Abstract
We investigated the effects of three botanicals with nematicidal properties (anise-Pimpinella anisum, parsley-Petroselinum crispum, and rocket-Eruca sativa) on the soil nematode community, in terms of trophic structure and nematode genera composition. We compared effects with those of fluopyram (synthetic nematicide) and Nemagold (bionematicide). We assessed the role of time, by sampling 15 and 45 days after treatments and analyzing nematode genera and microbial phospholipid fatty acid biomarkers (PLFA). Soil incorporation of botanicals reduced plant parasitic nematodes, increased bacterivores, especially the enrichment opportunists and among them Rhabditis, having no effect on fungivores and non-parasitic plant feeders. Neither the number nor the composition and dominance hierarchy of nematode genera were affected. Nemagold did not induce any significant change, while fluopyram decreased both free-living and parasitic nematodes, but with no uniform effect against all genera. The least affected genus was the fungivorous Aphelenchus. While most microbial PLFAs increased with time, the abundances of nematode genera did not change, except the Meloidogyne incognita second stage juveniles, which emerged in soil only 45 days after treatments. The low enrichment index and high channel index values of the fluopyram soil samples indicated a stressful environment. The opposite was observed in the botanical treatments, especially parsley and rocket.
1. Introduction
Soil incorporation of plant materials that act as biofumigants, has been effectively practiced to control plant-parasitic nematodes since 1870 [1]. Even though, there are several studies dealing with the response of plant-parasitic nematodes to soil disinfectants, either chemical [2] or organic [3,4], the majority of these studies neglect effects on non-target soil organisms involved in several ecosystem processes. Interestingly, even bio fumigation in some cases can lead to destabilization of the soil food web, as described for isothiocyanates affecting adversely a wide range of soil biota along with the plant-parasitic nematodes [5]. Thus, the efficacy of plant-based soil amendments should always be tested in relation to the impacts on soil communities that are vital for soil functionality, such as soil microbes and non-pathogenic free-living nematodes. Soil bacteria and fungi, as well as the free-living nematodes that graze on them, are the main counterparts of significant soil processes such as decomposition of organic residues, nitrogen mineralization, nutrient cycling, and formation of humic substances [6,7,8]. The ecological significance of soil free-living nematodes as bioindicators for evaluating the impact of stressors on soil conditions has been highlighted in numerous studies [9,10]. In fact, their importance lies in their high abundance and diversity, variety of trophic types and reproductive strategies, and in their contribution to soil nutrient turnover [6,11].
We recently published the nematicidal potential of Pimpinella anisum (anise), Petroselinum crispum (parsley), and Eruca sativa (rocket), used as soil amendments in tomato pots that were artificially inoculated with root-knot nematodes, and compared their nematicidal activity against the plant-parasitic nematode Meloidogyne incognita to that of the synthetic nematicide fluopyram and the bionematicide Nemagold [3]. Briefly, we showed that soil amending with rocket, anise and parsley resulted in decreased numbers of second-stage juveniles (J2) of M. incognita in soil, as well as females and galls in host roots. Moreover, all three botanicals had a positive effect on tomato root growth and favored the growth of microbial biomass and enzymatic activities. In the frame of a holistic approach, in the present paper we provided complementing results, focusing on all nematode functional guilds. Indeed, the study of the soil nematode community both in terms of trophic types and life strategies gives a better insight in the structure of the soil food web and hence the nutritional state of the soil [6,12]. More specifically, we quantified the short (15 days) and long-term effects (45 days) on the soil nematode community by analyzing its trophic and functional structure, as well as the composition of nematode genera. Furthermore, we aimed to estimate whether the time post-treatment plays a crucial role in shaping the structure of the soil communities. To answer this, we used the microbial phospholipid fatty acid (PLFA) biomarkers as well as the nematode genera recorded in all our experimental pots.
2. Materials and Methods
2.1. Preparation of Nematode Inoculum
Meloidogyne incognita population was reared on tomato plants cv. Belladonna at the six-leaf stage. Eggs were extracted from tomato plant roots according to Hussey and Barker (1973) [13] and second-stage juveniles (J2) were allowed to hatch in modified Baermann funnels at 28 °C. All freshly hatched (24 h) second-stage juveniles were used in the experiments.
