Simple Summary
Livestock activity plays a crucial role in the Amazon region of Ecuador. However, the rapid expansion of extensive pastures has led to serious problems in the environment. Grasses, such as Panicum maximum, are widely used by producers in Orellana Province, but poor management practices have resulted in inefficient production systems, as evidenced by decreased yield. Our study demonstrates that in silvopastoral systems, resources are used very efficiently, and growing conditions are improved, leading to greater productive responses. Furthermore, we observed that guinea grass responded well in silvopastoral systems. Therefore, these plants should be managed through well-planned grazing to achieve a high yield and better chemical composition.
Abstract
Climate change has increased the interannual and seasonal variation in the average temperature and precipitation rate, which determine forage availability globally. Similar patterns of change have occurred in tropical regions, and Ecuador is no exception. This region includes other influencing factors, such as the conversion of tropical forests to extensive pastures. Therefore, this study was carried out to evaluate guinea grass (Panicum maximum) cultivated under silvopastoral systems with different management strategies in both of the seasons of the study region in Ecuador (rainy season and dry season). A randomized complete block design was used for the experiment, with three repetitions and three treatments. Agronomic measures, forage production, chemical composition, animal responses, and soil properties were evaluated. Data were analyzed with the Mixed model of SAS. Differences in all evaluated parameters were observed between treatments, and silvopastoral systems (SPSs) produced better results than conventional grass monoculture. Furthermore, there was a strong seasonal effect on forage production, chemical composition, and soil properties. Therefore, management of Panicum maximum with well-planned grazing can enhance animal responses and help to retain natural resources, lowering the pressure on forests.
1. Introduction
The conversion of a tropical forest to extensive pastures for cattle grazing is one of the primary causes of deforestation, land degradation, greenhouse gas emissions (GHGs), depletion of carbon (C) stocks, and a reduction in biodiversity [1,2,3]. This global process generates an increase in the interannual and seasonal variation of factors that determine the forage availability, and as a consequence, animal productivity is reduced [4,5].
The sustainability of production systems depends on the development of techniques that increase production without necessarily resulting in an increase in the area used for pasture [6]. However, most grasslands are in tropical developing countries, where they are particularly important for the livelihoods of around 1 billion poor people [7].
The Ecuadorian Amazon is one of the most biodiverse areas in the world and has been referred to as “the most important source of fresh water and biodiversity” for its global climate regulatory function as a greenhouse gas sink [8,9,10].
Farmers in the Northern Ecuadorian Amazon (NEA) use land in a variety of ways, including forest, pasture, annual and perennial crops, and fallow land (which generally regrows into secondary forest). Deforestation is pervasive throughout the NEA and has largely been the result of smallholder farm expansion [11,12,13]. According to [10], the effect of colonization on the Ecuadorian Amazon has included the interference and use of forests for timber resources and the establishment of pastures and small orchards. Therefore, livestock has turned soils into degraded lands that are dependent on mechanization and agrochemicals.
Orellana Province has two well-defined seasons: a rainy season (>2942 mm, from February to August) and a dry season (<1000 mm, from September to January). As reported by previous authors [14,15], the predominant income-generating activities for producers in the Amazon region are concentrated in agriculture (56.5%) and livestock (10%), and 30% of the production is under a mixed production system (agriculture–livestock). However, these activities employ intensive systems that involve substantial amounts of natural resources and labor, but they have very low productivity and rentability.
According to [16], the adaptation of production systems and the mitigation of greenhouse gas emissions are key challenges resulting from the effects of global climate changes on agriculture. Therefore, the adoption of intensification strategies for livestock production in tropical grassland areas depends on farmers’ knowledge of soil, water resources, plant and animal management, and the beef market [7].
Consequently, because of the dependence on livestock activity, it is necessary to generate production models that are more sustainable. The use of silvopastoral systems can provide several benefits, such as diversification of production [17], recovery of degraded areas [18], and improvement of animal welfare [19]. Therefore, all these advantages must help to increase the synergies between all biotic components (soil–tree–forage) [1,10,20].
Panicum maximum constitutes the most commonly used grass species in tropical areas of Ecuador. This guinea grass is characterized by its persistence in intensive management conditions and high productivity, which are consequences of its photosynthetic and water efficiency and high phenotypic plasticity [6,16,21,22].
The behavior of the forage component in silvopastoral systems has not been studied in Orellana Province. Therefore, this work aimed to assess guinea grass (Panicum maximum) grown under silvopastoral systems using different management strategies. Measures of variables, such as forage production, chemical composition, soil properties, and animal responses, were compared with those resulting from a conventional monoculture system for livestock, which was used as a reference.
2. Materials and Methods
2.1. Study Area
Location: According to [15], Orellana Province covers a surface area of 21.730 km2 (18.6% of a total of 45.47% of the Amazon region). The climate in this region is characterized by humid tropical rainforest conditions [23]. The average annual rainfall is 2942 mm with an average annual temperature of 29.7 °C, and its altitude is 275 m above sea level. During the experimental period in this study were recorded average rainfalls every year as follows: rainy season at 1969 ± 81 mm with an average temperature of 26.48 °C, whereas in the dry season was observed 945 ± 50 mm, whose temperature was 27.03 °C (Figure 1) [24].
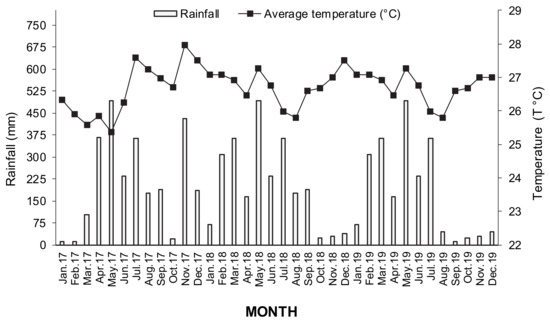
Figure 1.
Month mean temperatures and rainfall from January 2017 through December 2019 in the experimental area in Orellana Province.
The estimated population for 2018 was 157,520 [25]. The ethnic groups comprise Native Amerindians (Kichwa, Shuar, and Waodani; 31%), mestizo (mixed descendants of Spanish colonists and indigenous Amerindians; 57.5%), and a small Afro-Ecuadorian population (4.9%) [15,26].
Livelihoods: According to the estimates of [27], agricultural land use in Orellana Province accounts for a total surface area of 606,307 ha, distributed as follows: 80% (485.039 ha) mountains and forests; 7.2% (43,582 ha) permanent crops; 4.6% (28,049 ha) other uses; 4.2% (25,162 ha) cultivated pastures; 3.1% (19,034 ha) natural pastures; and 0.82% (4959 ha) transitory crop and fallow.
2.2. Description of Silvopastoral Systems
Silvopastoral systems are production structures that integrate forestry, pastures, and livestock production in time and space [3,28,29]. These systems have well-documented environmental benefits, such as increased input of organic matter and improved physical and chemical properties of the land. The system that forms has more balanced and stable nutrient cycling because of biological N2 fixation. Biodiversity is enhanced, and water quality is improved. Furthermore, livestock is protected from heat stress [1,29,30]. Therefore, the silvopastoral system currently constitutes a very efficient land use because of its multiple environmental, socioeconomic, and productivity advantages.
