Abstract
Soil alkalization triggers ion toxicity and osmotic and alkaline (high pH) stresses in plants, damaging their growth and productivity. Therefore, we investigated whether priming with abscisic acid (ABA) increases the tolerance of alfalfa seedlings to alkaline stress, and then examined the underlying molecular mechanisms. Alfalfa seedlings were pretreated with ABA (10 μM) for 16 h and then subjected to alkaline stress using a 15 mM Na2CO3 solution (pH 10.87). Compared with the control, ABA pretreatment significantly alleviated leaf damage and improved the fresh weight, water content, and survival rate of alfalfa seedlings under alkaline conditions. Abscisic acid pretreatment reduced accumulation of reactive oxygen species (ROS), increased activities of the antioxidant enzymes superoxide dismutase (SOD) and peroxidase (POD), maintained higher ratios of K+/Na+, Ca2+/Na+, and Mg2+/Na+, and increased accumulation of proline. In addition, ABA upregulated the expression of genes involved in proline biosynthesis (P5CS) and the sequestration of Na+ in vacuoles (NHX1 and AVP) under alkaline conditions. Abscisic acid priming increased tolerance to alkaline stress by maintaining homeostasis of ROS and metal ions and upregulating osmoprotection and the expression of stress tolerance-related genes.
1. Introduction
Saline–alkaline (SA) stress is one of the major abiotic factors severely limiting plant growth and development, and lowers grain yield [1,2]. Approximately 955 × 106 ha of arable land worldwide face the threat of soil salinization–alkalization [3,4]. High pH, in addition to high Na+ content (Na2CO3 or NaHCO3), characterizes SA stress [5,6] which has more complex adverse effects on plants than neutral saline stress [7]. Saline–alkaline stress results in osmotic damage to the cell membrane systems by producing excess levels of reactive oxygen species (ROS) and increasing oxidation [5,8,9,10]. A higher pH in the rhizosphere resulting from SA stress causes severe damage to the root cells and disturbs ion homeostasis, resulting in plant nutrient deficiencies [5,10,11,12]. However, our understanding of the physiological and molecular mechanisms underlying plant responses to SA stress is still limited [1,8,9,10].
To adapt to the stresses, plants generally employ multiple cross-talk strategies, including the regulation of metabolism and gene expression for physiological and morphological adaptation [13,14]. At present, the mechanisms of salt tolerance have been extensively characterized by investigating the efflux of Na+ from roots to soil, sequestration of Na+ in vacuoles, retrieval of Na+ from the xylem, increased tolerance to high Na+ concentrations, and accumulation of compatible solutes [13,15]. Plants usually increase the concentration of cell fluid and lower the osmotic potential by actively accumulating a variety of organic substances, such as proline and soluble sugars, thus maintaining cell swelling and normal physiological metabolic activities [13,16]. Plants usually respond to SA stress by maintaining K+ uptake, increasing Na+ compartmentalization [13,17], and increasing Ca2+ and Mg2+ levels [2,10,18]. These findings indicate that ion homeostasis plays an important role in plant response to SA stress. Plants can also increase their tolerance to alkalinity by increasing the activities of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX), and nonenzymatic antioxidant compounds, including ascorbic acid (ASH) and glutathione (GSH) [9,10,19,20].
Alfalfa (Medicago sativa L.) is considered to be a pioneer pasture plant because of its high yield and its tolerance to saline and SA stress [2,13]. In general, SA stress inhibits seed germination and restricts alfalfa seedling growth [1,6,12,21] and destroys the ion balance [12] and ROS homeostasis [1,6], ultimately affecting the normal growth and metabolism of alfalfa. Consistent with these results, SA stress in the field also inhibits alfalfa shoot dry mass and stem length, and the Ca2+/Na+ ratio was identified as a critical marker in evaluating SA tolerance [2]. Moreover, several abiotic stress-responsive genes in alfalfa have been reported, such as the genes encoding ROS-scavenging enzymes (CuZnSOD, POD) [15], antiporters/ATPase protein (NHX1, AVP) [15,22,23], and proline biosynthase (P5CS) [15,24]. These findings confirm that stress tolerance is associated with ROS, ion homeostasis, osmotic balance, and relative gene expression.
As a small liposoluble phytohormone, abscisic acid (ABA) plays an important role in plant responses to various abiotic stresses, such as salinization [25], drought [26], and chilling injury [27]. Priming (also known as hardening, sensitization, and potentiation) is thought to be a major pathway for the rapid acquisition of defense capabilities by plants in response to stress [28,29]. ABA pretreatment (priming) can significantly improve plant tolerance to abiotic stresses. Under salt stress, Brassica napus seeds pretreated with ABA show earlier germination and greater radical initiation than a mock control [30]. Gurmani et al. [31] found that seed-soaking with ABA improves the salt tolerance of rice by inhibiting the absorption of Na+ and Cl−. ABA priming increases the tolerance of rice to alkaline stress [11,32]. Liu et al. [9]. reported that ABA pretreatment significantly increases antioxidant enzyme activity and improves the survival rate of rice seedlings. Exogenous ABA increases salt tolerance of alfalfa [33] and alleviates cold stress injury [34]. However, as a defense against alkaline stress, the use of ABA priming in alfalfa seedlings has not yet received much attention.
In this study, we investigated the priming effect of ABA on alkaline tolerance in alfalfa seedlings, and elucidated the underlying mechanisms. The priming with ABA significantly increased alkaline tolerance in alfalfa seedlings by increasing proline content, maintaining ROS and ion homeostasis, and upregulating the expressions of the stress tolerance-related genes.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Alfalfa (Medicago sativa L. ‘Longmu No. 803’) seeds were sterilized with a 6% sodium hypochlorite solution for 10 min, rinsed with distilled water three times, and then germinated in plastic pots (25 cm diameter × 20 cm depth) filled with vermiculite. The seedlings were cultured in a tray soaked with distilled water for 10 d and then Hoagland’s nutrient solution for another 7 days. Twenty-one uniformly grown seedlings were secured onto seven custom sponges, three plants on each custom sponge, in a 320 mL plastic cup. Each treatment consisted of three plastic cups. A controlled growth chamber was set to 25 °C day/20 °C night with a 12 h photoperiod at 350 μmol photons m−2 s−1 light intensity. All experiments were performed with three biological replicates.