2.2. Experimental Set Up and Sampling
A clay loam soil with 1.3% organic matter and pH 7.8, free of root-knot nematodes, was collected from the upper 15 cm from the Farm of the School of Agriculture, Aristotle University of Thessaloniki. The experimental protocol is described in detail in Ntalli et al., 2019 [3]. For the experimental treatments we used anise (Pimpinella anisum) seed while for rocket (Eruca sativa) and parsley (Petroselinum crispum) we used the aerial parts at harvest. All three botanical materials were ground in a mortar with a pestle before mixing into the soil. All crops were organic local cultures at Thessaloniki and the botanical material was fresh. The treatments were performed at EC50 values increased by 25% in order to ensure an acceptable efficacy under field conditions (75%) [14,15,16]. The synthetic nematicide Velum Prime SC (a.i: fluopyram 40% w/v, Bayer Crop Science) and the natural nematicidal formulation Nemagold (a.i: Tagetes erecta extract 80% w/w & Seaweed extract 10% w/w, Atlantica Agricultura Natural) were used at the recommended doses for nematode control. Water served as the control. More specifically, the experimental treatments consisted of (1) Pimpinella anisum seed meal at 5.9 mg g−1 of soil, (2) Petroselinum crispum green manure at 30.9 mg g−1 of soil, (3) Eruca sativa green manure at 25.0 mg g−1 of soil, (4) Velum Prime SC at 0.1 μL kg−1 of soil, (5) Nemagold at 12.8 μL kg−1 of soil, and (6) water. The artificial inoculation of soil with the nematodes was performed in 10 L volume plastic bags, representing experimental treatments. The soil in each plastic bag was inoculated with a nematode suspension of 15,000 J2, mixed thoroughly by shaking and allowed to equilibrate in dark at 10 °C for 24 h. Subsequently, the soil was sieved through a 3 mm sieve, returned into the plastic bags, and treated with the respective botanical pastes or test solutions of commercial products or water control. After an additional 24 h equilibration period at 10 °C the soil from plastic bags was sieved again to ensure uniform distribution, and then it was placed in plastic pots (1500 g, 4 replications for each treatment), where six-leaf stage tomato plants, cv. Belladonna, were transplanted (one plant per pot). These plants were a kind offer of AGRIS SA and were used for the experiments exactly after arrival in the lab without prior handling. The experiment was maintained at 27 °C, 60% RH and 16 h photoperiod. Pots were watered with 80 mL of water every 3 days for a total of 45 days. Treatments were arranged in a completely randomized design with four replicates. The experiment was performed twice.
For analyses of PLFAs and soil nematodes, two soil samplings were conducted with a cylindrical soil corer (12 cm depth and 20 mm diameter). A composite soil sample, consisting of four cores, was taken from each pot carefully to avoid damaging the roots. The first sampling took place in the middle and the second in the end of the M. incognita biological cycle, i.e., 15 and 45 days After Application (15 DAA, 45 DAA) of treatments.
2.3. Phospholipid Fatty Acid Analysis
The extraction and analysis of the PLFA biomarkers from soil samples was performed according to the method described by Ntalli et al., (2018) [4]. Overall, 24 fatty acid methyl esters were consistently present in all samples, including the internal standard 19:0. These fatty acids were assigned to functional groups as follows [4,17,18]: i-15:0, a-15:0, 15:0, i-16:0, i-17:0, 17:0 (Gram-positive bacteria); cy17:0, 16:1ω7c (Gram-negative bacteria); 18:2ω9,12 (fungi); 20:5ω3 and 20:4ω6 (protozoa); 20:0, 21:0, 22:0, and 23:0 (microeukaryotes, e.g., algae, nematodes). The remaining PLFAs may have derived from several sources and were considered only for the estimation of total microbial biomass. For example, 18:1ω9t, 18:1ω9c may have been derived from both Gram-negative bacteria and fungi, 16:0 from bacteria and fungi, while 12:0, 14:0, 17:1ω7, 18:0, and 18:2ω6t are mainly of microbial origin.
