Quality Variation of the Moldovan Origanum vulgare L. ssp. vulgare L. and Origanum vulgare L. ssp. hirtum (Link) Ietsw. Varieties in Drought Conditions
Abstract
:1. Introduction
2. Materials and Methods
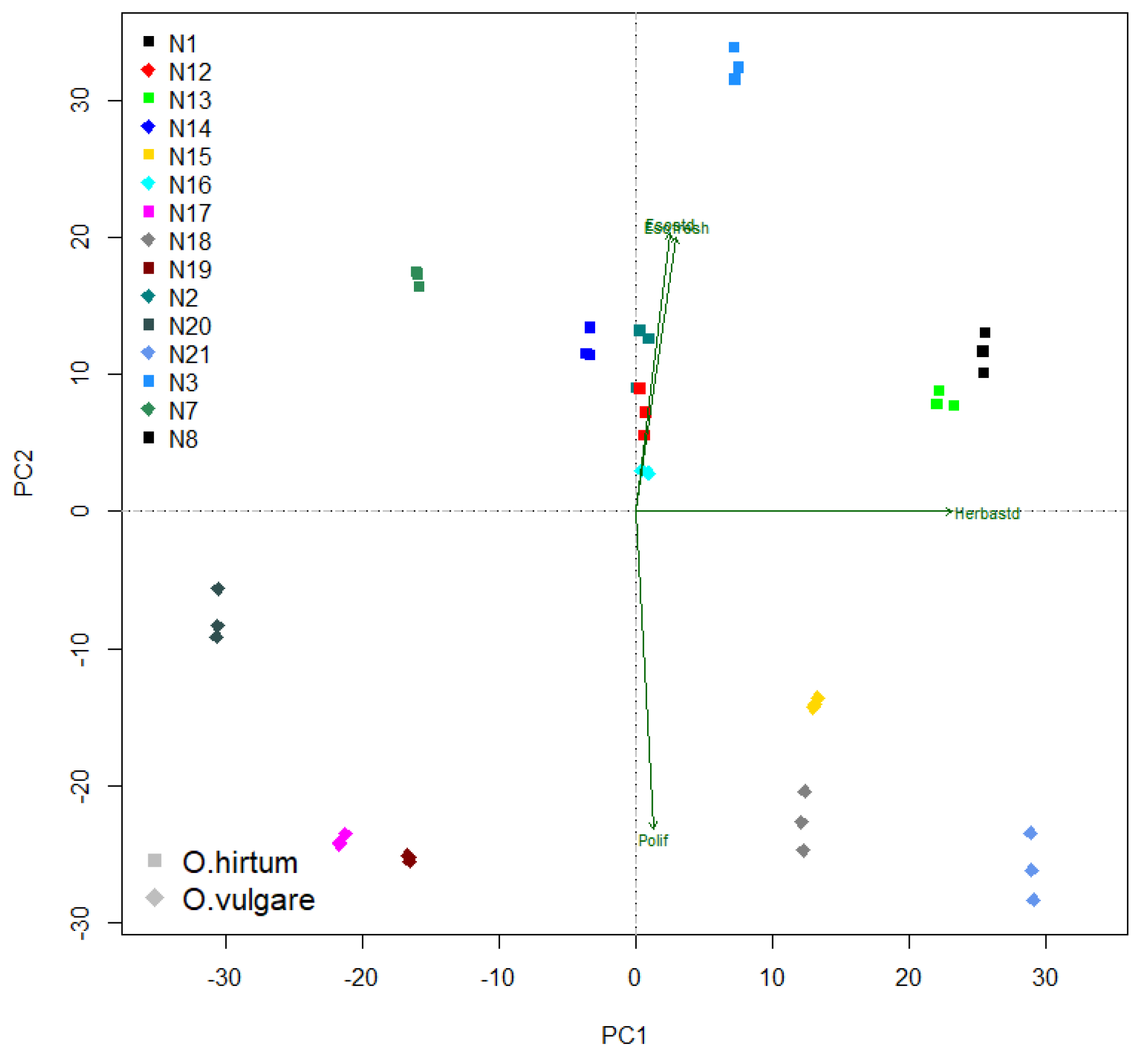
3. Results
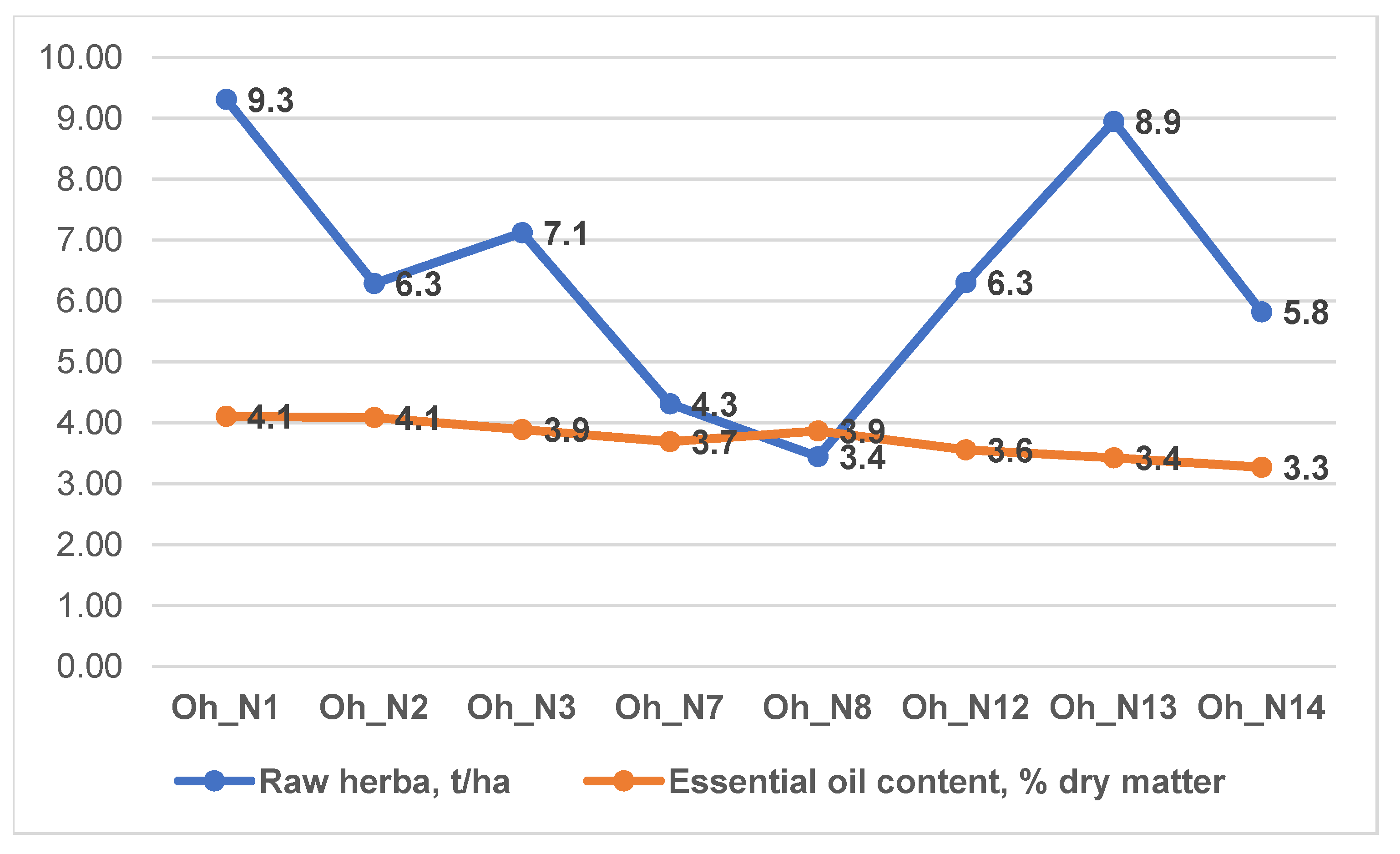
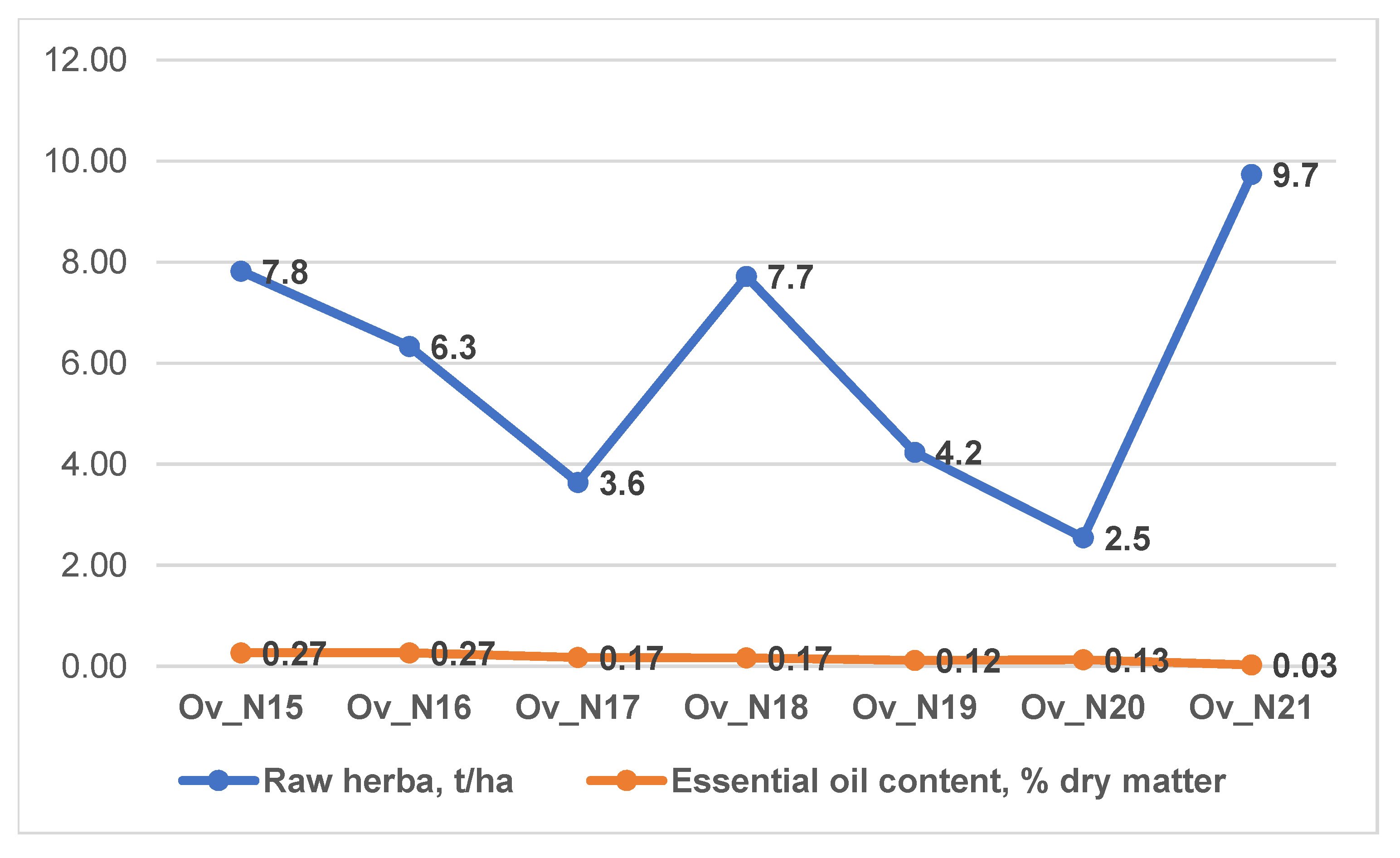
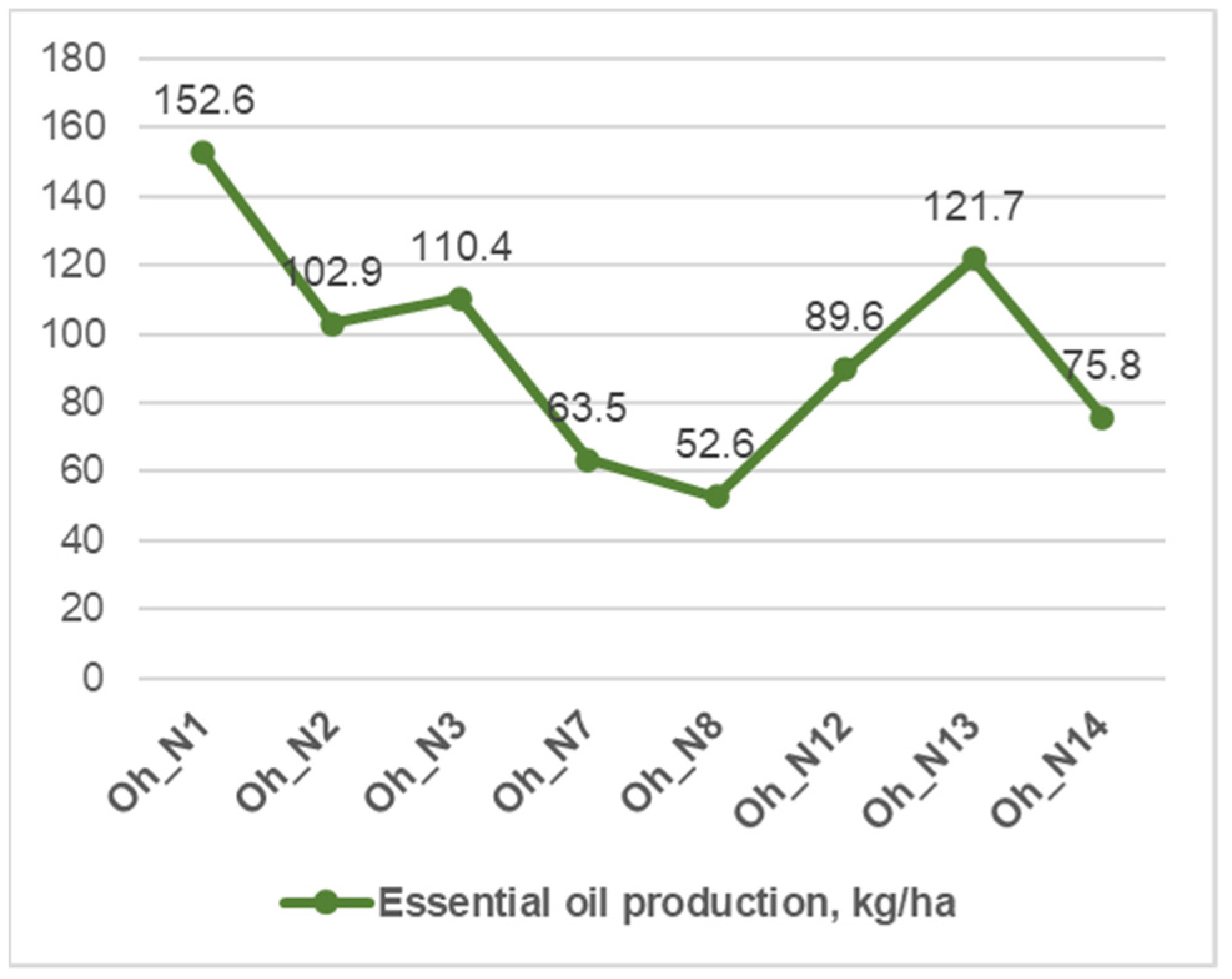
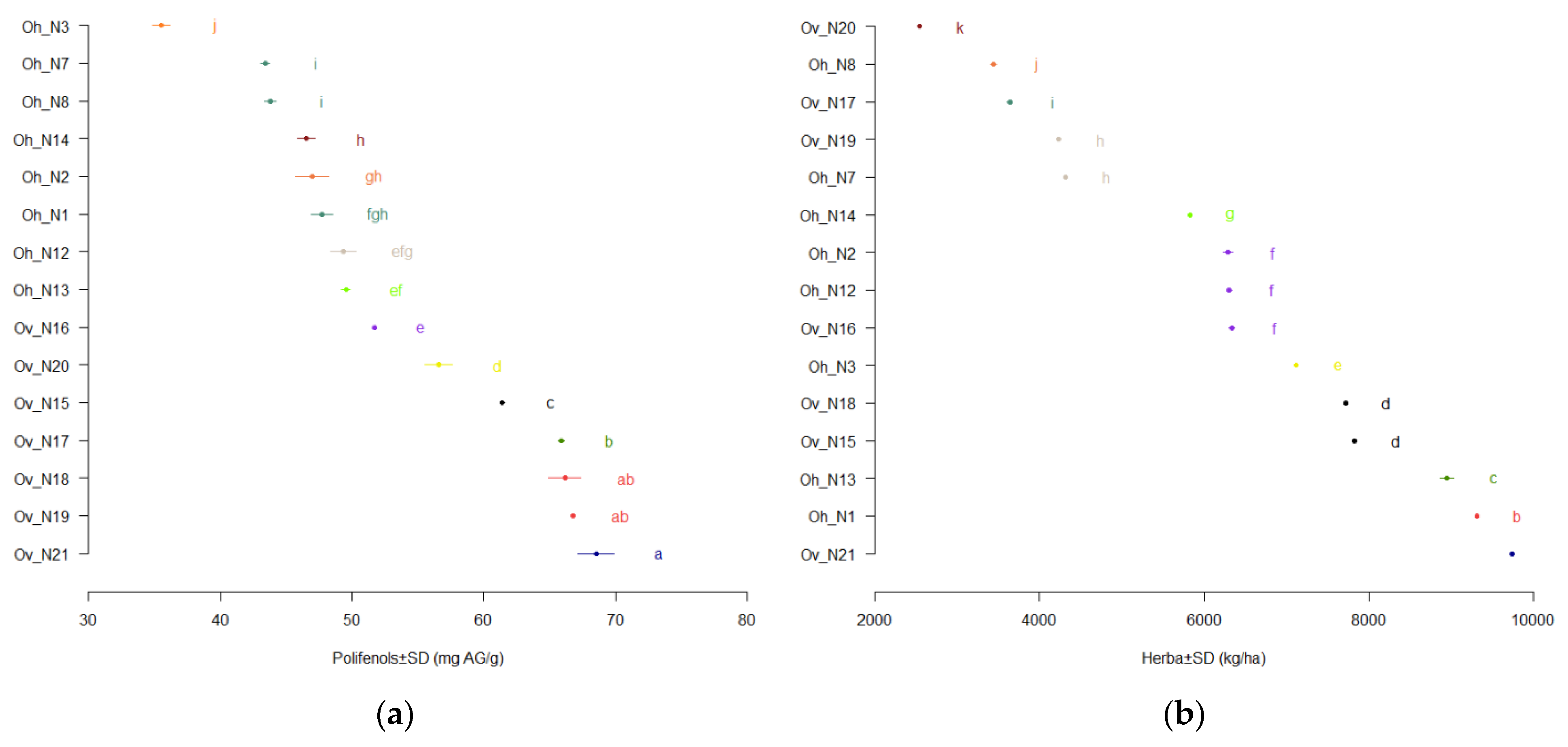
3.1. Productivity of O. vulgare ssp. hirtum and O. vulgare ssp. vulgare Varieties
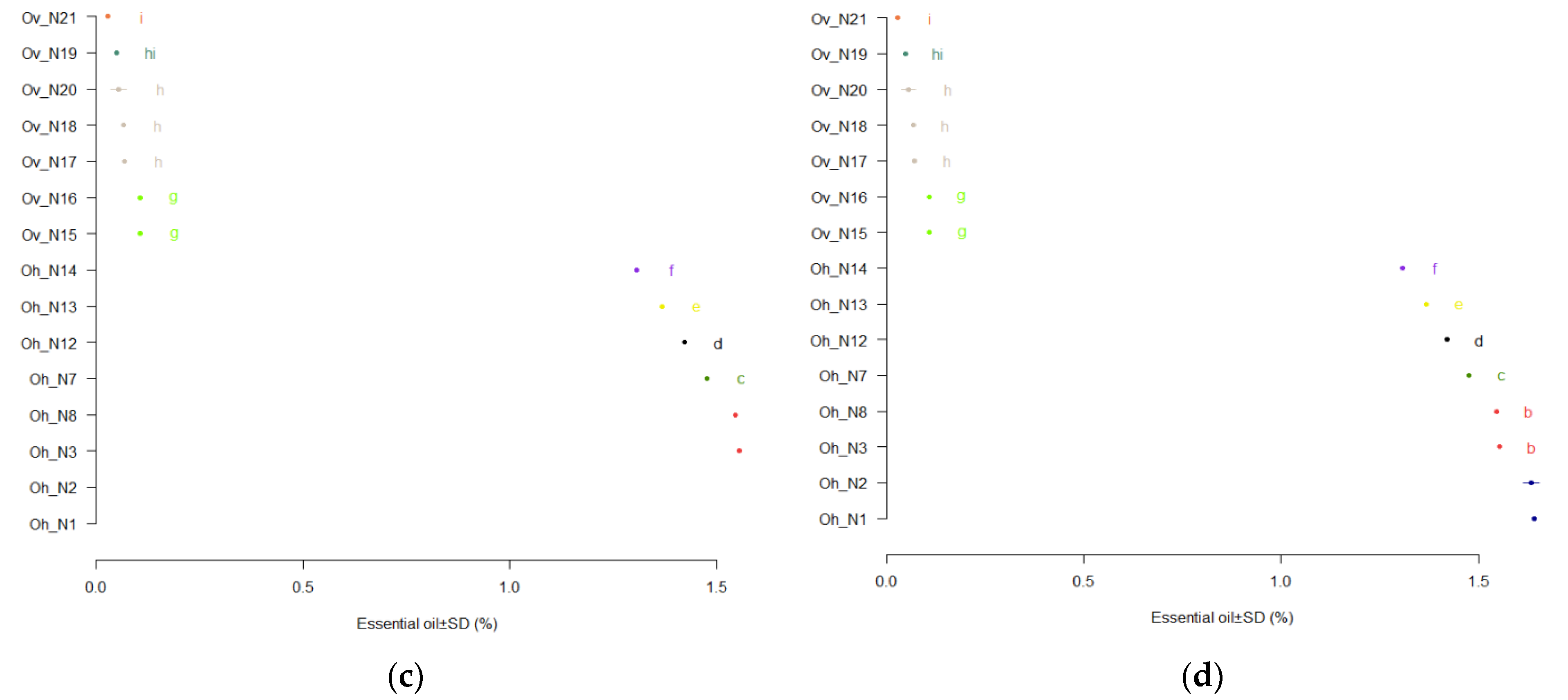
3.2. Qualitative and Quantitative Composition of Essential Oil
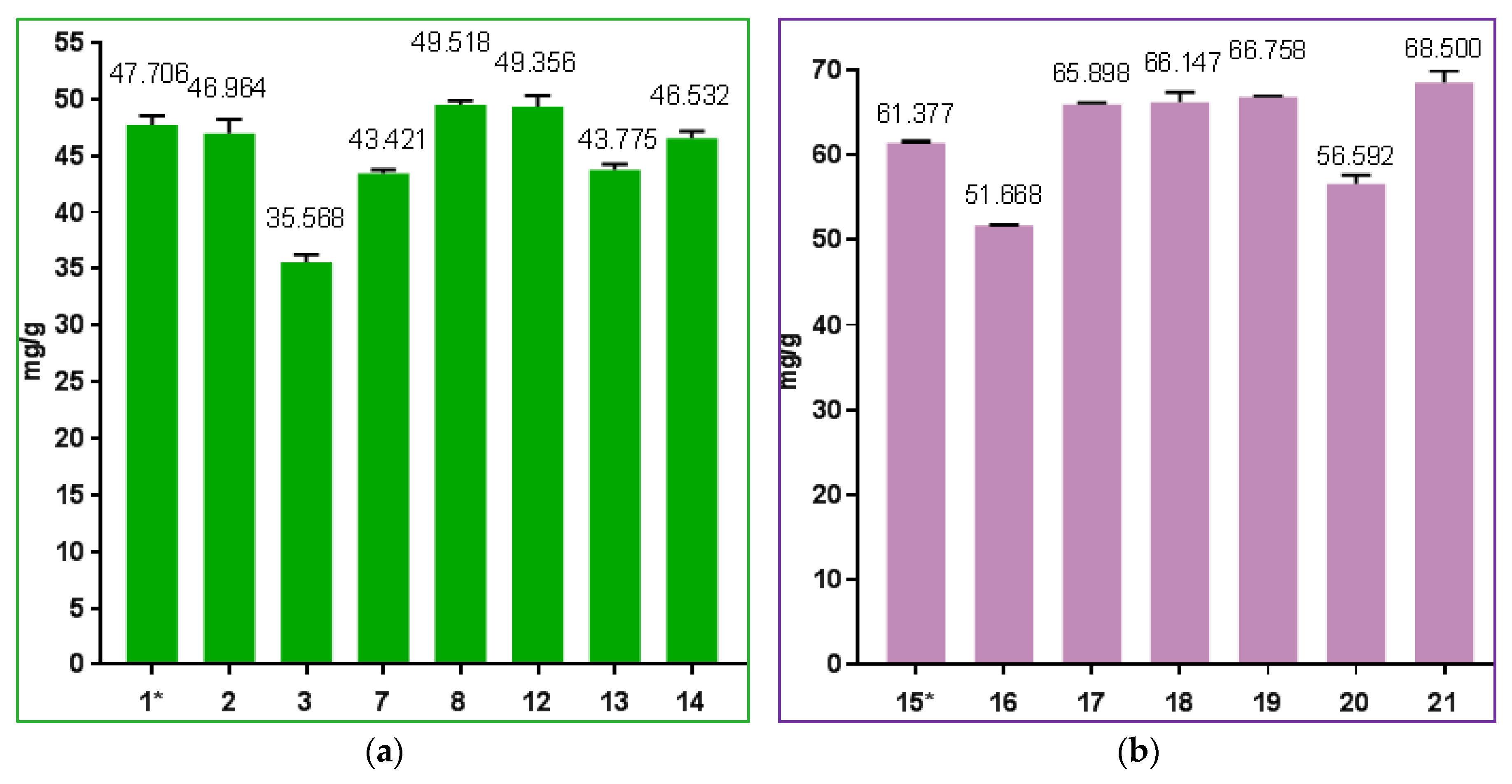
3.3. Polyphenols Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and Biological Properties of Satureja cuneifolia and Thymus spinulosus Ten.: Two Wild Officinal Species of Conservation Concern in Apulia (Italy). A Preliminary Survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and Antimicrobial Properties of Essential Oils from Four Wild Taxa of Lamiaceae Family Growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Fazio, A.; Musarella, C.M.; Mendoza-Fernández, A.J.; Mota, J.F.; Spampinato, G. Seed germination and antioxidant pattern in Lavandula multifida (Lamiaceae): A comparison between core and peripheral populations. Plant Biosyst. 2018, 152, 398–406. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Petek, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Herak Ćustić, M.; Satovic, Z. Medicinal plants of the family of Lamiaceae as functional foods—A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Özkan, M. Glandular and eglandular hairs of Salvia recognita & Mey. (Lamiaceae) in Turkey. Bangladesh J. Bot. 2008, 37, 93–95. [Google Scholar]