2.3. Experimental Design and Treatments
The guinea grass Panicum maximum cv. Mombaça was grown under silvopastoral systems using different management strategies on a total surface area of 10 ha and divided into paddocks of 1 ha. No lime, fertilizer, or herbicides were applied to the plants at any time. The experiment followed a randomized complete block design with three repetitions for each of the three treatments. We used an area of 1 ha for every treatment for a total experimental area of 9 ha:
- -
- Silvopastoral systems with guinea grass (Panicum maximum cv. Mombaça) surrounded by a fixed fence (SPS1);
- -
- Silvopastoral systems with guinea grass (Panicum maximum cv. Mombaça) surrounded by a mobile fence (SPS2);
- -
- Conventional system with guinea grass (Panicum maximum cv. Mombaça) as a monoculture (without trees) surrounded by wire fences (Control).
SPS1 was divided into three paddocks of 3333 m2 using permanent electrical wires. SPS2 was divided into four paddocks of 2500 m2 by mobile electrical wires. By contrast, the Control treatment was not divided to simulate a conventional system with guinea grass as a monoculture (Figure 2).
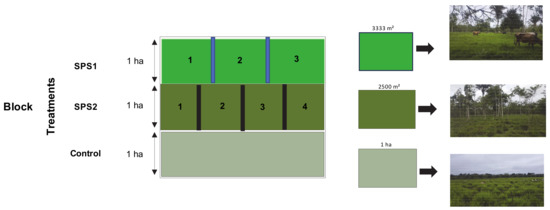
Figure 2.
Scheme of experimental design of guinea grass Panicum maximum under silvopastoral systems in combination with different management systems.
Then, we established a rotational grazing system with three days of occupation in each treatment. Furthermore, during the rainy season (February to August; HP), a grazing frequency of one every 35 days was adopted, while in the dry season (September to January; LP), we allowed 45 days between grazing.
To study the production performance, 90 steers of the mestizo Brahman breed (Bos indicus) with an average live weight (LW) of 275 ± 29 kg were used. Brahman is a breed of cattle that is widely used in the tropical region of Ecuador. Subsequently, the animals were divided into groups balanced for weight; 30 animals were assigned at random to each repetition, and subdivisions of 10 animals were established for every treatment. Consequently, in all treatments the animal rotations were according to weather conditions (rainy season and dry season). Therefore, throughout the experimental period (2017 to 2019) in the rainy season were applied six rotations every year. Meanwhile, in the dry season were applied three rotations every year, giving a total of 30 animal rotations (2017 to 2019).
Before the experimental period, all animals were administered 1% doramectin injectable solution (1 mL/50 kg BW) to protect them against ectoparasites, and they were dewormed with levamisole injection (1 mL/10 kg BW) to guard them against internal parasites. Water and a mineral mixture were provided ad libitum during the experiment.
All animal care was performed in accordance with the World Organization for Animal Health 2016 (Animal Welfare) and the current Ecuadorian regulations in Organic Law on Animal Health No. 56, published in the Official Gazette, Supplement 27, 3 July 2017. The study was conducted from January 2017 to December 2019.
2.4. Measured Variables
2.4.1. Agronomic Measurements and Forage Production
We performed agronomic evaluations according to two seasons (HP and LP). Canopy height was measured using a centimeter-graduated ruler at several random points per area before each harvest. The tiller population density (%/m2) of Panicum maximum was obtained by counting the number of tillers using three 1.00 × 0.25 m metallic frames.
From each treatment were randomly chosen a total of 20 points according to rainy season or dry season, and by visual assessment were recorded the total of tillers to determine the plant coverage.
Herbage mass was assessed through the double sampling technique described in [31] every 35 d (HP) or 45 d (LP) from January 2017 to December 2019, giving a total of 30 evaluation periods. For direct measurements, we used six 0.25 m2 quadrats to collect 40 random samples in each treatment before every grazing throughout the experimental period. Herbage was harvested at the ground level.
We also used a visual scale as an indirect measurement method: the scale ranges from 1 to 3, where 1 is the lowest herbage mass and 3 is the highest [28,31]. Furthermore, several subsamples of approximately 500 g were obtained and then dried in a forced-air ventilation oven at 60 °C for 72 h to measure the dry weight and calculate the dry mass (kg ha−1) [32,33,34].
2.4.2. Chemical Composition
Before every evaluation in each experimental period, samples of fresh grass were collected in duplicate and frozen at −20 °C for the determination of chemical composition. Prior to the analysis, the frozen fresh grass samples were conditioned at 60 °C for 24 h and then ground and homogenized through a cyclone mill (Retsch SM2000, Retsch, Haan, Germany) with a 1 mm mesh.
The chemical analysis was carried out in duplicate according to official reference methods and expressed on a dry matter basis [35]. Dry matter (DM) was determined at 103 °C for 24 h, and ashes were burnt at 550 °C for 5 h. Crude protein (CP) was calculated as a percentage of N × 6.25 by the Kjeldahl method. Crude fiber (CF) was determined by the method of [36] using the Ankom200 Fiber Analyzer (Ankom Technology, Fairport, Monroe, NY, USA).
2.4.3. Stocking Rate, Herbage Allowance, and Animal Responses
The LWs of the steers were recorded during the entire experimental period (according to grazing frequency and in both seasons) using a balance with a capacity of 15,000 kg and an accuracy of ±50 kg (BG-GANDO-01, BalanzasGalicia, Galicia, Spain), and the body condition scores (BCSs) were determined according to [37]. Calculations for the described animal pasture system were performed using the methodology of [38], where animal unit (AU) is defined as one animal, 454 kg per cow, or its equivalent.
Using the data obtained from the forage production (dry matter (DM), kg ha−1) and LW, we calculated the stocking rate and adjusted the AU in each repetition of every treatment [39,40]. The herbage allowance was estimated on the basis of green herbage mass and LW. We used an adjustment of 0.7 for grazing because the study zone has an annual average precipitation of about >2000 mm [39]. Average daily gain (ADG) was determined for the season by the post- and pre-weight difference divided by the number of days (HP = 35 d or LP = 45 d). Finally, we calculated the gain per area (GPA = stocking ratio × time interval (HP or LP)) [28].
Additionally, we created a forest inventory to register the predominant species and number of trees in paddocks, and the spacing between trees and tree density (ha−1) were determined.
2.4.4. Soil Macrofauna, Physical and Chemical Properties
Samples of soil were collected in duplicate in both seasons always at 0800 to 1000 h throughout the experimental period (2017–2019) to determine the macroinvertebrate composition and physical and chemical properties in accordance with the Tropical Soil Biology and Fertility Institute (TSBF) method [41]. One soil monolith (25 × 25 × 10 cm) was obtained at every sampling point at 0–10 and 10–20 cm depths.
These samples were immediately transported to the biological science laboratory ENA-ESPOCH in Ecuador and manually hand-sorted, during which the number of macroinvertebrates per m2 was determined. Then, the weight was measured using a balance with a capacity of 2000 g and a precision of ±0.5 (GRAM FC, BalanzasGalicia, Galicia, Spain) to determine the biomass per m2 using the standardized ISO 23611-5 method [42].