2.4. Nematode Extraction and Analysis
Nematodes were extracted from 100 mL of each composite soil sample that was previously mixed gently by hand to break up soil aggregates. For extraction, the Cobb’s sieving and decanting method was used as modified by S’Jacob and van Bezooijen (1984) [19], where a cotton wool filter is used in the last step. After counting total nematode numbers of nematodes, they were fixed with 4% formaldehyde and later we randomly selected 150 nematodes from each sample and identified them to genus level with the identification key of Bongers (1994) [20]. Nematode genera were assigned to trophic groups according to Yeates et al., (1993) [21], and classified along the colonization-persistence gradient (c–p values) following Bongers (1990) [22] and Bongers and Bongers (1998) [23]. Regarding nematode functional indices, we estimated the Maturity Index (MI) for free living nematodes and the Plant Parasitic Index (PPI) for plant feeding nematodes, both indicating the successional stage of communities, according to Bongers (1990) [22]. The Enrichment index (EI) and the Channel Index (CI), indicating the enrichment of soil and the dominant decomposition pathway respectively, were calculated according to Ferris et al., (2001) [24].
2.5. Data Analysis
The experiment was performed twice, and ANOVA indicated that the mean values of measured variables did not differ significantly between the two experimental series. In this context, in our study we presented the results of one of the two experiments, thus four genuine replicates (mean values, standard error) per treatment.
We applied a repeated-measures ANOVA to determine the effect of treatment, time after treatment and their interaction on soil nematode abundances and nematode community indices. In all analyses, means were compared using Fisher’s LSD test at p < 0.05. To further explore whether the time after treatment or the treatment per se is more important for data variability a principal components analysis (PCA) was conducted on the basis of nematode genera and PLFA biomarkers. Before all analyses, data were transformed appropriately, when necessary, in order to meet the assumptions of ANOVA. Statistical analyses were conducted using Statistica 7 for Windows (StatSoft, Tulsa, OH, USA).
3. Results
The abundance of nematode trophic groups under different treatments is presented in Figure 1. Bacterivores were the most abundant among nematode trophic groups, followed by fungivores, plant-parasites, and non-parasitic plant feeders. No carnivorous and omnivorous genera were recorded. The populations of bacterial feeders were enhanced following treatment with parsley (837.5%) and rocket (715.2%), and to a lesser extent with anise (248.7%). On the contrary, fluopyram had a strong negative impact on them (−93.2%), as their abundance was almost diminished (50 individuals per 100 mL of soil). The abundance of fungal feeders did not differ significantly among treatments, although their numbers were reduced after fluopyram application. Both the bacterivore and fungivore trophic groups were not affected by time and presented the same pattern at both samplings. The plant-parasitic nematodes, comprised mainly of M. incognita J2 juveniles and few specimens of the ectoparasite Bitylenchus. At 15 DAA newly hatched J2 had not emerged yet, therefore only low numbers of Bitylenchus were recorded (below 28 individuals per 100 mL of soil) that did not differ significantly among treatments (Figure 1). At 45 DAA, the J2 emergence increased the total number of plant parasites in the control (222 individuals per 100 mL of soil), revealing the differences between treatments. Specifically, plant parasites were significantly lower in all the botanical treatments (rocket: −90.9%, parsley: −89.5%, and anise: −68.2%), as well as in fluopyram (−85.9%) when compared to the control. P. crispum and E. sativa were the most effective, even compared to fluopyram. Finally, the group of non-parasitic plant feeders was negatively affected by Nemagold (−66%), fluopyram (−68%), and P. anisum (−59%) treatments on both sampling occasions.
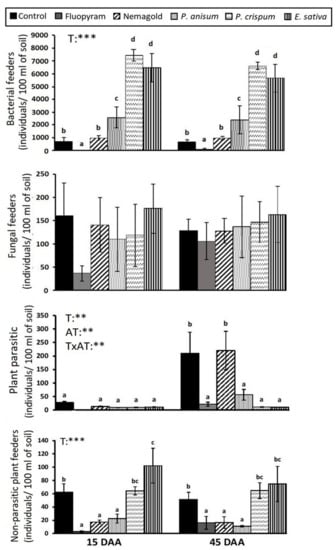
Figure 1.