- Chishti, S.; Kaloo, Z.A.; Sultan, P. Medicinal importance of genus Origanum: A review. Pharmacogn. Phytother. 2013, 5, 170–177. [Google Scholar]
- Sezik, E.; Tümen, G.; Özek, K.T.; Baser, K.H.C. Essential oil composition of four Origanum vulgare subspecies of Anatolian Origin. J. Essent. Oil Res. 1993, 5, 425–431. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Gonceariuc, M.; Balmuş, Z.; Benea, A.; Bârsan, V.; Sandu, T. Biochemical diversity of the Origanum vulgare ssp. vulgare L. and Origanum vulgare ssp. hirtum (Link) Ietswaart genotypes from Moldova. J. ASM Life Sci. 2015, 2, 92–100. [Google Scholar]
- Weglarz, Z.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Baczek, K. The Quality of Greek Oregano (O. vulgare L. ssp. hirtum (Link) Ietsw.) and Common Oregano (O. vulgare L. ssp. vulgare) cultivated in the temperate climate of central Europe. Foods 2020, 9, 1676. [Google Scholar] [CrossRef]
- Alkan, M. Chemical composition and insecticidal potential of different Origanum spp. (Lamiaceae) essential oils against four stored product pests. Turk. J. Entomol. 2020, 44, 149–163. [Google Scholar] [CrossRef]
- Asdal, A.; Galambosi, B.; Bjorn, G.; Olsson, K.; Pihlik, U.; Radušiene, J. Spice—And medicinal plants in the Nordic and Baltic countries. In Report from a Project Group at the Nordic Gene Bank; Conservation of Genetic Resources; NGB: Alnarp, Norway, 2006; p. 157. [Google Scholar]
- Asensio, C.M.; Grosso, N.R.; Juliani, H.R. Quality characters, chemical composition and biological activities of oregano (Origanum spp.) essential oils from central and southern Argentina. Ind. Crop. Prod. 2015, 63, 203–213. [Google Scholar] [CrossRef]