2.2. Alkaline Stress Treatment and Abscisic Acid Application
Based on a preliminary experiment, we selected 15 mM of Na2CO3 (pH = 10.87, electrical conductivity (EC) = 2.72 mS cm−1) to simulate alkaline stress (Figure S1), and 10 μM of ABA and fluridone as the treatment concentration (Figure S2). The EC and pH of solutions were measured using a DDS-12 conductivity meter (Lida Inc., Shanghai, China) and a PHS-25 pH meter (Baiyuan Inc., Beijing, China), respectively. ABA (Sigma, Inc., St. Louis, MO, USA) or fluridone (Sigma, Inc., St. Louis, MO, USA, an ABA biosynthesis inhibitor, as the negative control) was dissolved in a small amount of absolute ethanol and then diluted with deionized water to the desired concentration.
For the ABA priming experiment, the eighteen-day-old alfalfa seedlings were separately transferred to the plastic cups containing 0 (control) or 10 μM ABA or fluridone in a dark environment for 16 h, then transferred to solutions containing 0 (unstressed) or 15 mM Na2CO3 in the absence of ABA or fluridone. The seedlings were sampled for analysis after 24 h, 36 h, 48 h, and 60 h.
2.3. Measurement of Leaf Withering Rate, Seedling Survival, and Growth under Alkaline Conditions
The leaf withering rate per seedling with or without ABA pretreatment was recorded after 24, 36, 48, and 60 h of Na2CO3 stress. The survival of alfalfa seedlings with or without ABA pretreatment was determined after 48 h and 60 h of Na2CO3 stress. Individual seedlings were classified as dead when all the leaves were dry and brown. The total fresh weight of individual plants was measured after 60 h of Na2CO3 treatment. The samples were then dried in a forced air-driven oven at 105 °C for 20 min, followed by 70 °C, until a stable mass was reached, before the determination of the dry weight of individual plants. The water content was calculated by the following formula: (fresh weight − dry weight)/fresh weight × 100%.
2.4. Measurement of Chlorophyll Contents, Malondialdehyde, Reactive Oxygen Species, and Antioxidant Enzyme Activities
The chlorophyll content was determined according to the method of Zhang et al. [8]. Total chlorophyll content was calculated using the following formula: (8.02 OD663 nm + 20.21 OD645 nm) × V/1000 W. The contents of the ROS (H2O2 and O2•−), SOD, and POD were measured by the method of Zhang et al. [8]. The malondialdehyde (MDA) content was determined by the method of Liu et al. [9]. The MDA content was calculated using the following formula: 6.45 × (A532 − A600) − 0.56 × A450.
2.5. Measurement of Proline and Metal Ion Contents
The proline content was determined according to the modified method by Bates et al. [35]. Specifically, approximately 50 mg of a dry powder sample from shoots was homogenized in 5 mL of 3% (w/v) sulfosalicylic acid in 15 mL plastic tubes, and then the mixture was placed in a boiling water bath for 10 min. The plastic tubes were cooled and then centrifuged at 3000× g for 20 min. Approximately 2 mL of the supernatant was mixed with 2 mL of glacial acetic acid and 2 mL of acidic ninhydrin reagent and then boiled for 30 min. The plastic tubes were cooled, 4 mL of toluene was added to each tube, and the absorbance at 520 nm was determined with a spectrophotometer (T6, Puxi General Instrument Co., Ltd., Beijing, China).
To determine the contents of metal ions, approximately 0.1 g of dry powder from each sample was digested with a mixture of HNO3 and HClO4 (v/v = 2:1) and then diluted to 100 mL. The total contents of the cations Na+, K+, Ca2+, and Mg2+ in the mixture were determined as previously described using an inductively coupled plasma mass spectrometer (ICPS-7500, Shimadzu Corporation, Kyoto, Japan) [2]. The ratios of K+/Na+, Ca2+/Na+, and Mg2+/Na+ in alfalfa leaves were also calculated.
2.6. RNA Isolation and Reverse Transcription Quantitative PCR
Fresh alfalfa leaves (0.2 g) were harvested from each treatment, frozen in liquid nitrogen, and ground using a benchtop ball mill at 50 Hz for 30 s. Total RNA was extracted using TRIzol reagent (TaKaRaBio, Co., Ltd., Tokyo, Japan), and the first-strand cDNA was biosynthesized using EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen Technologies, Co., Ltd., Beijing, China) following the manufacturer’s instructions. The POD, CuZnSOD, AVP, NHX1, and P5CS accession numbers (GenBank/miRBase) and primers are shown in Table S1 [15,36,37,38]. These genes are involved in antioxidant enzyme synthesis, ion transport, and proline synthesis. The housekeeping gene Actin 2 (GenBank ID: JQ028730.1) was selected as the internal standard due to its stable expression levels in different tissues under various different conditions [38]. The PCR reaction mixture (20 μL) contained 1.6 μL of cDNA template, 0.4 μL of 10 μM specific forward primer, 0.4 μL of 10 μM specific reverse primer, 10 μL of 2× SYBR PremixEx Taq (TaKaRaBio, Co., Ltd., Tokyo, Japan), and 7.6 μL of double-distilled H2O and was run in a PCR max machine (Eco TM 48, Illumina, Saffron Walden, UK). The procedure was conducted as follows: 1 cycle at 95 °C for 30 s; 40 cycles at 95 °C for 15 s, 58 °C for 30 s, and 72 °C for 30 s; and 1 cycle at 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s for melting curve analysis. The level of relative expression was calculated using the 2−△△CT method [39].