Soil chemical properties were also determined. Total N content (TN) was measured by Kjeldahl digestion and steam distillation. Macronutrients (Ca, Mg, K, Na) were extracted with 1 N ammonium acetate and analyzed through elemental analysis by atomic absorption spectrophotometry (Analyst 400, PerkinElmer, Wellesley, MA, USA). Micronutrients (Fe, Zn, Cu, Mn, B) were extracted with an unbuffered potassium chloride solution and quantified by the Mehlich I method and atomic absorption.
2.4.5. Statistical Analysis
Data were analyzed using Proc Mixed from SAS v. 9.4 (SAS Institute Inc., Cary, NC, USA). Fixed effects included silvopastoral systems (SPS1, SPS2, and Control), season (HP and LP), and their interactions. Block and residual error were considered random effects. If a significant effect was observed for the silvopastoral system, an orthogonal polynomial contrast analysis was performed to compare Control vs. SPS1, Control vs. SPS2, and SPS1 vs. SPS2. Means were separated using PDIFF from SAS adjusted by Dunnett’s test with significance declared at p < 0.05 and a tendency assigned at p < 0.10.
3. Results
3.1. Agronomic Measurements and Forage Production
Table 1 shows the results of guinea grass (Panicum maximum cv. Mombaça) evaluated in different silvopastoral systems.

Table 1.
Agronomic measurements and forage production of guinea grass Panicum maximum cv. Mombaça in the different silvopastoral systems.
Differences in agronomic measurements between treatments were not significant. However, the percentages of plant coverage were higher in SPS1 and SPS2 compared with that in Control (average −10%), as shown in Table 1. Although the Control group had less coverage than the other silvopastoral systems, we did not observe significant differences based on the season or its interaction with plant coverage (p = 0.372). The plant height (cm) of guinea grass (Panicum maximum cv. Mombaça) was not significantly affected by treatment, season, or their interaction (T × S; p > 0.05). However, plant heights in the Control group were lower than those in SPS1 and SPS2 in both evaluated seasons.
Consequently, when fresh mass production (kg ha−1) was analyzed, no significant differences were found between treatments (p > 0.05), but it was lower in the Control group in comparison with SPS1 and SPS2, which had similar fresh masses (14,493 and 15,146 kg ha−1, respectively), as shown in Table 1. However, there were statistical tendencies that reflected seasonal effects (p = 0.089), with higher fresh mass values (kg ha−1) in the dry season (from September to January) for all treatments, as shown in Figure 3. Therefore, we observed differences (p < 0.05) in DM (kg ha−1) due to the varying seasons: DM was higher in the LP season than in the HP season (5712 ± 1036 vs. 3694 ± 951 kg ha−1, respectively). Moreover, a slight tendency was associated with the interaction between treatment and season (T × S; Table 1).
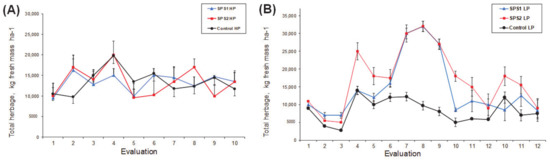
Figure 3.
Total fresh mass of guinea grass Panicum maximum cv. Mombaça evaluated under silvopastoral systems and different seasons at the year p = 0.089. HP (35 days) (A); LP (45 days) (B).
3.2. Chemical Composition
The results for chemical composition are shown in Table 2. No differences in ash, OM (organic matter), CP, CF, EE, or NFE N-free extract (or nonfiber carbohydrate) (p > 0.05) were detected between treatments. However, DM significantly differed between treatments (p = 0.012; Table 2): the DM values of the Control were higher than those of SPS1 and SPS2 (37.36 ± 3.8% vs. 31 ± 3.8%). Seasonal effects produced statistical tendencies (p < 0.06), as illustrated in Table 2: we observed higher values of DM in the HP season compared with the LP season (from February to August) (35.55 ± 3.8% vs. 31.61 ± 3.8%, respectively).

Table 2.
Chemical composition of guinea grass Panicum maximum cv. Mombaça evaluated under different silvopastoral systems.
Our results show that for guinea grass (Panicum maximum) cultivated under different silvopastoral systems, higher ash content was observed in the HP season compared with the LP season regardless of treatment. Because ash content is closely related to organic matter (OM) content, the OM values were also different (p < 0.003) between seasons, and higher OM content was obtained in LP in all treatments. This high OM content might have served as a source for bacterial growth and improved animal performance. Another important element for ruminant nutrition is protein content. We did not detect statistically significant differences between treatments (p > 0.05), but there was a statistical tendency due to the seasonal effect (p = 0.006; Table 2), in which higher CP values were obtained in the LP season in comparison with the HP season (8.1 ± 0.6% vs. 6.7 ± 0.6% of CP on a DM basis). The T × S interaction did not lead to significant differences (p > 0.05). CF values did not differ according to treatment, season, or their interaction (T × S), although the Control had slight numerical differences compared with the other systems assessed (SPS1 and SPS2).
3.3. Stocking Rate, Herbage Allowance, and Animal Responses
Data analysis shows that the season had a strong influence on usable herbage mass (cut/kg DM ha−1, Figure 4). We obtained higher values of usable herbage mass in LP than in HP (2178 ± 852 vs. 821 ± 852 DM kg ha−1, respectively). Although there were no statistically significant differences between treatments, the Control had lower herbage production compared with the other systems (601 ± 1065 vs. 2021 ± 910 DM kg ha−1; p = 183; Table 3). Therefore, the same dynamic follows for the total usable herbage/year in the different systems studied. There were numerical differences between the Control and the other treatments (Table 3), although they were not statistically significant (p > 0.05).
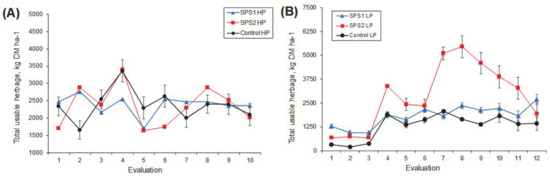
Figure 4.
Total usable herbage of guinea grass Panicum maximum under silvopastoral systems evaluated according to the season at the year p = 0.05. HP (A); LP (B).

Table 3.
Least square mean of guinea grass Panicum maximum evaluated in different silvopastoral systems.
We determined differences in forage demand on the basis of 3% LW of DM. Treatment, season, and their interaction had statistically significant effects on forage demand (p < 0.05). However, marked differences were observed in the Control group due to factors that are described in more detail in the discussion. In general terms, the silvopastoral systems SPS1 and SPS2 needed fresher forage mass to meet nutritional requirements.
Statistical tendencies were found in the stocking rate (AU ha−1) due to seasonal effects (0.88 ± 0.3 vs. 0.35 ± 0.3; p ≤ 0.065). Specifically, a higher stocking rate was observed in the LP season because of greater herbage mass production (Table 3). However, the stocking rates were similar when the different treatments were compared using orthogonal contrast (p = 0.416). Although differences for the interaction (T × S) were not detected, SPS1 and SPS2 were associated with a higher stocking rate compared with the Control (1.15 ± 0.4 and 1.22 ± 0.4 vs. 0.28 ± 0.4, respectively) in the LP season.