- Gonceariuc, M.; Balmuş, Z.; Sandu, T.; Romanciuc, G.; Gonceariuc, N. Essential oil of Origanum vulgare ssp. vulgare L. and Origanum vulgare ssp. hirtum (Link) Ietswaart from Moldova: Content and chemical composition. IJAIR 2014, 3, 659–663. [Google Scholar]
- Skoufogianni, E.; Solomou, A.D.; Danalatos, N.G. Ecology, Cultivation and Utilization of the Aromatic Greek Oregano (Origanum vulgare L.): A Review. Not. Bot. Horti Agrobot. 2019, 47, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Dutra, T.V.; Castro, J.C.; Menezes, J.L.; Ramos, T.R.; Prado, I.N.; Machinski Junior, M.; Mikcha, J.M.G.; Abreu, F. A bioactivity of oregano (Origanum vulgare) essential oil against Aliciclobacillus ssp. Ind. Crop. Prod. 2019, 129, 345–349. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.J.; Heredia, B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Olmedo, R.; Nepote, V.; Grosso, N.R. Antioxidant activity of fractions from oregano essential oils obtained by molecular distillation. Food Chem. 2014, 156, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Aktumsek, A.; Zengin, G.; Oskay, M.; Ceylan, R.; Uysal, S. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two O. vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crop. Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Grosso, N.R.; Lante, A.; Lomolino, G.; Zygadlo, J.A.; Nepote, V. Chemical composition, antioxidant activity and anti-lipase activity of Origanum vulgare and Lippia turbinata essential oils. J. Food Sci. Technol. 2013, 48, 642–649. [Google Scholar] [CrossRef]