2.7. Statistical Analyses
The leaf withering rate and survival rate in Figure 1 were analyzed using a Student’s t-test, and the asterisks denote a significant difference compared with control plants at the level of * p < 0.05 or ** p < 0.01. The other data were analyzed using one-way ANOVA, followed by Duncan’s multiple range tests. p-values < 0.05 were considered as statistically significant. All tests were performed in SPSS v 20.0 for Windows (SPSS, Chicago, IL, USA).
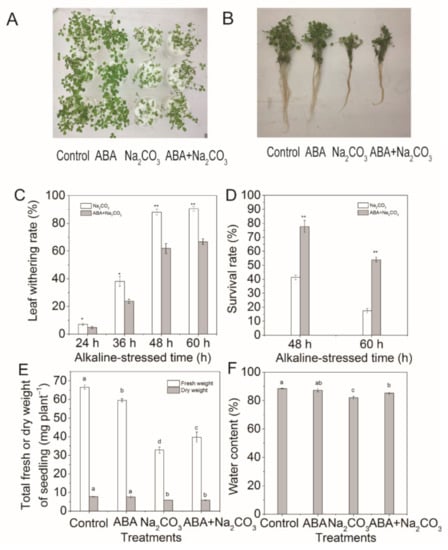
Figure 1.
Abscisic acid (ABA) priming protected alfalfa (Medicago sativa L.) seedlings from wilting and death under alkaline conditions. Eighteen-day-old alfalfa seedlings were root-drenched with 10 μM ABA or without 10 μM ABA (Control) for 16 h and then exposed to alkaline stress (15 mM Na2CO3). (A,B) Photographs of seedling growth were taken after 60 h of alkaline treatment, (C) leaf withering (%) was recorded at 24 h, 36 h, 48 h, and 60 h, (D) survival rate of alfalfa seedlings was determined after 48 h and 60 h of alkaline treatment, (E) total fresh or dry weight of seedlings and (F) water content were measured after 60 h of alkaline treatment. Values are the mean ± standard error, n = 3. Asterisks denote a significant difference compared with control plants (* p < 0.05, ** p < 0.01) based on Student’s t-test. Different letters above the columns indicate significant differences (p < 0.05) at each time point based on Duncan’s test.
3. Results
3.1. Priming with Abscisic Acid Alleviated Alkaline Damage
Our preliminary experiment showed that increasing the Na2CO3 concentration significantly increases MDA content and decreases chlorophyll content (Figure S1). The MDA content was the highest and the chlorophyll content was the lowest under 15 mM Na2CO3 treatment conditions. Therefore, the solution of 15 mM Na2CO3 was selected for the simulation of alkaline stress in this study.
Compared with the control treatment (non-ABA pretreatment), ABA pretreatment significantly reduced the rates of leaf wilting by 33.33%, 37.50%, 29.73%, and 26.32% at 24 h, 36 h, 48 h, and 60 h after alkaline stress treatment, respectively (Figure 1C). Furthermore, ABA pretreatment also increased the survival rate of alfalfa seedlings by 1.88-fold and 3.99-fold at 48 h and 60 h, respectively (Figure 1D), fresh weight by 21.11%, and water content by 3.91% at 60 h after alkaline stress treatment (Figure 1E,F).
In addition, ABA pretreatment significantly decreased the Na+ content (Figure S2A), but significantly increased the K+/Na+ (Figure S2E), Ca2+/Na+ (Figure S2F), and Mg2+/Na+ (Figure S2G) ratios and Ca2+ content (Figure S2C) in alfalfa leaves when 10 μM and 20 μM concentrations of ABA were applied under alkaline stress. The Na+ content under alkaline stress showed no significant differences among the ABA concentrations of 10 μM, 20 μM, and 30 μM (Figure S2A). The contents of ions in the leaves of fluridone-treated seedlings were similar to those in the control treatment (Figure S2A–G).
3.2. Priming with Abscisic Acid Reduced Accumulation of Reactive Oxygen Species and Increased Activities of Antioxidant Enzymes
Alkaline stress induced high accumulation of MDA (Figure 2A), H2O2 (Figure 2B), and O2•− (Figure 2C), which was reduced significantly by ABA pretreatment.
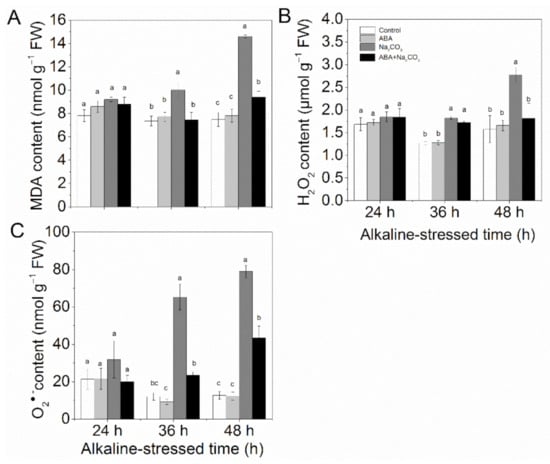
Figure 2.
Abscisic acid (ABA) priming decreased MDA content and ROS accumulation in alfalfa (Medicago sativa L.) seedlings under alkaline stress. Eighteen-day-old alfalfa seedlings were root-drenched with 10 μM ABA or without ABA (Control) for 16 h and then exposed to unstressed (Control and ABA) or alkaline-stressed (Na2CO3 and ABA + Na2CO3, 15 mM Na2CO3) conditions. Accumulation of (A) malonaldehyde (MDA), (B) H2O2, and (C) O2•− in the leaves was determined after 24 h, 36 h, and 48 h of alkaline treatment. Values are the mean ± standard error, n = 3. Different letters above the columns indicate significant differences (p < 0.05) at each time point based on Duncan’s test.
In addition, the activities of the antioxidant enzymes SOD (at 24 h, 36 h, and 48 h; Figure 3A) and POD (at 36 h and 48 h; Figure 3B) were significantly increased by ABA pretreatment under alkaline stress.