Herbage allowance was affected by treatment (Table 3) and was higher in the Control group than in SPS1 and SPS2 (p ≤ 0.04). In addition, herbage allowance varied (0.30 ± 0.1 kg DM kg live weight−1 in HP; 0.43 ± 0.1 kg DM kg live weight−1 in LP). Notably, when we applied orthogonal contrast, we found differences (p < 0.03) between the Control and the other treatments (p ≤ 0.029), whereas SPS1 and SPS2 were similar (p = 0.899). Thus, the evaluation of the interaction of T × S treatments resulted in greater herbage allowances in the LP season than in the HP season, but the differences were not statistically significant (p > 0.778; Table 3).
Average daily gain (kg AU−1 d−1) varied among treatments (p < 0.001; Table 3). The results obtained with SPS1 were better than those with SPS2 and the Control. Statistically significant differences (p < 0.001) were found using orthogonal contrast for Control vs. SPS1 (p < 0.001) and SPS1 vs. SPS2 (p ≤ 0.020). Furthermore, the season affected the average daily gain; in the HP season, an average of 0.543 ± 0.02 kg AU−1 d−1 was obtained, whereas the corresponding value in the LP season was 0.658 ± 0.02 kg AU−1 d−1. In all treatments, a better animal performance was observed in the LP season than in the HP season, although the differences were not significant (T × S; p < 0.108, Table 3).
Gain per area (GPA) varied among treatments. The Control had lower values (4.60 LW ha−1) compared with the SPS1 and SPS2 silvopastoral systems (28.44 ± 9.6 kg LW ha−1 and 24.82 ± 9.6 kg LW ha−1, Table 3). Analysis through orthogonal contrasts revealed differences between the Control and other treatments (p < 0.02). By contrast, there were no statistically significant differences between SPS1 and SPS2 (p > 0.788). We observed that the season had a strong influence on GPA (p < 0.02): higher values (kg LW ha−1) were observed in the LP season compared with the HP season (33.34 ± 8.9 vs. 5.23 ± 8.9, respectively). Furthermore, there were statistically significant differences due to treatment. Mcal values in the Control were higher than those in the other silvopastoral systems (p = 0.008, Table 3). However, the season × treatment interaction was not associated with differences in GPA.
In addition, tree densities (ha−1) differed between systems. In the SPS1 system, the distance between groves was 6 × 6, and the density was 138 trees ha−1. By contrast, a higher density was observed in SPS2, which had 312 trees ha−1 (distance between groves: 4 × 4; Table 4).

Table 4.
Predominant species of trees found in silvopastoral systems evaluated with guinea grass.
In general, all paddocks with silvopastoral systems in this region of Ecuador have a high or low tree density. However, currently, monoculture systems predominate among the fragile ecosystems in this region. An abundance of scientific evidence shows that these practices are inefficient, unsustainable, and not environmentally friendly. Another important focus of our work is the interesting diversity of native species in combination with other components in silvopastoral systems. Therefore, the silvopastoral system in combination with the appropriate management of pastures is key to livestock sustainability. Our results demonstrate that effective planning, management, and execution of these practices improve the production yield in a form that is friendly to our resources, resulting in economic benefits.
3.4. Soil Macrofauna, Physical and Chemical Properties
The results of soil physical and chemical analyses are shown in Table 5. The pH values were not affected by treatment, season, or their interaction. However, higher numerical values of OM were found in SPS1 and SPS2 compared with the Control (Table 5). Macronutrients were not affected by treatment, but we observed increases in the level of the exchangeable Ca in SPS1. However, nitrogen and phosphorus levels did not vary among treatments. Potassium concentrations were lower in SPS1 compared with the Control and SPS2 (Figure 5). By contrast, the T × S interaction affected macronutrients, such as P, Ca, Mg, and K (p < 0.05; Table 5). The contents of all elements were lower during the LP season than during the HP season.

Table 5.
Soil physical and chemical properties in different silvopastoral systems with Panicum maximum cv. Mombaça.
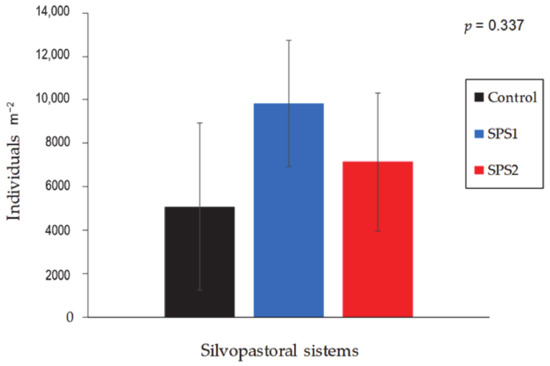
Figure 5.
Soil macrofauna (individuals m−2) found in the different silvopastoral systems with guinea grass Panicum maximum cv. Mombaça.
Micronutrients were not affected by treatment, season, or their interaction, with the exception of Fe. We found that the season and T × S interaction affected the Fe level. The Fe levels in the LP season were higher than those in the HP season (48.0 ± 8.9 vs. 16.5 ppm, respectively).
The soil macrofauna was not statistically affected by treatment, season, or their interaction (p > 0.05; Figure 5). However, more individuals (per m−2) were detected in the SPS1 and SPS2 silvopastoral systems than in the Control.
4. Discussion
4.1. Agronomic Measurements and Forage Production
In many regions of the world, grasslands provide food and services and are the basis of farmer livelihood. Therefore, these lands constitute economically important and ecologically friendly production systems [20]. Panicum maximum is widely used in livestock systems in the tropical region of Ecuador. This guinea grass has high persistence and productivity due to its high photosynthetic and water efficiency, and it has high forage production potential with adequate nutritional value; it is also highly adaptable to different climate conditions and poor soils [6,22].
Similar plant coverage and height measurements were obtained for all treatments; however, the values were lower in the monoculture of guinea grass than in SPS1 and SPS2. Similar data were obtained in the comparison between seasons. Slightly higher agronomic measurements (plant height and coverage) were observed in the rainy season, but the Control had lower agronomic values compared with the silvopastoral systems evaluated in the same conditions.
As reported in [43,44], this species of guinea grass becomes taller when water is highly available, and its productivity is thus highly dependent on this factor. In [6], different Panicum cultivars were compared, and the cv. Mombaça was found to have the greatest height. According to [16], seasonal variations in temperature and water availability occur during the year. Therefore, this affects the annual forage production and should be considered in the planning of livestock farms.
We did not find significant effects of treatments on fresh mass (kg ha−1; Table 1). However, seasonal effects resulted in a statistical tendency (p < 0.10). Greater production was obtained in the LP season. In [16], similar data were reported in regard to seasonal effects on the production of fresh mass (kg ha−1), but the levels of production from Panicum maximum (30,000 kg DM ha−1 year−1) were higher than the measurements in our study (15,490 kg DM ha−1 year−1). In the present work, higher forage production was recorded during the LP season (11,757 vs. <10,000 kg DM ha−1 year −1).
Therefore, the cultivar Mombaça is demonstrated to have high potential for cultivation in areas with a water deficit and high temperatures. In Orellana Province of Ecuador, despite the low precipitation in the LP season and high evaporation, the growth of Panicum maximum may be able to yield acceptable forage levels in terms of fresh mass. Furthermore, silvopastoral systems could contribute to improving the physical and chemical conditions of the livestock production system and form a system more balanced and stable through nutrient cycling.
