency is characterized by an epigenetic component that can be directed through artificial selection to increase yield. Proc. Natl. Acad. Sci. USA 2009, 106, 20109–20114. [Google Scholar] [CrossRef]
- Juszczuk, I.M.; Flexas, J.; Szal, B.; Dąbrowska, Z.; Ribas-Carbo, M.; Rychter, A.M. Effect of mitochondrial genome rearrangement on respiratory activity, photosynthesis, photorespiration and energy status of MSC16 cucumber (Cucumis sativus) mutant. Physiol. Plant. 2007, 131, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Jones, J.G. Effect of selection for dark respiration rate of mature leaves on crop yields of Lolium perenne cv. S23. Ann. Bot. 1982, 49, 313–320. [Google Scholar] [CrossRef]
- Amthor, J.S. Respiration and Crop Productivity; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Ramankutty, N.; Evan, A.T.; Monfreda, C.; Foley, J.A. Farming the planet: 1. Geographic distribution of global agricultural lands in the year 2000. Glob. Biogeochem. Cycles 2008, 22, GB1003. [Google Scholar] [CrossRef]
- Atkin, O.K.; Bloomfield, K.J.; Reich, P.B.; Tjoelker, M.G.; Asner, G.P.; Bonal, D.; Bonisch, G.; Bradford, M.G.; Cernusak, L.A.; Cosio, E.G.; et al. Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 2015, 206, 614–636. [Google Scholar] [CrossRef]
- Aryal, N.R.; Anderson, K. Which Winegrape Varieties Are Grown Where? A Global Empirical Picture; University of Adelaide Press: Adelaide, Australia, 2013; p. 700. [Google Scholar]
- Coombe, B.G. Growth stages of the grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Berry, Z.C.; Goldsmith, G.R. Diffuse light and wetting differentially affect tropical tree leaf photosynthesis. New Phytol. 2020, 225, 143–153. [Google Scholar] [CrossRef]
- Salter, W.T.; Merchant, A.M.; Richards, R.A.; Trethowan, R.; Buckley, T.N. Rate of photosynthetic induction in fluctuating light varies widely among genotypes of wheat. J. Exp. Bot. 2019, 70, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Delignette-Muller, M.L.; Dutang, C. fitdistrplus: An R package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-131. 2017. Available online: https://CRAN.R-project.org/package=nlme (accessed on 10 January 2021).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-5. 2017. Available online: CRAN.R-project.org/package=vegan (accessed on 10 January 2021).
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. smatr 3—An R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Keller, M. The Science of Grapevines; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Isaac, M.E.; Martin, A.R.; de Melo Virginio Filho, E.; Rapidel, B.; Roupsard, O.; van den Meersche, K. Intraspecific trait variation and coordination: Root and leaf economics spectra in coffee across environmental gradients. Front. Plant Sci. 2017, 8, 1196. [Google Scholar] [CrossRef] [PubMed]
- Milla, R.; Morente-López, J.; Alonso-Rodrigo, J.M.; Martín-Robles, N.; Stuart Chapin, F., III. Shifts and disruptions in resource-use trait syndromes during the evolution of herbaceous crops. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141429. [Google Scholar] [CrossRef] [PubMed]
- Morales-Castilla, I.; de Cortázar-Atauri, I.G.; Cook, B.I.; Lacombe, T.; Parker, A.; van Leeuwen, C.; Nicholas, K.A.; Wolkovich, E.M. Diversity buffers winegrowing regions from climate change losses. Proc. Natl. Acad. Sci. USA 2020, 117, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.H. Temperature and CO2 dependency of the photosynthetic photon flux density responses of leaves of Vitis vinifera cvs. Chardonnay and Merlot grown in a hot climate. Plant Physiol. Biochem. 2017, 111, 295–303. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).