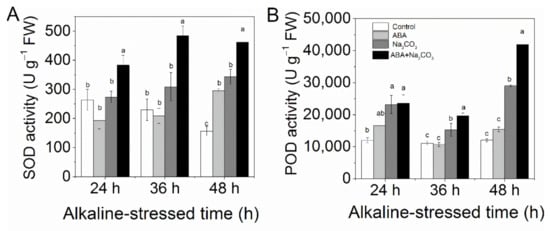
Figure 3.
Abscisic acid (ABA) priming increased the activity of antioxidant enzymes and upregulated the expression of ROS-scavenging genes in alfalfa (Medicago sativa L.) seedlings under alkaline stress. Eighteen-day-old alfalfa seedlings were root-drenched with 10 μM ABA or without ABA (Control) for 16 h and then exposed to unstressed (Control and ABA) or alkaline-stressed (Na2CO3 and ABA + Na2CO3, 15 mM Na2CO3) conditions. The activities of (A) superoxide dismutase (SOD) and (B) and peroxidase (POD) enzymes were measured at 24 h, 36 h, and 48 h of alkaline treatment. Values are the mean ± standard error, n = 3. Different letters on the columns indicate significant differences (p < 0.05) at each time point based on Duncan’s test.
3.3. Priming with Abscisic Acid Enhanced Ion Homeostasis and Proline Accumulation
Alkaline stress increased the accumulation levels of Na+ (Figure 4A) and K+ (Figure 4B) in alfalfa leaves but decreased the contents Ca2+ (Figure 4C) and Mg2+ (Figure 4D). ABA pretreatment increased the ratios of K+/Na+ (Figure 4E), Ca2+/Na+ (Figure 4F), and Mg2+/Na+ (Figure 4G) compared with the control treatment.
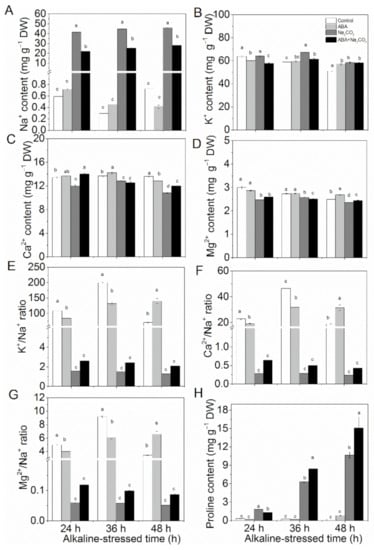
Figure 4.
Abscisic acid (ABA) priming regulated ion homeostasis in alfalfa (Medicago sativa L.) seedlings under alkaline stress. Eighteen-day-old alfalfa seedlings were root-drenched with 10 μM ABA or without ABA (Control) for 16 h and then exposed to unstressed (Control and ABA) or alkaline-stressed (Na2CO3 and ABA + Na2CO3, 15 mM Na2CO3) conditions. (A) Na+ content, (B) K+ content, (C) Ca2+ content, (D) Mg2+ content, (E) K+/Na+ ratio, (F) Ca2+/Na+ ratio, (G) Mg2+/Na+ ratio, and (H) proline contents were determined after 24 h, 36 h, and 48 h of alkaline treatment. Values are the mean ± standard error, n = 3. Different letters above the columns indicate significant differences (p < 0.05) at each time point based on Duncan’s test.
The contents of the osmoprotective compound proline increased significantly under alkaline stress (Figure 4H), which was further increased by ABA pretreatment by 33.42% at 36 h and 41.45% at 48 h after alkaline treatment.
3.4. Priming with Abscisic Acid Upregulated Stress Tolerance-Related Genes
Quantitative real-time PCR (qRT-PCR) showed that ABA pretreatment significantly upregulated expression of the antioxidant enzyme genes CuZnSOD (Figure 5A) and POD (Figure 5B), the stress tolerance-related genes NHX1 (Figure 5C) and AVP (Figure 5D), and the proline synthesis gene P5CS (Figure 5E) under alkaline stress. In addition, the relative expression of NHX1 in the ABA pretreatment reached to the highest value at 36 h and then decreased at 48 h (Figure 5C), whereas the expression of AVP peaked at 24 h and then gradually decreased at 36 and 48 h under alkaline stress (Figure 5D). Moreover, ABA pretreatment induced the highest expression of P5CS at 24 h, and then gradually decreased at 36 and 48 h under alkaline stress (Figure 5E).
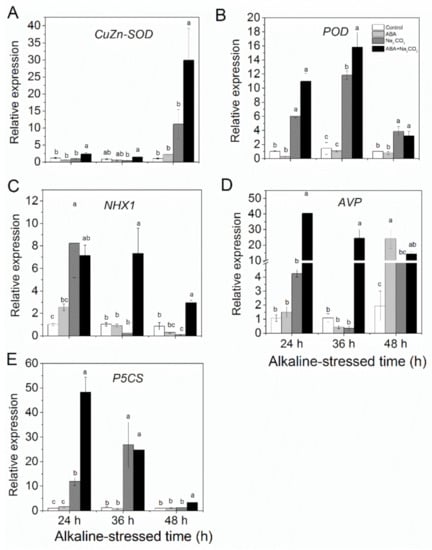
Figure 5.
Abscisic acid (ABA) priming upregulated the relative expression of the ion transport genes (A) CuZnSOD, (B) POD, (C) NHX1, (D) AVP, and (E) P5CS in alfalfa (Medicago sativa L.) leaves under alkaline stress. Eighteen-day-old alfalfa seedlings were root-drenched with 10 μM ABA or without ABA (Control) for 16 h and then exposed to unstressed (Control and ABA) or alkaline-stressed (Na2CO3 and ABA + Na2CO3, 15 mM Na2CO3) conditions. Values are the mean ± standard error, n = 3. Different letters above the columns indicate significant differences (0.05) at each time point based on Duncan’s test. The relative expression levels were analyzed by qRT-PCR and normalized against the internal reference gene Actin 2, which was stably expressed. The expression levels of unpretreated control (Control) at the indicated times were set as the unit to calculate the expression levels, shown as fold changes relative to the control at the indicated times.