4.2. Chemical Composition
Forage allows producers to feed livestock at a low cost [45]. The nutritive value of forage is influenced by chemical composition and digestibility, and for ruminants, protein is one of the most important elements [43]. However, grasses mature rapidly, and their protein content falls to very low values as the fiber content rises. Thus, chemical composition has an influence on forage digestibility and voluntary intake, affecting animal responses [45].
The CP content did not vary among treatments. However, the protein content in the conventional system was lower than that in the silvopastoral systems, and a statistical tendency due to seasonal effects was observed. Critical CP values (6.7%) were obtained in the HP season, potentially because of the large stem/leaf ratio and small number of leaves, in which CP content is largely concentrated. Furthermore, a large proportion of mature forage leads to decreases in nutritive quality because carbohydrates accumulate in the basal part of the stem [34].
Our DM and CP results are similar to those reported by [46,47,48]. By contrast, in [34,44,49], greater values of CP were reported in Panicum maximum, ranging from 8% to 15%. However, this high CP content was due to the use of fertilizer.
The low protein content often found in tropical grasses is an inherent characteristic of C4 plant metabolism, which is associated with survival under conditions of low soil fertility [43]. Therefore, the CP content of guinea grass (Panicum maximum) was better when it was cultivated under silvopastoral systems compared with the conventional system in both seasons evaluated. Moreover, critical CP values (<7%) were measured in the HP season; according to [46,50], low CP values might depress animal intake. Our hypothesis is that forest components in the paddocks recycle nutrients from deeper soil layers and enhance soil fertility. Therefore, this might explain why Panicum maximum had moderate CP content despite the lack of fertilizer. However, the contents were markedly higher under the silvopastoral systems than in the conventional monoculture system.
4.3. Stocking Rate, Herbage Allowance, and Animal Responses
Cattle typically prefer leaves and green herbage to stems and senescent tissue, respectively. Guinea grass (Panicum maximum) cultivated under different silvopastoral systems was affected by the season. Contrary to [34], the results of our study were improved in the dry season in comparison with the rainy season, although a greater growth rate was observed in normal conditions in the HP season due to soil temperature and high moisture. We surmise that the soil structure is related to the grade of water infiltration, which can cause water puddling and limit the growth of pastures [5]. By contrast, in the LP season, guinea grass was highly water efficient, as indicated by higher mass (DM kg ha−1). Additionally, other processes such as biological N2 fixation and recycling from legume trees may have contributed to this result [28].
In our study, we observed a greater stocking rate in the silvopastoral systems compared with the conventional system, as shown in Table 3 (0.7 vs. 0.3 AU ha−1, respectively), and the data show a strong relation between total usable mass (DM kg ha−1) and stocking rate. The results in [28] were different from ours. As noted in [30], a low stocking rate does not harm planted trees. In general, as the stocking rate increases, the individual animal performance decreases [20]. Therefore, the stocking rate has a large impact on the performance of grazing livestock [40]. The Society for Range Management (1974) defined stocking density as the relation between the number of animals (AU) and the area at a given time point [28,38,40].
There is a positive correlation between animal performance and herbage allowance [28,40]. In this study, treatment effects resulted in statistically significant differences (p < 0.05). In this case, herbage allowance values were greater in the conventional system in comparison with those in the silvopastoral systems (0.62 vs. 0.25 kg DM kg LW−1; Table 2). However, seasonal effects did not result in statistically significant differences. We observed that herbage allowance was higher in the HP season than in the LP season. According to [5,20,38,40,45], herbivores are highly effective in selecting food of higher nutritive value, and thus, intake is lower when herbage allowance is high and nutritive value is low.
Our hypothesis is that, in general, in the management of a conventional silvopastoral system, the forage quality of pastures decreases as a result of forage maturity and senescence [51]. Therefore, herbage allowance has been defined as the amount of available forage per animal in a point-in-time measurement [28,38,40]. Thus, forage allowance can be a powerful tool for explaining differences in animal performance [40]. In our case, this explains the high values obtained for the guinea grass (Panicum maximum) cultivated under the conventional system.
Forage mass, herbage allowance, and forage nutritive value can lead to average daily gain (ADG) variations of 50%–90%, depending on the specific conditions [7,20]. The average daily gain obtained under the conventional system in this study was similar to that reported by [28] (0.465 kg AU−1 d−1) in grass monocultures; this ADG is above the typical values reported for animal performance in signalgrass pastures (0.350 kg AU−1 d−1). As reported in [20], production from grazing animals depends on the provision of pastures during seasonal fluctuations within and between years.
Therefore, the management of guinea grass (Panicum maximum) under silvopastoral systems produced better animal responses relative to the conventional system (+49%, ranging from 0.612 ± 0.02 to 0. 732 ± 0.02 kg AU−1 d−1; Table 3). Contrary to our study, the authors of [28] reported lower daily gain in the dry season (0.3 kg vs. 0.6 kg AU−1 d−1), whereas we recorded greater daily gain in Panicum maximum in the LP season in comparison with the HP season (0.658 ± 0.02 vs. 0.543 ± 0.02 kg AU−1 d−1). Regarding these discrepancies, our theory is that the soil properties might be related to and have a strong influence on the productivity of guinea grass under silvopastoral systems.
The results for the above-described factors are coherent with the data on gain per area (GPA). As we anticipated, conventional systems were less efficient. Therefore, the animal performance under this condition is critical from productive, socioeconomic, and ecological points of view. The silvopastoral systems had better GPA values when compared with the conventional production system (average 24.82 ± 0.03 vs. 4.60 ± 0.03 kg LW ha−1 d−1).
Furthermore, these results might be comparable to those reported by [28] (30 kg live weight ha−1 28 d−1). We achieved average annual gains of 266 kg ha−1 in silvopastoral systems (SPS1 and SPS2) in comparison with 46 kg ha−1 in the conventional livestock system in this region of Ecuador. In addition, the season had a strong influence on animal performance. We observed that the GPA was better in the LP season than in the HP season (i.e., 33.34 ± 8.9 vs. 5.23 ± 4.1 kg ha−1). All of these results involving animal responses reflect the markedly complex interactions in silvopastoral systems. The potentially higher productivity could be due to the capture of more resources for growth (i.e., improved soil fertility), maintenance of organic matter, and physical properties. Soil fertility is crucial for global food security and environmental sustainability.
On the basis of our results, we conclude that silvopastoral systems in this region are characterized by the maintenance of native tree species and varying densities in paddocks, distributed as dispersed trees at different distances. In SPS1, the spacing between groves was 36 m and the tree density was 138 h−1, while in SPS2, the respective values were 16 m and 312 tree h−1.
Independent of the distances between groves, forestry might help to reduce solar radiation and temperature and increase water use efficiency [19]. This may explain why higher biomass was obtained in the LP season than in the HP season regardless of treatment.
However, the results in [1] suggest that the distance between trees in silvopastoral systems might affect physicochemical soil properties and macrofauna abundance. By contrast, in our work, the forestry component resulted in marked differences from the conventional system and produced better agronomic measurements, biomass, and animal performance.
Moreover, in this study, we observed that guinea grass (Panicum maximum) was highly shade-tolerant under silvopastoral systems. Finally, we aim to highlight the importance of adopting more efficient production methods to convert traditional cattle systems to eco-friendly systems that integrate silvopastoral components.