- Oniga, I.; Puscas, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Azizi, A.; Janke, S.; Schwarz, M.; Zeller, S.; Honermeier, B. Antioxidant capacity variation in the oregano (Origanum vulgare L.) collection of the German National Gene bank. Ind. Crop. Prod. 2016, 92, 19–25. [Google Scholar] [CrossRef]
- Cazzola, R.; Cestaro, B. Antioxidant spices and herbs used in diabetes. In Diabetes: Oxidative Stress and Dietary Antioxidants Diabetes; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 89–97. [Google Scholar]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpenes compound in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Béjaou, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum Desf. at different phenological stages. J. Med. Food 2013, 16, 1115–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottumukkala, V.R.; Mukhopadhyay, T.; Annamalai, N.; Radhakrishnan Sahoo, M.R. Chemical constituents and biological studies of Origanum vulgare L. Pharmacogn. Res. 2011, 3, 143–145. [Google Scholar]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Martucci, J.F.; Gende, L.B.; Neira, L.M.; Ruseckaite, R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatin films. Ind. Crop. Prod. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [Green Version]
- Reza Yousefi, A.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Azizi, A.; Ardalani, H.; Honermeier, B. Statistical analysis of the associations between phenolic monoterpenes and molecular markers, AFLPs and SAMPLs in the spice plant Oregano. Herba Pol. 2016, 62, 42–56. [Google Scholar] [CrossRef] [Green Version]