4. Discussion
The high salinity and alkalinity of saline–alkaline (SA) stress seriously inhibit plant growth by causing osmotic shock and ion toxicity [1,2,6,7]. Of the two SA stress factors, high alkalinity is the primary factor limiting plant growth [40]. In alfalfa, alkaline stress causes severe wilting of seedlings and oxidative damage [1,6] and disrupts the ion balance [2,7,12] and osmotic homeostasis [2,7]. Therefore, the method to improve the productivity of alfalfa is to improve the tolerance to SA stress. In the present study, we showed that ABA pretreatment significantly alleviated leaf damage and increased the fresh weight, water content, and survival rate of alfalfa seedlings under alkaline stress (Figure 1). Our study revealed that these effects of ABA priming on alfalfa might be associated with decreased ROS accumulation (Figure 2), thus increasing the antioxidant enzyme activities (Figure 3), changing ion and osmotic homeostasis (Figure 4), and upregulating the expression of stress tolerance-related genes (Figure 5). These data indicated a molecular basis of the ABA-primed alkaline tolerance in alfalfa seedlings.
Priming is a cost-effective and environmentally friendly agrobiological technique, which improves plant growth by increasing resistance to diverse biotic and abiotic stresses [28,41,42]. ABA is a potent priming phytohormone that increases the tolerance to various stresses in diverse plant species [31,43,44]. In alfalfa, ABA treatment has been shown to improve stress tolerance to salt [33] and cold [34]. However, few studies have reported on the effect of ABA priming against alkaline stress [9,11,32]. In the present study, ABA application significantly mitigated leaf withering and increased seedling survival rates (Figure 1), suggesting that ABA priming can be a new practical approach to improve alfalfa adaptive planting in soil with high alkalinity. Despite the promise of ABA priming for stress tolerance, the obstacles to the use of ABA as a priming agent include the prohibitively high cost—still several hundred US dollars per gram, and unstable characteristics in the field, both metabolically and related to light-sensitivity [45,46]. However, some representative ABA agonists have been discovered, such as pyrabactin [47,48], ABA mimic-1 (AM1)/quinabactin [45,49], and S7 [50]. These compounds induce stress responses in plants similar to that of ABA and increase plant tolerance to diverse abiotic stresses, including drought [45,49] and cold [46]. Although their effects on plant responses to SA stress are unclear, they may have great potential uses as agrochemicals in the future [50].
ROS accumulation is associated with most abiotic and biotic stresses and causes oxidative damage to plants [51]. Although small amounts of ROS are used as signal molecules, when the ROS homeostasis is disturbed, excessive accumulation of ROS leads to damage to plant cells, eventually resulting in cell death [19,20,52]. An et al. [1] found that antioxidation and detoxification in alfalfa have important roles in response to SA stress. In this study, ABA pretreatment significantly reduced the accumulation of ROS (Figure 2), increased the antioxidant enzyme activities of SOD and POD (Figure 3A,B), and enhanced the expression levels of ROS-scavenging genes under alkaline conditions (Figure 5A,B). These data suggest that ABA pretreatment increased tolerance to the alkaline conditions at least partly by activating the antioxidant defense system in alfalfa seedlings.
Plants exposed to saline stress usually accumulate extra sodium, thus resulting in imbalances among available nutrient elements and competitive absorption, translocation, or distribution, ultimately affecting normal plant metabolism [53,54]. Notably, compared with the control, the K+ content in alfalfa leaves increased after prolonged exposure to alkaline stress (Figure 4B), indicating that the maintenance of adequate K+ in plant tissues under salt stress depends on the selective uptake of K+ and the selective cellular compartmentalization and distribution of K+ and Na+ in the shoots [55]. Moreover, increasing evidence suggests that maintaining adequate Ca2+ and Mg2+ concentrations contributes to signaling and homeostasis of ions and enzyme activity under alkaline conditions [10]. A widely used critical marker of saline or SA tolerance is the ratio of K+/Na+ [56,57,58,59]. Additional important markers reflecting saline or SA tolerance are the ratios of Ca2+/Na+ [2,18] and Mg2+/Na+ [2,60]. In this study, ABA pretreatment resulted in a significant increase in the Ca2+content at 24 h and 48 h, and the Mg2+ content at 48 h (Figure 4C,D). In addition, the K+/Na+, Ca2+/Na+, and Mg2+/Na+ ratios in the ABA pretreatment were higher than those in the pretreatment without ABA under alkaline conditions (Figure 4E–G). Furthermore, some important proteins involved in the sequestration of Na+ in vacuoles, such as vacuolar Na+/H+ exchanger (NHX) and vacuolar H+-PPase (AVP), play pivotal roles in the leaves of plants against salinity [15]. In this study, ABA priming significantly decreased the Na+ content under alkaline conditions (Figure 4A) and increased the expression levels of the stress tolerance-related genes NHX1 and AVP (Figure 5C,D), indicating that ABA pretreatment could alleviate the toxicity of Na+ by preventing excessive cytosolic Na+ accumulation with the compartmentalization of Na+ into vacuoles via upregulating the corresponding vacuolar genes NHX and AVP [13,54,61,62]. Collectively, the results revealed that the maintenance of ion homeostasis is another molecular mechanism that can explain the ABA priming effect.
Proline is an osmotic substance participating in the physiological response that protects plants through osmotic regulation [63,64] and is also a nontoxic source of carbon, nitrogen, and energy reserves [65]. In addition, proline is an ROS scavenger that helps to protect cells from damage caused by stress [66]. In this study, the accumulation levels of proline were increased by ABA pretreatment in alfalfa leaves under alkaline stress (Figure 4H). Consistently, the expression level of P5CS was increased by the ABA pretreatment (Figure 5E). These findings are consistent with previous studies in which primed plants increase the proline content in response to salt stress [31,67]. Thus, ABA pretreatment may also increase alkaline tolerance by increasing osmoprotection in alfalfa leaves.