4.4. Soil Macrofauna, Physical and Chemical Properties
Ample scientific evidence supports the crucial role of silvopastoral systems in providing numerous benefits and ecosystem services [1,17,19,30,33]. According to [52], forest ecosystems are estimated to absorb up to 3 Pg of carbon (C) annually. Therefore, the presence of the forestry component is key to producing changes in the microclimate, improving the soil structure, and supplying nutrients to a production system.
Several studies have described multiple relationships between pastures and soil biota [1]. Isolated trees in several ecosystems can contribute to the accumulation of soil organic matter (SOM), carbon (C), and essential nutrients, such as nitrogen (N) in soil [53]. Evaluation of the guinea grass (Panicum maximum) under silvopastoral systems revealed numerical differences between treatments. The pH and OM values varied, but significant differences were not detected (p > 0.05). Therefore, the incorporation of legumes, shrubbery, or trees into pastures may improve the physical and physiological characteristics of soil and increase the biodiversity parameters within a silvopastoral system. In the quantification of biomass (fresh mass kg ha−1), we observed enhanced plant productivity and better stocking rates depending on the season.
Few changes in soil macronutrients were found between treatments, with the exception of Ca and K. We identified a seasonal effect in the silvopastoral systems (SPS1 and SPS2), which had better Ca content in the LP season in comparison with the HP season. Similar results were reported in [29,54], in which soil was evaluated in a tropical climate, and high levels of Ca were frequently measured in agroforestry systems. Similarly, we measured lower K levels in silvopastoral systems when compared with the conventional system.
Conventional monoculture systems are typically characterized by high levels of K. Therefore, practices that combine forest components with pastures can promote the availability of nutrients in the soil [55]. Furthermore, we suggest that pastures be grown only with grasses during the early stage of development (first 3 years) to prevent the depletion of soil nutrient reserves by fast-growing plants. In this study, we observed acidic soil pH in all treatments, but the Fe values were lower in the silvopastoral systems compared with those in the conventional silvopastoral system. This finding might be explained by the synergy between pastures and forest components in production systems, specifically in this region of Ecuador.
Another important factor is the soil structure, which contributes to the dynamics of organic matter and nutrient cycling as well as other properties, such as porosity, compaction, and water retention [29]. Soil structure has been defined as the size, shape, and characteristics of soil particles, aggregates, and pores across the size range from nanometers to centimeters [56]. We established two main types of soil structure: the Control, with sandy loam soil, and SPS1 and SPS2, which have silty loam soils. According to [57], the soil structure and texture influence soil water flow, availability, and storage.
The key to understanding why higher values were obtained in the season with lower precipitation (LP) for nearly all evaluated parameters might therefore be as follows. We performed observations in the experimental period during two seasons (HP and LP). In the HP season, the water flow is low; in other words, the water infiltration is slow, and we observed that an average of 3 days is needed for the water to drain. Sandy loam soil has a high rate of pluviometry puddles or frequent runoff, at which point the laminar erosion is extremely low.
Although guinea grass is characterized by high tolerance to extreme conditions, which are typical in tropical climates, we cannot rule out the possibility that conditions in the HP season lead to water stress [57], despite its association with greater water use efficiency [58,59]. By contrast, in the LP season, we observed that guinea grass is more efficient in periods of hydric deficit. In [6], the authors confirmed that the cultivation of guinea grass (Panicum maximum) may be possible in areas with marked water deficit and high temperatures. Thus, the soil structure might affect water use and efficiency, depending on the season of the year, and influence root distribution and the ability to take up water and nutrients.
The authors of [1] found a positive relationship between biomass production and soil macrofauna abundance, which indicates the importance of this key plant functional group. In [57], soil macrofauna enhanced aeration, porosity, infiltration, and aggregate stability. In our study, we demonstrated the importance of silvopastoral systems. In a comparison between silvopastoral systems and the conventional livestock system with only monoculture pastures, we observed differences in the number of individuals per m2.
According to [29], the soil in deforested areas, such as conventional systems, generally affects communities of soil macrofauna. By contrast, a high density of trees improves environmental and feeding conditions. Therefore, a higher abundance of macrofauna can facilitate enhanced biomass production or diversify substrates for the roots of pastures.
5. Conclusions
The guinea grass Panicum maximum exhibited different behaviors according to weather conditions and management practices, and better results were obtained when it was managed under silvopastoral systems. Another important finding to highlight in this work is that a strong seasonal effect was identified. Thus, this type of system should be considered during the planning of grazing frequency. Accordingly, the animal responses obtained from this study confirmed that the use of environmentally friendly technology is more sustainable for improving production systems in this tropical region of Ecuador. Importantly, we highlighted that practices involving a conventional grass monoculture cause the rapid degradation of natural resources and raise pressure on the forest. Thus, the appropriate grazing management of guinea grass (Panicum maximum) in combination with silvopastoral systems results in additional ecosystem services when compared with grass monoculture.
Author Contributions