Mean abundance values (± st. error) of nematode trophic groups under different treatments and results of repeated-measures ΑΝOVA regarding “treatment” (T), “assessment time” (AT) and their interactive effect (T × AT). Different letters (a, b, c, d) indicate significant differences among treatments based on Fisher’s LSD post-hoc test (**: p < 0.01; ***: p < 0.001, for all cases n = 4).
The Maturity, Enrichment, and Plant Parasitic Indices (MI, EI, and PPI, respectively) were significantly affected by treatment, time after treatment and their interactive effect (Table 1). The Channel Index (CI) was affected only by treatment. The fluopyram samples presented the greatest values of MI and CI indices, and the lowest of the EI index in both sampling occasions. In botanical treatments, the EI values were greatest, and the CI values were lowest (Table 1).

Table 1.
Mean values (±st. error) of the Maturity Index (MI), Enrichment Index (EI), Channel Index (CI), and Plant Parasitic Index (PPI) under the different treatments for two assessment times, 15 and 45 days after application (15 DAA, 45 DAA). For each sampling occasion, within columns, means followed by the same letter are not significantly different (ANOVA and Fisher’s LSD post-hoc comparisons; **: p <0.01; ***: p < 0.001; ns: not significant, for all cases n = 4).
The composition of the nematode community under different treatments is given in the rank abundance graphs of Figure 2. The same number of genera (11–12) was recorded in all treatments on both samplings. The genera that we recorded were the bacterivores Rhabditis, Mesorhabditis, Acrobeloides, Chiloplacus, Panagrolaimus, and Cervidellus, the fungivores Ditylenchus, Aphelenchus, and Aphelenchoides, the soil-dwelling J2 juveniles of the endoparasite M. incognita, the ectoparasite Bitylenchus, and finally the non-parasitic plant feeder Malenchus.
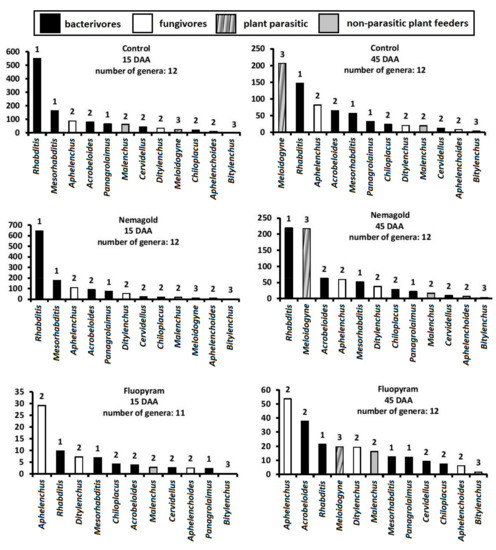
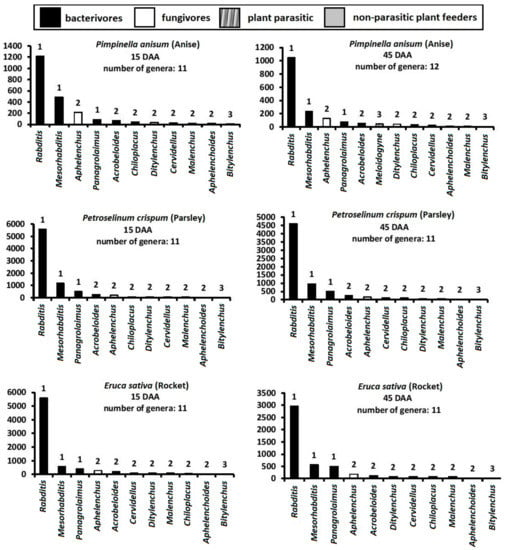
Figure 2.