- Azizi, A.; Hadian, J.; Gholami, M.; Friedt, W.; Honermeier, B. Correlations between genetic, morphological, and chemical diversities in a germplasm collection of the medicinal plant Origanum vulgare L. Chem. Biodivers. 2012, 9, 2784–2801. [Google Scholar] [CrossRef]
- Elezi, F.; Plaku, F.; Ibraliu, A.; Stefkov, G.; Karapandzova, M.; Kulevanova, S.; Aliu, S. Genetic variation of oregano (Origanum vulgare) for etheric oil in Albania. J. Agric. Sci. 2013, 4, 449–454. [Google Scholar]
- Gavalas, N.P.; Kalburtji, K.L.; Kokkini, S.; Veresoglou, D.S.; Mamolos, A.P. Ecotypic variation in plant characteristics for Origanum vulgare subsp. hirtum populations. Biochem. Syst. Ecol. 2011, 39, 562–569. [Google Scholar] [CrossRef]
- Kokkini, S. Autumn essential oils of Greek oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]
- Vokou, D.; Kokkini, S.; Bessiere, J.M. Geographic variation of Greek oregano (O. vulgare ssp. hirtum) essential oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Hanlidou, E.; Lanaras, T. Essential oil composition of Greek oregano (Origanum vulgare ssp. hirtum) and Turkish (O. onites) Oregano: A tool for their distinction. J. Essent. Oil Res. 2004, 16, 334–338. [Google Scholar] [CrossRef]
- Ietswaart, J.H. A Taxonomic Revision of the Genus Origanum (Labiatae), 1st ed.; Leiden University Press: The Hague, The Netherlands; Boston, MA, USA; London, UK, 1980; pp. 1–153. [Google Scholar]
- DECISION. No. 43 of 15.01.2013 for the Approval of the Regulation on Testing and Admission of Varieties in the Catalog of Plant Varieties. Available online: http://lex.justice.md/index.php?action=view&view=doc&lang=1&id=346378 (accessed on 18 July 2020).