Conceptualization, R.L.G.M.; methodology, R.L.G.M.; validation, W.E.C.G. and A.E.G.P.; formal analysis, N.R.O.N. and S.A.G.R.; investigation, R.L.G.M., W.E.C.G., P.A.V.Z. and A.E.G.P.; resources, R.L.G.M., W.E.C.G., A.E.G.P. and N.R.O.N.; data curation, S.A.G.R., R.L.G.M. and A.E.G.P.; writing—original draft preparation, R.L.G.M., W.E.C.G., A.E.G.P., N.R.O.N. and S.A.G.R.; writing—review and editing, R.L.G.M., P.A.V.Z. and A.E.G.P.; visualization, R.L.G.M. and W.E.C.G.; supervision, R.L.G.M.; project administration, R.L.G.M.; funding acquisition, R.L.G.M. and W.E.C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Development of a Production System Climate-Smart to Determine the Synergy between Mitigation, Adaptation, and Food Security in Orellana Province.” This project was realized from 2017 to 2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All authors are grateful to the livestock producers that participated in this project; to Vicente Baez, Ismael Gaviria, and Francisco Armijos; and to INAMHI for its contributed weather information of Orellana Province.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vázquez, E.; Teutscherová, N.; Lojka, B.; Arango, J.; Pulleman, M. Pasture diversification affects soil macrofauna and soil biophysical properties in tropical (silvo)pastoral systems. Agric. Ecosyst. Environ. 2020, 302, 107083. [Google Scholar] [CrossRef]
- Baldassini, P.; Despósito, C.; Piñeiro, G.; Paruelo, J. Silvopastoral systems of the Chaco forests: Effects of trees on grass growth. J. Arid Environ. 2018, 156, 87–95. [Google Scholar] [CrossRef]
- Bussoni, A.; Alvarez, J.; Cubbage, F.; Ferreira, G.; Picasso, V. Diverse strategies for integration of forestry and livestock production. Agrofor. Syst. 2019, 93, 333–344. [Google Scholar] [CrossRef]
- Murgueitio, E.R.; Chará, J.O.; Barahona, R.R.; Cuartas, C.C.; Naranjo, J.R. Intensive Silvopastoral Systems (ISPS), mitigation and adaptation tool to climate change. Trop. Subtrop. Agroecosystems 2014, 17, 501–507. [Google Scholar]
- Delgado, I.; Randel, P. Supplementation of Cows Grazing Tropical Grass Swards with Concentrates Varying in Protein Level and Degradability. J. Dairy Sci. 1989, 72, 995–1001. [Google Scholar] [CrossRef]
- De Lima Veras, E.L.; Difante, G.D.S.; Chaves Gurgel, A.L.; Graciano da Costa, A.B.; Gomes Rodrigues, J.; Marques Costa, C.; Emerenciano Neto, J.V.; Gusmão Pereira, M.D.; Ramon Costa, P. Tillering and structural characteristics of Panicum cultivars in the Brazilian semiarid region. Sustainability 2020, 12, 3849. [Google Scholar] [CrossRef]
- Cardoso, A.; Barbero, R.P.; Romanzini, E.P.; Teobaldo, R.W.; Ongaratto, F.; Fernandes, M.H.M.D.R.; Ruggieri, A.C.; Reis, R.A. Intensification: A Key Strategy to Achieve Great Animal and Environmental Beef Cattle Production Sustainability in Brachiaria Grasslands. Sustainability 2020, 12, 6656. [Google Scholar] [CrossRef]
- Lessmann, J.; Fajardo, J.; Muñoz, J.; Bonaccorso, E. Large expansion of oil industry in the Ecuadorian Amazon: Biodiversity vulnerability and conservation alternatives. Ecol. Evol. 2016, 6, 4997–5012. [Google Scholar] [CrossRef]
- Torres, B.; Günter, S.; Acevedo-Cabra, R.; Knoke, T. Livelihood strategies, ethnicity and rural income: The case of migrant settlers and indigenous populations in the Ecuadorian Amazon. For. Policy Econ. 2018, 86, 22–34. [Google Scholar] [CrossRef]
- Huera-Lucero, T.; Labrador-Moreno, J.; Blanco-Salas, J.; Ruiz-Téllez, T. A Framework to Incorporate Biological Soil Quality Indicators into Assessing the Sustainability of Territories in the Ecuadorian Amazon. Sustainability 2020, 12, 3007. [Google Scholar] [CrossRef]
- Ojeda, T.; Zhunusova, E.; Günter, S.; Dieter, M. Measuring forest and agricultural income in the Ecuadorian lowland rainforest frontiers: Do deforestation and conservation strategies matter? For. Policy Econ. 2020, 111, 102034. [Google Scholar] [CrossRef]
- Sellers, S.; Bilsborrow, R.; Salinas, V.; Mena, C. Population and development in the Amazon: A longitudinal study of migrant settlers in the Northern Ecuadorian Amazon. Acta Amaz. 2017, 47, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Valdiviezo, R.; Hern, H.; Tafur, V.; Eche, D.; Jácome, E. Off-Farm Employment, Forest Clearing and Natural Resource Use: Evidence from the Ecuadorian Amazon. Sustainability 2020, 12, 4545. [Google Scholar] [CrossRef]
- Lòpez, V.; Espíndola, F.; Calles, J.; Ulloa, J. Atlas “Amazonía Ecuatoriana Bajo Presión”; EcoCiencia: Quito, Ecuador, 2013; Available online: https://biblio.flacsoandes.edu.ec/libros/digital/56384.pdf (accessed on 5 January 2020).
- GADPO. Development and Land Management Plan of the Province of Orellana. 2015. Available online: www.gporellana.gob.ec/wp-content/uploads/2015/11/pdyot-2015-2019_orellana_actualizado.pdf (accessed on 18 September 2020).
- Pezzopane, J.R.M.; Santos, P.M.; Evangelista, S.R.M.; Bosi, C.; Cavalcante, A.C.R.; Bettiol, G.M.; de Miranda Gomide, C.; Pellegrino, G.Q. Panicum maximum cv. Tanzânia: Climate trends and regional pasture production in Brazil. Grass Forage Sci. 2017, 1, 104–117. [Google Scholar]
- Cubbage, F.W.; Balmelli, G.; Bussoni, A.; Noellemeyer, E.; Pachas, A.N.; Fassola, H.; Colcombet, L.; Rossner, B.; Frey, G.E.; Dube, F.; et al. Comparing silvopastoral systems and prospects in eight regions of the world. Agrofor. Syst. 2012, 86, 303–314. [Google Scholar] [CrossRef]
- Almeida, L.L.D.S.; Frazão, L.A.; Lessa, T.A.M.; Fernandes, L.A.; de Carvalho Veloso, Á.L.; Lana, A.M.Q.; De Souza, I.A.; Pegoraro, R.F.; Ferreira, E.A. Soil carbon and nitrogen stocks and the quality of soil organic matter under silvopastoral systems in the Brazilian Cerrado. Soil Tillage Res. 2021, 205, 104785. [Google Scholar] [CrossRef]
- Gomes, F.J.; Pedreira, B.C.; Santos, P.M.; Bosi, C.; Lulu, J.; Pedreira, C.G.S. Microclimate effects on canopy characteristics of shaded palisadegrass pastures in a silvopastoral system in the Amazon biome of central Brazil. Eur. J. Agron. 2020, 115, 126029. [Google Scholar] [CrossRef]
- Boval, M.; Dixon, R. The importance of grasslands for animal production and other functions: A review on management and methodological progress in the tropics. Animal 2012, 6, 748–762. [Google Scholar] [CrossRef]
- Akiyama, Y.; Yamada-Akiyama, H.; Yamanouchi, H.; Takahara, M.; Ebina, M.; Takamizo, T.; Nakagawa, H.; Sugita, S.-I. Estimation of genome size and physical mapping of ribosomal DNA in diploid and tetraploid guineagrass (Panicum maximum Jacq.). Grassl. Sci. 2008, 54, 89–97. [Google Scholar] [CrossRef]
- Gurgel, A.L.C.; Difante, G.D.S.; De Araujo, A.R.; Montagner, D.B.; Euclides, V.P.B.; Da Silva, M.G.P. Carbon and Nitrogen Stocks and Soil Quality in an Area Cultivated with Guinea Grass under the Residual Effect of Nitrogen Doses. Sustainability 2020, 12, 9381. [Google Scholar] [CrossRef]
- Holdridge, L.R. Life Zone Ecology; Tropical Science Center: San Jose, Costa Rica, 1967. [Google Scholar]
- INEC. Proyecciones Poblacionales. Available online: https://www.ecuadorencifras.gob.ec/proyecciones-poblacionales/ (accessed on 15 December 2019).
- González-Andrade, F.; Roewer, L.; Willuweit, S.; Sánchez, D.; Martinez-Jarreta, B. Y-STR variation among ethnic groups from Ecuador: Mestizos, Kichwas, Afro-Ecuadorians and Waoranis. Forensic Sci. Int. Genet. 2009, 3, e83–e91. [Google Scholar] [CrossRef] [PubMed]
- INEC-ESPAC. Encuesta de Superficie y Producción Agropecuaria. Usos del Suelo. Available online: https://www.ecuadorencifras.gob.ec/estadisticas-agropecuarias-2/ (accessed on 11 November 2019).