Rank abundance graphs for nematode genera at different treatments 15 and 45 days after application (15 DAA, 45 DAA). Genera are ranked from the most to the least abundant. Numbers above bars indicate the c–p value of each genus. For all cases n = 4.
At 15 DAA, all soil samples from all treatments except fluopyram were overdominated by Rhabditis (cp-1 bacterivore). Despite the much greater abundance of almost all nematode genera—especially of Rhabditis—in the botanical treatments, the composition of the community was almost the same as in the control and Nemagold-treated soil. In the fluopyram-treated soil, the hierarchy of genera changed, since the abundance of all genera was reduced, and the least affected genus was Aphelenchus, the most abundant fungal feeder.
At 45 DAA, the most important change in the composition of the nematode community was the emergence of J2 M. incognita, mainly in the control and the Nemagold treated soil, where this species became dominant along with Rhabditis. Low numbers of J2 M. incognita were present in the fluopyram and anise treatments, and zero in parsley and rocket, where the community composition was almost the same as at 15 DAA. Another change in the fluopyram treatment of the second sampling, is the predominance of the cp-2 bacterivore Acrobeloides over the cp-1 bacterivores Rhabditis and Mesorhabditis.
In Figure 3, we present the ordination of soil samples, microbial PLFA biomarkers and nematode genera on a PCA biplot. The two first PCA axes accounted for 73.37 % of total data variability. The differentiation of samples due to treatment is obvious along the first axis, which explained 60.81 % of data variability. Rocket and parsley samples from both samplings are ordinated towards the left end of first axis, showing a positive correlation with almost all PLFA biomarkers and nematode genera. The samples from fluopyram, control and Nemagold treatments occupy the right half-plane of PCA, while the anise samples are ordinated almost at the center of the first axis. The ordination of samples along the second axis reflects the time effect, since the samples from 15 DAA and 45 DAA occupy the lower and the upper part of the biplot, respectively. Soil samples from control, Nemagold and fluopyram treatments at 45 DAA are positively correlated with the increased numbers of the M. incognita juveniles. The fact that the second axis explains only 12.56 % of total data variability, indicates that the treatment effect is much stronger than the effect of assessment time on the alterations of the soil microbial and nematode community.
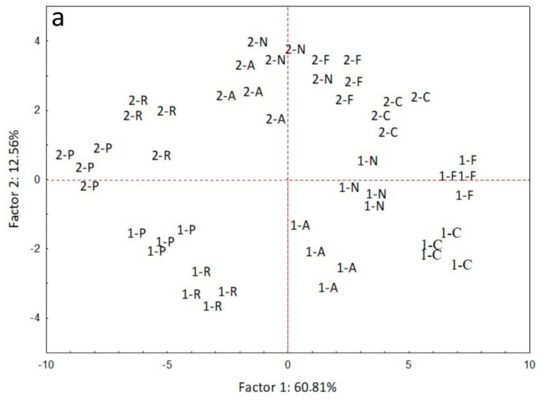
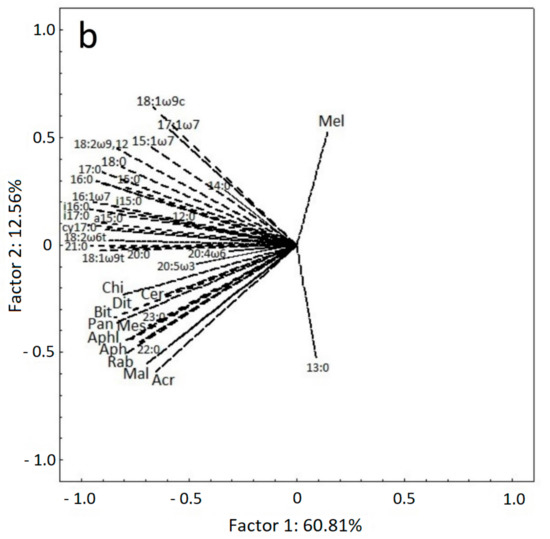
Figure 3.
(a) Ordination of soil samples based on (b) PLFA biomarkers and nematode genera on a PCA biplot. In (a), the first symbol indicates the assessment time (1: 15DAA, 2: 45DAA) and the second symbol indicates the treatment (C: control, F: fluopyram, N: Nemagold, P: P. crispum, R: E. sativa, A: P. anisum). For all cases n = 4.