- Miron, T.L.; Gazi, I.; Plaza del Moral, M. Romanian aromatic plants as sources of antioxidants. Innov. Rom. Food Biotechnol. 2010, 6, 18–24. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2019. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research, R package version 1.9.12; Northwestern University: Evanston, IL, USA, 2019. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R Package Version 1.3–2.24; R Foundation Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Vidican, R.; Păcurar, F.; Vâtcă, S.D.; Pleșa, A.; Stoian, V. Arbuscular Mycorrhizas Traits and Yield of Winter Wheat Profiled by Mineral Fertilization. Agronomy 2020, 10, 846. [Google Scholar] [CrossRef]
- Vâtcă, S.; Vidican, R.; Gâdea, Ș.; Horvat, M.; Vâtcă, A.; Stoian, V.A.; Stoian, V. Blackcurrant Variety Specific Growth and Yield Formation as a Response to Foliar Fertilizers. Agronomy 2020, 10, 2014. [Google Scholar] [CrossRef]
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2018, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Pârlici, R.M.; Maxim, A.; Mang, S.M.; Camele, I.; Mihalescu, L.; Stoian, V. Alternative Control of Phragmidium rubi-idaei Infecting Two Rubus Species. Plants 2021, 10, 1452. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5-6; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Puia, C.; Vidican, R.; Szabó, G.; Stoian, V. Potential of biofertilisers to improve performance of local genotype tomatoes. Ital. J. Agron. 2017, 12, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Horváth, H.; Szabó, K.; Bernáth, J.; Kókai, Z. Evaluation of selected Origanum vulgare subsp. hirtum (Link) Ietswaart clones, by “electronic nose”. Acta Hortic. 2002, 576, 61–64. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Karioti, A.; Bergonzi, M.C.; Pescitelli, G.; Di Bari, L.; Skaltsa, H. Polar constituents from the aerial parts of Origanum vulgare L. ssp hirtum growing wild in Greece. J. Agric. Food Chem. 2006, 54, 5388–5392. [Google Scholar] [CrossRef]
- Bonfanti, C.; Ianni, R.; Mazzaglia, A.; Lanza, C.M.; Napoli, E.M.; Ruberto, G. Emerging cultivation of oregano in Sicily: Sensory evaluation of plans and chemical composition of essential oils. Ind. Crop. Prod. 2012, 35, 160–165. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Formisano, C.; Mignola, E.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from three chemotypes of Origanum vulgare L. ssp. hirtum (Link) Ietswaart growing wild in Campania (Southern Italy). Molecules 2009, 14, 2735–2746. [Google Scholar] [CrossRef] [Green Version]
- De Mastro, G.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential oil diversity of Origanum vulgare L. populations from Southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, A.; Guerrini, A.; Tacchini, M.; Vinciguerra, V.; Leone, C.; Maresca, I.; Simonetti, G.; Sacchetti, G.; Angiolella, L. Chemical Composition and Bio-efficacy of Essential Oils from Italian Aromatic Plants: Mentha suaveolens, Coridothymus capitatus, Origanum hirtum and Rosmarinus officinalis. Nat. Prod. Commun. 2016, 11, 1517–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stešević, D.; Jaćimović, Z.; Šatović, Z.; Šapčanin, A.; Jančanf, G.; Kosovićb, M.; Damjanović-Vratnicab, B. Chemical characterization of wild growing Origanum vulgare populations in Montenegro. Nat. Prod. Commun. 2018, 13, 1357–1362. [Google Scholar] [CrossRef] [Green Version]
- Mockute, D.; Bernotiene, G.; Judzentiene, A. The essential oil of Origanum vulgare L. ssp. vulgare growing wild in Vilnius district (Lithuania). Phytochemistry 2001, 57, 65–69. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pave, Z.I.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A recent insight regarding the phytochemistry and bioactivity of Origanum vulgare L. essential oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Sarrou, E.; Tsivelika, N.; Chatzopoulou, P.; Tsakalidis, G.; Menexes, G.; Mavromatis, A. A conventional breeding of Greek oregano (Origanum vulgare ssp. hirtum) and development of improved cultivars for yield potential and essential oil quality. Euphitica 2017, 213, 2–16. [Google Scholar] [CrossRef]