- Costa, S.B.D.M.; de Mello, A.C.; Dubeux, J.C., Jr.; dos Santos, M.V.; Lira, M.D.A.; Oliveira, J.T.; Apolinário, V.X. Livestock performance in warm-climate silvopastures using tree legumes. Agron. J. 2016, 5, 2026–2035. [Google Scholar] [CrossRef]
- Rodríguez, L.; Suárez, J.C.; Rodriguez, W.; Artunduaga, K.J.; Lavelle, P. Agroforestry systems impact soil macroaggregation and enhance carbon storage in Colombian deforested Amazonia. Geoderma 2021, 384, 114810. [Google Scholar] [CrossRef]
- Brienza Junior, S.; Gazel Yared, J.A. Agroforestry systems as an ecological approach in the Brazilian Amazon development. For. Ecol. Manage. 1991, 45, 319–323. [Google Scholar] [CrossRef]
- Haydock, K.P.; Shaw, N.H. The comparative yield method for estimating dry matter yield of pasture. Aust. J. Exp. Agric. 1975, 76, 663–670. [Google Scholar]
- National Research Council. Basic Problems and Techniques in Range Research; The National Academies Press: Washingon, DC, USA, 1962; Available online: https://www.nap.edu/catalog/20268 (accessed on 15 August 2020).
- Santos, D.D.C.; Júnior, R.G.; Vilela, L.; Pulrolnik, K.; Bufon, V.B.; França, A.F.D.S. Forage dry mass accumulation and structural characteristics of Piatã grass in silvopastoral systems in the Brazilian savannah. Agric. Ecosyst. Environ. 2016, 233, 16–24. [Google Scholar] [CrossRef]
- Núñez Delgado, J.; Ñaupari Vasquez, J.; Flores Mariazza, E. Comportamiento nutricional y perfil alimentario de la producción lechera en pastos cultivados (Panicum maximum Jacq). Rev. Investig. Vet. Perú 2019, 1, 178–192. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Russel, A.J.F.; Doney, J.M.; Gunn, R.G. Subjective assessment of body fat in live sheep. J. Agric. Sci. 1969, 72, 451–454. [Google Scholar] [CrossRef]
- Scarnecchia, D.L.; Kothmann, M.M. A Dynamic Approach to Grazing Management Terminology. J. Range Manag. 1982, 35, 262–264. [Google Scholar] [CrossRef]
- Jerry, L.; Holechek, J.L. An Approach for Setting the Stocking Rate An Approach for Setting the Stocking Rate. Rangelands 1988, 10, 1–10. [Google Scholar]
- Sollenberger, L.E.; Moore, J.E.; Allen, V.G.; Pedreira, C.G.S. Reporting Forage Allowance in Grazing Experiments. Crops Sci. 2005, 45, 896–900. [Google Scholar] [CrossRef]
- Baillie, I.C.; Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods. J. Ecol. 1990, 78, 547. [Google Scholar] [CrossRef]
- ISO. ISO 23611-5. Soil Quality-Sampling of Soil Invertebrates-Part 5: Sampling and Extraction of Soil Macro-Invertebrates. 2011. Available online: https://www.iso.org/standard/50777.html (accessed on 2 August 2020).
- National Research Council; National Research Council Staff; Committee on Animal Nutrition. Nutrient Requirements of Beef Cattle: Update 2000; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. The Animal and Its Food, 7th ed.; Prentice Hall/Pearson: London, UK, 2010; pp. 1–15. [Google Scholar]
- Homen, M.; Entrena, I.; Arriojas, L.; Ramia, M. Biomasa y valor nutritivo del pasto Guinea Megathyrsus maximus (Jacq.) B. K. Simon & S. W. L. Jacobs. ‘Gamelote’ en diferentes períodos estado Miranda. Zootec. Trop. 2010, 2, 255–265. [Google Scholar]
- Kenneth, J.M.; Collins, M.; Jerry, N.C.; Redfearn, D. Forages, The Science of Grassland Agriculture, II, 7th ed.; John Wiley & Sons Ltd: Chichester, UK, 2020. [Google Scholar]
- Lagunes, F.J.; Fox, D.; Blake, R.; Pell, A. Evaluation of Tropical Grasses for Milk Production by Dual-Purpose Cows in Tropical Mexico. J. Dairy Sci. 1999, 82, 2136–2145. [Google Scholar] [CrossRef]
- Phimphachanhvongsod, V.; Ledin, I. Performance of Growing Goats Fed Panicum maximum and Leaves of Gliricidia sepium. Asian-Australas. J. Anim. Sci. 2002, 15, 1585–1590. [Google Scholar] [CrossRef]
- Ajayi, F.T.; Babayemi, O.J.; Taiwo, A.A. Effects of supplementation of Panicum maximum with four herbaceous forage legumes on performance, nutrient digestibility and nitrogen balance in West African dwarf goats. Anim. Sci. J. 2008, 6, 673–679. [Google Scholar] [CrossRef]
- Seresinhe, T.; Pathirana, K.K. Associative effects of tree legumes and effect of cutting height on the yield and nutritive value of Panicum maximum cv. Guinea. Trop. Grassl. 2000, 34, 103–109. [Google Scholar]
- Moore, J.E.; Mott, G.O.; Matches, A.G. Structural Inhibitors of Quality in Tropical Grasses. In Anti-Quality Components of Forages; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 1973; pp. 53–98. Available online: https://acsess.onlinelibrary.wiley.com/doi/abs/10.2135/cssaspecpub4.c4 (accessed on 1 May 2020).
- Bernardino, F.S.; Tonucci, R.G.; Garcia, R.; César, J.; Neves, L.; Rocha, G.C. Forage yield and performance of beef steers in a silvopastoral system: Effects of forage offers and nitrogen fertilization. Rev. Bras. Zootec. 2011, 40, 1412–1419. [Google Scholar] [CrossRef]
- Ibrahim, M.; Guerra, L.; Casasola, F.; Neely, C. Importance of silvopastoral systems for mitigation of climate change and harnessing of environmental benefits. CIPAV 2010, 11, 189–196. [Google Scholar]
- Avendaño-Yáñez, M.D.L.L.; López-Ortíz, S.; Perroni, Y.; Pérez-Elizalde, S. Leguminous trees from tropical dry forest generate fertility islands in pastures. Arid. Land Res. Manag. 2017, 32, 57–70. [Google Scholar] [CrossRef]
- Alfaia, S.S.; Ribeiro, G.A.; Nobre, A.D.; Luizão, R.C.; Luizão, F.J. Evaluation of soil fertility in smallholder agroforestry systems and pastures in western Amazonia. Agric. Ecosyst. Environ. 2004, 102, 409–414. [Google Scholar] [CrossRef]
- Martínez, J.; Cajas, Y.S.; León-Peláez, J.D.; Osorio, N.W. Silvopastoral Systems Enhance Soil Quality in Grasslands of Colombia. Appl. Environ. Soil Sci. 2014, 2014, 359736. [Google Scholar] [CrossRef]
- Regelink, I.C.; Stoof, C.R.; Rousseva, S.; Weng, L.; Lair, G.J.; Kram, P.; Nikolaidis, N.P.; Kercheva, M.; Banwart, S.A.; Comans, R.N. Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247–248, 24–37. [Google Scholar] [CrossRef]
- Bronick, C.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Del Pozo Rodriguez, P. Bases Ecofisiológicas para el Manejo de los Pastos Tropicales. Pastos 2002, 32, 109–137. [Google Scholar]
- Baruch, Z. Responses to drought and flooding in tropical forage grasses. Plant Soil 1994, 164, 97–105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).