4. Discussion
The free-living nematode community of our experiment was dominated by microbial feeders, mainly bacterivores. Predators and omnivores are highly sensitive to soil disturbances [23] and were absent from our samples as expected. Plant parasitic nematodes, at least the ones that can be found in the soil, had a quite low contribution at the beginning of our experiment and 15 days after application of treatments. This was partly due to our experimental set-up; the sieving of soil before its inoculation with M. incognita removed root fragments and probably the nematodes associated with them. Even if some of them survived this procedure, the roots of tomato plants at 15 DAA were too small to support large populations of plant feeding nematodes. Regarding the M. incognita juveniles, their numbers at 15 DAA were trivial, because the time needed for this nematode to complete its biological cycle inside the tomato roots is more than 20 days [25].
The treatments altered the nematode community, both in terms of trophic structure and composition of genera. The abundance of bacterivorous nematodes was significantly greater in the pots amended with parsley and rocket and to a lesser extend in the pots amended with anise. The incorporated organic matter was utilized primarily by soil bacteria, which consequently increased their biomass, as reported in Ntalli et al., (2019) [3], favoring also bacterial feeders through the soil food chain [26]. Indeed, the three plant macerates provide bacteria with a source of easily degradable carbon. The most favored nematodes were the cp-1 bacterivores, mainly the dominant Rhabditis, followed by Mesorhabditis and Panagrolaimus. These genera have short life cycles and high reproductive potential, they mirror more closely than other nematodes the bloom of bacteria and are therefore described as enrichment opportunists [12]. Their short generation time allowed them to proliferate even during fifteen days after treatment application. The overdominance of Rhabditis in our botanical treatments (about 80% of the total community) is the reason for the high values of the Enrichment Index (EI) and the low values of the Channel Index (CI). Despite the changes in trophic structure after botanical amendments, i.e., the increase of bacterivores and especially of Rhabditis, the composition of nematode genera did not differ from the one in the control and Nemagold treatments. For example, at 15 DAA, the cp-1 bacterivores Rhabditis, Mesorhabditis, and Panagrolaimus, the cp-2 bacterivore Acrobeloides and the cp-2 fungivore Aphelenchus were the most abundant genera in all treatments except fluopyram. Moreover, Rhabditis was also the dominant genus in the control and Nemagold treatments, at least at 15 DAA, i.e., before the emergence of the Meloidogyne juveniles. This is due to the ability for quick colonization of the cp-1 bacterivores in recently planted and/or disturbed soils. Even at 45 DAA, the genera composition in the botanical treatments did not change, since time did not affect significantly the free-living nematodes, while the juveniles of the plant-parasite M. incognita did not emerge effectively, especially in the parsley and the rocket treatments. On the contrary, the emergence of the M. incognita juveniles was the main change in the composition of the nematode community over time in control and in the Nemagold treatment, which proved the least efficient against M. incognita.
The application of fluopyram decreased the abundance of all nematodes, both the free-living and the parasitic, as also shown by Waldo et al. (2019) [27]. According to our results presented in Ntalli et al. (2019) [5], fluopyram application had no effect on soil microbes, indicating that its negative effect on nematodes was direct and not via their food resources. However, this effect was not uniform against all nematode genera, and thus changes in trophic structure and genera composition occurred. Bacterivores were the most affected genera, especially those belonging to the cp-1 functional guild and mainly Rhabditis. No significant effect against fungal feeders was recorded, and the least affected genus was the cp-2 fungivore Aphelenchus. Waldo et al. (2019) [27] also showed that fluopyram decreased significantly the abundance of bacterial feeding nematodes, not affecting fungal feeders. These changes in the nematode community resulted in the low EI and high CI values in the fluopyram treatment. Another change in the composition of the nematode community was the emergence of M. incognita juveniles at 45 DAA. In the fluopyram treatment, their number was significantly lower than in control and Nemagold treatments, but higher than those recorded in parsley and rocket treatments. Indeed, although fluopyram was more efficient than anise, parsley, and rocket against root knot nematodes in terms of root galling and numbers of females in roots [3], in terms of J2 juveniles in soil it was less efficient. This might be due to the degradation half-life of fluopyram inside the soil (38.4–40 days) [28] being slightly shorter than the time needed for the eggs to hatch inside the newly formed egg sacks.