- Cirino, I.C.S.; Menezes-Silva, S.M.P.; Silva, H.T.D.; Souza, E.L.; Siqueira-Júnior, J.P. The essential oil from Origanum vulgare L. and its individual constituents carvacrol and thymol enhance the effect of tetracycline against Staphylococcus aureus. J. Chemother. 2014, 60, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Shafiee-Hajiabad, M.; Novak, J.; Honermeier, B. Content and composition of essential oil of four Origanum vulgare L. Accessions under reduced and normal light intensity conditions. J. Appl. Bot. Food Qual. 2016, 89, 126–134. [Google Scholar]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Kosakowska, O.; Weglarz, Z.; Pióro-Jabrucka, E.; Jaroslaw, L.; Przybyl, J.L.; Krasniewska, K.; Gniewosz, M.; Baczek, K. Antioxidant and antibacterial activity of essential oils and hydroethanolic extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef] [PubMed]
- Król, B.; Kołodziej, B.; Kędzia, B.; Hołderna-Kędzia, E.; Sugier, D.; Luchowska, K. Date of harvesting affects yields and quality of Origanum vulgare ssp. hirtum (Link) Ietswaart. J. Sci. Food Agric. 2019, 99, 5432–5443. [Google Scholar] [CrossRef] [PubMed]
- Atalay, F.; Tatar, A.; Dincer, B.; Gündoğdu, B.; Köyceğiz, S. Protective effect of carvacrol against paclitaxel-induced ototoxicity in rat model. Turk. Arch. Otorhinolaryngol. 2021, 58, 241–248. [Google Scholar] [CrossRef]
- Hernandez, H.; Fraňková, A.; Sýkora, T.; Klouček, P.; Kouřimská, L.; Kučerová, I.; Banout, J. The effect of oregano essential oil on microbial load and sensory attributes of dried meat. J. Sci. Food Agric. 2017, 97, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.D.S.; Quintans-Júnior, L.J.; De Santana, W.A.; Martins Kaneto, C.; Pereira Soares, M.B.; Villarreal, C.F. Anti-inflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Scafati, O.T.; Senatore, F. Chemical composition and biological activity of essential oils of Origanum vulgare L. subsp. vulgare L. under different growth conditions. Molecules 2013, 18, 14948–14960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A Mini-Review. Front. Nutr. 2018, 5, 21–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güneş-Bayır, A.; Aksoy, A.; Koçyiğit, A. The Importance of Polyphenols as Functional Food in Health. Bezmialem Sci. 2019, 7, 157–163. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]

| Months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | Anuală |
| 0.6 | 4.4 | 7.8 | 11.3 | 14.4 | 21.5 | 22.8 | 23.5 | 20.2 | 14.2 | 4.6 | 2.9 | 12.7 °C |
| 9 | 23 | 19 | 4 | 69 | 86 | 85 | 5 | 75 | 81 | 32 | 74 | 562 mm |
| Components | Rt, min | Sample Area % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 * | 2 | 3 | 7 | 8 | 12 | 13 | 14 | ||
| α-Thuene | 4.27 | 0.93 | 1.08 | 1.01 | 1.02 | 0.65 | 0.84 | 0.57 | 0.49 |
| α-Pinene | 4.41 | 0.34 | - | 0.37 | 0.38 | 0.24 | 0.32 | 0.21 | 0.19 |
| Camphene | 4.70 | 0.08 | 0.39 | 0.08 | - | - | - | - | - |
| β-Phellandrene | 5.17 | 0.28 | - | 0.31 | 0.36 | 0.26 | 0.38 | 0.23 | 0.11 |
| β-Pinene | 5.25 | 0.35 | 0.31 | 0.37 | 0.37 | 0.37 | 0.44 | 0.32 | 0.22 |
| β-Mircene | 5.49 | 1.28 | 0.31 | 1.17 | 1.28 | 0.98 | 1.26 | 0.96 | 0.87 |
| α-Phellandrene | 5.81 | 0.17 | 1.31 | 0.16 | 0.18 | 0.14 | 0.17 | 0.13 | 0.13 |
| β-Terpinene | 6.09 | 0.77 | 0.18 | 0.7 | 0.82 | 0.67 | 1.28 | 1.02 | 0.62 |
| p-Cymene | 6.28 | 2.88 | 0.86 | 2.99 | 3.03 | 2.67 | 4.48 | 3.12 | 2.78 |
| Sylvestrene | 6.38 | 0.22 | 2.66 | 0.22 | 0.27 | 0.18 | 0.25 | 0.20 | 0.19 |
| trans-Ocimene | 6.53 | - | 0.32 | - | - | - | - | - | - |
| cis-Ocimene | 6.79 | 0.06 | - | 0.08 | - | 0.07 | 0.09 | 0.07 | 0.06 |
| γ-terpinene | 7.09 | 3.54 | - | 3.20 | 3.95 | 3.41 | 8.76 | 7.49 | 3.64 |
| 4-Thujanol | 7.29 | 0.75 | 3.99 | 0.81 | 0.78 | 0.98 | 0.83 | 0.75 | 0.63 |
| Linalool | 8.08 | 0.35 | 0.58 | 0.58 | 0.38 | 0.76 | 0.51 | 0.43 | 0.44 |
| Borneol | 9.82 | 0.26 | - | 0.21 | - | 0.27 | 0.36 | 0.40 | 0.29 |
| 4-Terpineol | 10.13 | 0.40 | - | 0.51 | 0.52 | 0.50 | 0.40 | 0.40 | 0.34 |
| Thymol methyl ester | 11.86 | 0.14 | - | 0.13 | - | 0.12 | 0.05 | 0.06 | 0.10 |
| Thymol | 13.14 | 0.33 | - | 0.34 | - | 0.37 | 0.39 | 0.44 | 0.35 |
| Carvacrol | 13.78 | 83.91 | - | 82.87 | 83.12 | 83.82 | 76.46 | 80.62 | 86.26 |
| Thymol acetate | 15.23 | - | 84.27 | 0.08 | - | 0.09 | 0.05 | 0.08 | 0.10 |