In our study, although ABA priming created alkaline tolerance in alfalfa seedlings by the root-drenching with ABA, there is still a limitation to practical application in SA fields for alfalfa planting. Therefore, we may need to determine the effect of ABA agonists and develop more effective treatment methods in the future studies, such as spraying onto the leaves, which should provide new approaches to enhance alkaline tolerance and improve alfalfa production.
5. Conclusions
In summary, ABA priming increases the tolerance to alkaline stress in alfalfa seedlings by maintaining homeostasis of ROS and metal ions and by upregulating osmoprotection and genes related to stress tolerance. The study provides new insight into the ABA priming mechanisms responsible for increasing alkaline stress tolerance in alfalfa and may provide a reference when applying signal molecules for increasing the alkaline tolerance of other plants in future studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agriculture11070608/s1, Table S1: Primer pairs for stress-responsive genes used in reverse transcription qPCR; Figure S1: Effects of different concentrations of Na2CO3 on the (A) malondialdehyde (MDA) and (B) chlorophyll contents in the leaves of alfalfa (Medicago sativa L.) seedlings. Different letters above the columns indicate significant differences (p < 0.05) among concentrations based on Duncan’s test. Figure S2: Abscisic acid (ABA) priming alleviated damage to alfalfa (Medicago sativa L.) seedlings under alkaline conditions. Eighteen-day-old alfalfa seedlings were also root-drenched with an ABA concentration of 0, 10, 20, or 30 μM or with fluridone (an ABA biosynthesis inhibitor, 10 μM) for 16 h and then exposed to alkaline stress (15 mM Na2CO3). (A) Na+ content; (B) K+ content; (C) Ca2+ content; (D) Mg2+ content; (E) K+/Na+ ratio; (F) Ca2+/Na+ ratio; (G) Mg2+/Na+ ratio. Values are the mean ± standard error, n = 3. Different letters on the columns indicate significant differences (p < 0.05) (a to g) between ABA treatments and fluridone treatment based on Duncan’s test.
Author Contributions
Conceptualization, C.-J.J. and Z.-W.L.; validation, C.-J.J. and Z.-W.L.; investigation, T.-J.W., M.-M.W., Y.-Y.J., G.-H.Z., M.L., and H.-Y.Y.; writing—original draft, T.-J.W.; writing—review and editing, C.-J.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (41271522); the National Basic Research Program (973 Program) of China (2015CB150800); the Program of Science and Technology development of Jilin province (20200201018JC); Major Science and Technology Innovation Project of Shandong Province (2019JZZY010726); special fund project for science and technology cooperation high-tech industrialization of Jilin Province and Chinese Academy of Sciences (20190725009).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- An, Y.M.; Song, L.L.; Liu, Y.R.; Shu, Y.J.; Guo, C.H. Denovo transcriptional analysis of alfalfa in response to saline-alkaline stress. Front. Plant Sci. 2016, 7, 931. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.J.; Jiang, C.J.; Jin, Y.Y.; Zhang, G.H.; Wang, M.M.; Liang, Z.W. Ca2+/Na+ ratio as a critical marker for field evaluation of saline-alkaline tolerance in alfalfa (Medicago sativa L.). Agronomy 2020, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.; Greene, R.; Dalal, R.; Murphy, B.W. Soil carbon dynamics in saline and sodic soils: A review. Soil Use Manag. 2009, 26, 2–11. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, K.; Singh, B.; Singh, R.P. New Approaches to Enhance Eco-Restoration Efficiency of Degraded Sodic Lands: Critical Research Needs and Future Prospects. Ecol. Restor. 2011, 29, 322–325. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.X.; Yan, C.R.; Zhong, X.L.; Gu, F.X.; Liu, Q.; Xia, X.; Li, H.R. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, R.C.; Sun, H.; Cao, C.Y.; Zhang, T.J.; Kang, J.M.; Wang, Z.; Li, M.N.; Gao, Y.L.; Li, X.; Yang, Q.C. Identification of alkali-responsive proteins from early seedling stage of two contrasting Medicago species by iTRAQ-based quantitative proteomic analysis. Environ. Exp. Bot. 2019, 157, 26–34. [Google Scholar] [CrossRef]
- Peng, Y.L.; Gao, Z.W.; Gao, Y.; Liu, G.F.; Sheng, L.X.; Wang, D.L. Eco-physiological characteristics of alfalfa seedlings in response to various mixed salt-alkaline stresses. J. Integr. Plant Biol. 2008, 50, 29–39. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.-L.; Zhang, R.-X.; Yuan, H.-Y.; Wang, M.-M.; Yang, H.-Y.; Ma, H.-Y.; Liu, D.; Jiang, C.-J.; Liang, Z.-W. Root Damage under Alkaline Stress Is Associated with Reactive Oxygen Species Accumulation in Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1580. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Zhang, H.; Jin, Y.-Y.; Wang, M.-M.; Yang, H.-Y.; Ma, H.-Y.; Jiang, C.-J.; Liang, Z.-W. Abscisic acid primes rice seedlings for enhanced tolerance to alkaline stress by upregulating antioxidant defense and stress tolerance-related genes. Plant Soil 2019, 438, 39–55. [Google Scholar] [CrossRef]
- Yin, Z.P.; Zhang, H.; Zhao, Q.; Yoo, M.J.; Zhu, N.; Yu, J.L.; Guo, S.Y.; Miao, Y.C.; Chen, S.X.; Qi, Z.; et al. Physiological and comparative proteomic analyses of saline-alkali NaHCO3-responses in leaves of halophyte Puccinellia tenuiflora. Plant Soil 2019, 437, 137–158. [Google Scholar] [CrossRef]