When we considered both the nematode genera and the microbial biomarkers, in order to elucidate the alterations of the soil community due to treatment and time, our PCA results showed that the effect of treatment was much stronger than the effect of time. The effect of treatment is evident by the increase of bacteria and bacterivorous nematodes after rocket and parsley application. On the other hand, the effect of time is reflected first on the proliferation of soil microbes at 45 DAA due to the growth of tomato roots, as shown in Ntalli et al. (2019) [3], and second on the emergence of J2 M. incognita juveniles in all treatments except rocket and parsley. It is well known that large numbers of bacterivorous nematodes are associated with enhanced N-mineralization rates, since by consuming bacteria the bacterial feeders assimilate more nitrogen than necessary, excreting the excess as ammonia [6]. Thus, rocket, parsley and to a lesser extend anise seem to improve soil fertility, affecting at the same time negatively the reproduction of M. incognita, at least for 45 days after their application.
5. Conclusions
The incorporation of anise, parsley, and rocket in the soil, apart from significantly reducing the numbers of the J2 Μ. incognita, increased significantly the abundance of bacterivorous nematode genera, mostly the ones that indicate soil enrichment, even 45 DAA. This implies a positive impact on soil fertility and productivity, since bacterivorous nematodes are associated with enhanced N-mineralization. The application of fluopyram decreased the nematode abundances, especially those of bacterial feeders, and induced changes in the composition of nematode genera, which indicate a stressful environment. Nemagold was less effective against plant parasites compared with other treatments, not inducing any significant change in the nematode community. Finally, the effect of time after application of treatments on the structure of the soil community is reflected on the proliferation of soil microbes 45DAA and on the emergence of J2 M. incognita juveniles in all treatments except rocket and parsley. Although this effect was significant, it did not mask the effects of the different treatments.
Author Contributions
Conceptualization, N.M. and N.N; investigation, K.T., G.B., and N.M.; data analysis, N.M.; writing—original draft preparation, N.M., M.D.A., and N.N.; writing—review and editing, M.D.A., U.M.-S., and T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to T. Koufakis and AGRIS SA for providing seeds and seedlings. Meloidogyne incognita eggs were kindly provided from nematode cultures of EA Tzortzakakis of Hellenic Agricultural Organization—Demeter, Crete.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Berkum, J.A.; Hoestra, H. Practical Aspects of Chemical Control of Nematodes in Soil. In Soil Disinfestation; Mulder, D., Ed.; Elsevier: Amsterdam, The Netherlands, 1979; pp. 53–120. [Google Scholar]
- Giannakou, I.O.; Karpouzas, D.G. Evaluation of chemical and integrated strategies as alternatives to methyl bromide for the control of root-knot nematodes in Greece. Pest Manag. Sci. 2003, 59, 883–892. [Google Scholar] [CrossRef]
- Ntalli, N.; Zioga, D.; Argyropoulou, D.M.; Papatheodorou, M.E.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Anise, parsley and rocket as nematicidal soil amendments and their impact on non-target soil organisms. Appl. Soil Ecol. 2019, 143, 17–25. [Google Scholar] [CrossRef]
- Ntalli, N.; Monokrousos, N.; Rumbos, C.; Kontea, D.; Zioga, D.; Argyropoulou, M.D.; Menkissoglu-Spiroudi, U.; Tsiropoulos, N.G. Greenhouse biofumigation with Melia azedarach controls Meloidogyne spp. and enhances soil biological activity. J. Pest Sci. 2018, 91, 29–40. [Google Scholar] [CrossRef]
- Ramirez, R.A.; Henderson, D.R.; Riga, E.; Lacey, L.A.; Snyder, W.E. Harmful effects of mustard bio-fumigants on entomopathogenic nematodes. Biol. Control 2009, 48, 147–154. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.C.; Scow, K.M. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralisation function. Appl. Soil Ecol. 2004, 25, 19–35. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Monokrousos, N.; Charalampidis, G.; Boutsis, G.; Sousanidou, V.; Papatheodorou, E.M.; Argyropoulou, M.D. Plant-induced differentiation of soil variables and nematode community structure in a Mediterranean serpentine ecosystem. Soil Res. 2014, 52, 593–603. [Google Scholar] [CrossRef]
- Ntalli, N.; Tsiafouli, M.A.; Tzani, K.; Mavridi, O.; Oplos, C.; Menkissoglu-Spiroudi, U.; Monokrousos, N. Whey: The soil bio-community enhancer that selectively controls root-knot nematodes. Plants 2019, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- Ferris, H.; Matute, M.M. Structural and functional succession in the nematode fauna of a soil food web. Appl. Soil Ecol. 2003, 23, 93–110. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T. Nematode indicators of organic enrichment. J. Nematol. 2006, 38, 3–12. [Google Scholar] [PubMed]
- Hussey, R.S.; Barker, K.R. Comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Ntalli, N.G.; Ferrari, F.; Menkissoglu-spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef]
- Aissani, N.; Urgeghe, P.P.; Oplos, C.; Saba, M.; Tocco, G.; Petretto, G.L.; Eloh, K.; Menkissoglu-spiroudi, U.; Ntalli, N.; Caboni, P. Nematicidal activity of the volatilome of Eruca sativa on Meloidogyne incognita. J. Agric. Food. Chem. 2015, 63, 6120–6125. [Google Scholar] [CrossRef]
- Caboni, P.; Saba, M.; Oplos, C.; Aissani, N.; Maxia, A.; Menkissoglu-spiroudi, U.; Ntalli, N. Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag. Sci. 2015, 71, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Findlay, R.H. Determination of microbial community structure using phospholipid fatty acid profiles. In Molecular Microbial Ecology Manual, 2nd ed.; Kowalchuk, G.A., Bruijn, F.J.D., Head, I.M., Akkermans, A.D.L., Elsas, J.D.V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 983–1004. [Google Scholar]
- Angelina, E.; Papatheodorou, E.M.; Demirtzoglou, T.; Monokrousos, N. Effects of Bacillus subtilis and Pseudomonas fluorescens inoculation on attributes of the lettuce (Lactuca sativa L.) soil rhizosphere microbial community: The role of the management system. Agronomy 2020, 10, 1428. [Google Scholar] [CrossRef]
- S’Jacob, J.J.; van Bezooijen, J. A Manual for Practical Work in Nematology; Department of Nematology, Wageningen Agricultural University: Wageningen, The Netherlands, 1984. [Google Scholar]
- Bongers, T. De Nematoden van Nederland; Pirola: Schoorl, The Netherlands, 1994. [Google Scholar]
- Yeates, G.W.; Bongers, T.; de Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar] [PubMed]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Eisenback, J.D.; Triantaphyllou, H.H. Root-knot Nematodes: Meloidogyne species and races. In Manual of Agricultural Nematology; Nickle, W.R., Ed.; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Gebremikael, M.T.; Steel, H.; Buchan, D.; Bert, W.; De Neve, S. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Sci. Rep. 2016, 6, 32862. [Google Scholar] [CrossRef] [PubMed]
- Waldo, B.D.; Grabau, Z.J.; Mengistu, T.M.; Crow, W.T. Nematicide effects on non-target nematodes in bermudagrass. J. Nematol. 2019, 51, e2019-09. [Google Scholar] [CrossRef] [PubMed]
- Matadha, N.Y.; Mohapatra, S.; Siddamallaiah, L.; Udupi, V.R.; Gadigeppa, S.; Raja, D.P.; Donagar, S.P.; Hebbar, S.S. Persistence and dissipation of fluopyram and tebuconazole on bell pepper and soil under different environmental conditions. Int. J. Environ. Anal. Chem. 2020, 26, 6077–6086. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).