- Wei, L.X.; Lv, B.S.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Liu, X.L.; Jiang, C.J.; Liang, Z.W. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol. Biochem. 2015, 90, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Ren, H.L.; Wei, Z.W.; Wang, Y.W.; Ren, W.B. Effects of neutral salt and alkali on ion distributions in the roots, shoots, and leaves of two alfalfa cultivars with differing degrees of salt tolerance. J. Integr. Agric. 2017, 16, 1800–1807. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, D.; Cornacchione, M.V.; Ferreira, J.F.S.; Suarez, D.L. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. 2017, 7, 42958. [Google Scholar] [CrossRef]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M.; Pessarakli, M. Selecting alfalfa cultivars for salt tolerance based on some physiochemical traits. Agron. J. 2014, 106, 1758–1764. [Google Scholar] [CrossRef]
- Adem, G.D.; Roy, S.J.; Zhou, M.X.; Bowman, J.P.; Shabala, S. Evaluating contribution of ionic, osmotic and oxidative stress components towards salinity tolerance in barley. BMC Plant Biol. 2014, 14, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anower, M.R.; Mott, I.W.; Peel, M.D.; Wu, Y.J. Characterization of physiological responses of two alfalfa half-sib families with improved salt tolerance. Plant Physiol. Biochem. 2013, 71, 103–111. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global plant stress signaling: Reactive oxygen species at the cross-road. Front. Plant Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Liu, D.; Liu, M.; Liu, X.L.; Cheng, X.G.; Liang, Z.W. Silicon priming created an enhanced tolerance in alfalfa (Medicago sativa L.) seedlings in response to high alkaline stress. Front. Plant Sci. 2018, 9, 716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.F.; Wang, D.L.; Jin, T.C.; Chang, Q.; Yin, D.X.; Xu, S.M.; Liu, B.; Liu, L.X. The Vacuolar Na+/H+ antiporter gene SsNHX1 from the Halophyte Salsola soda confers salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Mol. Biol. Rep. 2011, 29, 278–290. [Google Scholar] [CrossRef]
- Quan, W.L.; Liu, X.; Wang, H.Q.; Chan, Z.L. Physiological and transcriptional responses of contrasting alfalfa (Medicago sativa L.) varieties to salt stress. Plant Cell Tiss. Org. 2016, 126, 105–115. [Google Scholar] [CrossRef]
- Ehsanpour, A.A.; Fatahian, N. Effects of salt and proline on Medicago sativa callus. Plant Cell Tissue Organ Cult. 2003, 73, 53–56. [Google Scholar] [CrossRef]
- Amjad, M.; Akhtar, J.; Anwar-ul-Haq, M.; Yang, A.Z.; Akhtar, S.S.; Jacobsen, S.-E. Integrating role of ethylene and ABA in tomato plants adaptation to salt stress. Sci. Hortic. Amst. 2014, 172, 109–116. [Google Scholar] [CrossRef]
- Yin, C.Y.; Duan, B.L.; Wang, X.; Li, C.Y. Morphological and physiological responses of two contrasting Poplar species to drought stress and exogenous abscisic acid application. Plant Sci. 2004, 167, 1091–1097. [Google Scholar] [CrossRef]
- Chen, H.H.; Li, P.H.; Brenner, M.L. Involvement of abscisic Acid in potato cold acclimation. Plant Physiol. 1983, 71, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U. Priming of Induced Plant Defense Responses. Adv. Bot. Res. 2009, 51, 361–395. [Google Scholar]
- Pastor, V.; Luna, E.; Mauch-Mani, B.; Ton, J.; Flors, V. Primed plants do not forget. Environ Exp. Bot. 2013, 94, 46–56. [Google Scholar] [CrossRef]
- Gao, Y.P.; Bonham-Smith, P.C.; Gusta, L.V. The role of peroxiredoxin antioxidant and calmodulin in ABA-primed seeds of Brassica napusexposed to abiotic stresses during germination. J. Plant Physiol. 2002, 159, 951–958. [Google Scholar] [CrossRef]
- Gurmani, A.R.; Bano, A.; Khan, S.U.; Din, J.; Zhang, J.L. Alleviation of salt stress by seed treatment with abscisic acid (ABA), 6-benzylaminopurine (BA) and chlormequat chloride (CCC) optimizes ion and organic matter accumulation and increases yield of rice (Oryza sativa L.). Aust. J. Crop. Sci. 2011, 5, 1278–1285. [Google Scholar]
- Wei, L.-X.; Lv, B.-S.; Li, X.-W.; Wang, M.-M.; Ma, H.-Y.; Yang, H.-Y.; Yang, R.-F.; Piao, Z.-Z.; Wang, Z.-H.; Lou, J.-H.; et al. Priming of rice (Oryza sativa L.) seedlings with abscisic acid enhances seedling survival, plant growth, and grain yield in saline-alkaline paddy fields. Field Crop Res. 2017, 203, 86–93. [Google Scholar] [CrossRef]
- Yang, Y.X.; Liu, D.L.; Han, J.G.; Zhao, G.Q.; Han, J.; Wang, X.S. Effects of ABA on the content of mineral element and proline of two alfalfa varieties under NaCl stress condition. Pratacultural Sci. 2010, 27, 57–61. (In Chinese) [Google Scholar]
- Wang, Y.Z.; Ren, W.; Xu, A.K.; Wang, Z.F.; Deng, B. Physiological responses to exogenous SA and ABA in alfalfa varieties under chilling stress. Acta Agric. Boreali-Sin. 2012, 27, 144–149. (In Chinese) [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Felix, K.; Su, J.C.; Lu, R.F.; Zhao, G.; Cui, W.T.; Wang, R.; Mu, H.L.; Cui, J.; Shen, W.B. Hydrogen-induced tolerance against osmotic stress in alfalfa seedlings involves ABA signaling. Plant Soil 2019, 445, 409–423. [Google Scholar] [CrossRef]
- Zhang, C.M.; Shi, S.L.; Liu, Z.; Yang, F.; Yin, G.L. Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J. Plant. Physiol. 2019, 232, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Fu, Y.Y.; Ban, L.P.; Wang, Z.; Feng, G.Y.; Li, J.; Gao, H.W. Selection of reliable reference genes for quantitative real-time RT-PCR in alfalfa. Genes Genet. Syst. 2015, 90, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lv, B.S.; Li, X.W.; Ma, H.Y.; Sun, Y.; Wei, L.X.; Jiang, C.J.; Liang, Z.W. Differences in growth and physiology of rice in response to different saline-alkaline stress factors. Agron. J. 2013, 105, 1889. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Conrath, U. Priming for stress resistance: From the lab to the field. Curr. Opin. Plant Biol. 2007, 10, 425–431. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Sripinyowanich, S.; Klomsakul, P.; Boonburapong, B.; Bangyeekhun, T.; Asami, T.; Gu, H.Y.; Buaboocha, T.; Chadchawan, S. Exogenous ABA induces salt tolerance in indica rice (Oryza sativa L.): The role of OsP5CS1 and OsP5CR gene expression during salt stress. Environ. Exp. Bot. 2013, 86, 94–105. [Google Scholar] [CrossRef]
- Wang, G.J.; Miao, W.; Wang, J.Y.; Ma, D.R.; Li, J.Q.; Chen, W.F. Effects of exogenous abscisic acid on antioxidant aystem in weedy and cultivated rice with different chilling sensitivity under chilling stress. J. Agron. Crop Sci. 2013, 199, 200–208. [Google Scholar] [CrossRef]
- Cao, M.; Liu, X.; Zhang, Y.; Xue, X.; Zhou, X.E.; Melcher, K.; Gao, P.; Wang, F.; Zeng, L.; Zhao, Y.; et al. An ABA-mimicking ligand that reduces water loss and promotes drought resistance in plants. Cell Res. 2013, 23, 1043–1054. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.M.; Jin, R.; Cao, M.J.; Liu, X.D.; Chan, Z.L. Exogenous application of ABA mimic 1 (AM1) improves cold stress tolerance in bermudagrass (Cynodon dactylon). Plant Cell Tiss. Org. 2016, 125, 231–240. [Google Scholar] [CrossRef]
- Zhao, Y.; Chow, T.F.; Puckrin, R.S.; Alfred, S.E.; Korir, A.K.; Larive, C.K. Chemical genetic interrogation of natural variation uncovers a molecule that is glycoactivated. Nat. Chem. Biol. 2007, 3, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.; et al. Abscisic Acid Inhibits Type 2C Protein Phosphatases via the PYR/PYL Family of START Proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, M.; Peterson, F.C.; Defries, A.; Park, S.Y.; Endo, A.; Nambara, E.; Volkman, B.F.; Cutler, S.R. Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proc. Natl. Acad. Sci. USA 2013, 110, 12132–12137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, M.K.; Kim, R.; Moon, S.J.; Lee, Y.; Han, S.; Lee, S.; Kim, B.-G. Selection and functional identification of a synthetic partial ABA agonist, S7. Sci. Rep. 2020, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, E.; Razmjoo, J.; Zahedi, M. Effect of salt stress on growth and ion accumulation of alfalfa (Medicago sativa L.) cultivars. J. Plant Nutr. 2018, 41, 818–831. [Google Scholar] [CrossRef]
- Lei, Y.T.; Xu, Y.X.; Hettenhausen, C.; Lu, C.K.; Shen, G.J.; Zhang, C.P.; Li, J.; Song, J.; Lin, H.H.; Wu, J.Q. Comparative analysis of alfalfa (Medicago sativa L.) leaf transcriptomes reveals genotype-specific salt tolerance mechanisms. BMC Plant Biol. 2018, 18, 35. [Google Scholar] [CrossRef] [Green Version]
- Carden, D.E.; Walker, D.J.; Flowers, T.J.; Miller, A.J. Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol. 2003, 131, 676. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.H. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct. Plant Biol. 2007, 2, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Fakhrfeshani, M.; Shahriari-Ahmadi, F.; Niazi, A.; Moshtaghi, N.; Zare-Mehrjerdi, M. The effect of salinity stress on Na+, K+ concentration, Na+/K+ ratio, electrolyte leakage and HKT expression profile in roots of Aeluropus littoralis. J. Plant Mol. Breed. 2015, 3, 1–10. [Google Scholar]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Horie, T. A conserved primary salt tolerance mechanism mediated by HKT transporters: A mechanism for sodium exclusion and maintenance of high K+/Na+ ratio in leaves during salinity stress. Plant Cell Environ. 2010, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N.; Silk, W.K.; Läuchli, A. Growth and development of sorghum leaves under conditions of NaCl stress: Possible role of some mineral elements in growth inhibition. Planta 1995, 196, 699–705. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ Antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- Ding, M.Q.; Hou, P.C.; Shen, X.; Wang, M.J.; Deng, S.R.; Sun, J.; Xiao, F.; Wang, X.Y.; Zhou, X.Y.; Lu, C.F.; et al. Salt-induced expression of genes related to Na+/K+ and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant Mol. Biol. 2010, 73, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Majid, M.; Ali, A.; Essia, B. Effect of salinity on sodium and chloride uptake, proline and soluble carbohydrate contents in three alfalfa varieties. IOSR-JAVS 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Anower, M.R.; Peel, M.D.; Mott, I.W.; Wu, Y. Physiological processes associated with salinity tolerance in an alfalfa half-sib family. J. Agron. Crop Sci. 2017, 203, 506–518. [Google Scholar] [CrossRef]
- Joyce, P.A.; Aspinall, D.; Paley, L.G. Photosynthesis and the accumulation of proline in response to water deficit. Funct. Plant Biol. 1992, 19, 249–261. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ortega, C.O.; Ochoa-Alfaro, A.E.; Reyes-Agüero, J.A.; Aguado-Santacruz, G.A.; Jiménez-Bremont, J.F. Salt stress increases the expression of P5CS gene and induces proline accumulation in cactus pear. Plant Physiol. Bioch. 2008, 46, 82–92. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).