| β-Caryophyllene | 16.46 | 2.10 | - | 2.34 | 2.49 | 1.95 | 1.75 | 1.48 | 1.66 |
| α-Caryophyllene | 17.27 | 0.11 | 2.59 | 0.12 | - | 0.12 | 0.15 | 0.14 | 0.17 |
| Germacrene D | 17.95 | 0.35 | - | 0.80 | 0.65 | 0.63 | 0.17 | 0.11 | 0.08 |
| β-Bisabolene | 18.56 | 0.11 | 0.78 | 0.22 | 0.39 | 0.30 | 0.22 | - | 0.21 |
| δ-Cadinene | 18.92 | 0.07 | 0.37 | 0.12 | - | 0.09 | - | - | - |
| Spathulenol | 20.18 | - | - | 0.11 | - | 0.16 | - | - | - |
| Caryophyllene oxide | 20.41 | 0.10 | - | 0.07 | - | 0.09 | 0.07 | 0.05 | 0.10 |
| Components number | 25 | 15 | 28 | 17 | 26 | 24 | 23 | 24 | |
| Identification, % | 99.88 | 100 | 99.97 | 99.99 | 99.89 | 99.68 | 99.28 | 99.99 | |
| Components | Rt., min | Sample, Area% | ||||||
|---|---|---|---|---|---|---|---|---|
| 15 * | 16 | 17 | 18 | 19 | 20 | 21 | ||
| Sabinene | 5.17 | 2.69 | 1.37 | 0.72 | - | 1.84 | 1.44 | 1.05 |
| 1-Octen-3-ol | 5.22 | - | 0.49 | 0.27 | - | 0.28 | - | 0.41 |
| β-Mircene | 5.48 | - | 0.15 | - | - | 0.34 | - | - |
| α-Phellandrene | 6.10 | - | - | - | - | 0.21 | - | - |
| p-Cymene | 6.27 | 0.33 | 0.46 | 0.23 | - | 0.77 | - | 0.32 |
| trans-Ocimene | 6.54 | 1.97 | 1.78 | 0.96 | - | 2.14 | 1.43 | 1.21 |
| cis-Ocimene | 6.79 | 2.64 | 1.86 | 0.72 | - | 0.87 | - | 1.33 |
| γ-terpinene | 7.07 | 0.67 | 0.51 | - | - | 0.62 | - | 0.35 |
| 4-Thujanol | 7.29 | 0.90 | 0.64 | 0.22 | - | 0.18 | - | 0.69 |
| Linalool | 8.08 | 2.47 | 6.31 | 7.06 | 4.91 | 4.50 | 9.28 | 5.74 |
| Borneol | 9.82 | - | 0.21 | - | - | - | - | - |
| 4-Terpineol | 10.13 | 1.63 | 2.21 | 1.52 | - | 3.34 | 3.33 | 2.13 |
| α-Terpineol | 10.47 | 0.34 | 0.67 | 0.66 | - | 0.68 | 1.44 | 0.59 |
| Linalylacetate | 12.15 | - | 2.96 | 3.03 | 4.28 | 2.78 | 4.91 | 2.36 |
| Lavandulyl acetate | 13.05 | - | 0.56 | 0.54 | - | 0.45 | 1.12 | 0.54 |
| Carvacrol | 13.39 | - | 3.02 | 3.64 | - | 2.10 | 11.43 | 2.35 |
| β -Bourbonene | 15.55 | 1.21 | 1.22 | 0.95 | 1.98 | 1.03 | 1.50 | 1.37 |
| Elemene | 15.71 | 0.69 | - | 0.50 | - | 0.55 | - | 0.59 |
| β-Caryophyllene | 16.50 | 15.78 | 12.85 | 15.30 | 6.61 | 8.77 | 8.89 | 14.38 |
| β-Cubebene | 17.08 | 0.52 | - | 0.26 | - | 0.27 | - | - |
| α-Caryophyllene | 17.28 | 1.76 | 1.42 | 2.24 | - | 1.64 | 1.69 | 1.55 |
| (+)Aromadendrene | 17.50 | 1.46 | 1.27 | 0.34 | - | 0.58 | - | 0.82 |
| Germacrene D | 18.08 | 31.13 | 28.82 | 24.69 | 33.02 | 25.97 | 21.63 | 32.04 |
| β -Bergamotene | 18.20 | 0.54 | - | 0.39 | - | 0.55 | - | - |
| Bicyclogermacrene | 18.36 | 2.96 | 2.82 | 3.01 | 10.00 | 6.39 | 5.81 | 2.75 |
| β -Farnesene | 18.60 | 11.41 | 8.57 | 12.21 | 16.60 | 15.56 | 9.83 | 9.42 |
| Cadinene | 18.74 | 0.62 | - | 0.74 | - | 0.38 | - | 0.33 |
| Cadinene | 18.92 | 2.01 | 2.27 | 2.38 | 2.71 | 1.79 | 2.02 | 2.41 |
| cis Bisabolene | 19.36 | 0.84 | 0.31 | 0.54 | - | - | - | - |
| GermacreneD-4ol | 20.18 | - | - | 4.88 | 4.03 | 3.47 | 3.45 | 6.28 |
| (-)Spatulenol | 20.24 | 6.55 | 5.54 | - | 6.64 | 2.04 | 2.33 | - |
| Caryophyllene oxide | 20.41 | 1.35 | 1.36 | 1.81 | - | 1.26 | 2.15 | 1.48 |
| Cubenol | 21.09 | 0.73 | - | 0.35 | - | - | - | 0.73 |
| Cadinol | 21.62 | 2.57 | 2.46 | 3.02 | 4.03 | 3.16 | 2.79 | 2.33 |
| α -Cadinol | 22.00 | 2.99 | 3.19 | 3.60 | 5.02 | 2.75 | 3.53 | 3.56 |
| Trans-Phytol | 30.07 | 0.21 | 0.18 | 0.28 | - | 0.30 | - | - |
| Number identified components | 28 | 29 | 31 | 12 | 33 | 20 | 28 | |
| Total, identified components, % | 98.97 | 95.48 | 97.06 | 99.83 | 97.56 | 100 | 99.11 | |
| Oh Varieties | Essential Oil Content % in: | Polyphenols Content, mg/g | Raw Materials Production, kg/ha | |
|---|---|---|---|---|
| Fresh Raw Matter | Standard Humidity | |||
| Oh_N1 * | 1.43 ± 0 a | 1.64 ± 0 a | 47.7 ± 0.82 ab | 9308.67 ± 11.02 a |
| Oh _N2 | 1.37 ± 0.01 b | 1.63 ± 0.02 a | 46.97 ± 1.25 b | 6287 ± 54.06 d |
| Oh _N3 | 1.27 ± 0 d | 1.56 ± 0 b | 35.51 ± 0.65 d | 7119.67 ± 21.96 c |
| Oh _N7 | 1.23 ± 0 f | 1.48 ± 0 c | 43.42 ± 0.33 c | 4309.33 ± 12.06 f |
| Oh _N8 | 1.29 ± 0 c | 1.55 ± 0 b | 43.78 ± 0.46 c | 3442.67 ± 37 g |
| Oh_N12 | 1.29 ± 0 c | 1.42 ± 0 d | 49.36 ± 0.96 a | 6300.67 ± 29.14 d |
| Oh_N13 | 1.25 ± 0.01 e | 1.37 ± 0 e | 49.52 ± 0.35 a | 8948 ± 80.07 b |
| Oh_N14 | 1.11 ± 0 g | 1.31 ± 0 f | 46.53 ± 0.63 b | 5820.67 ± 19.73 e |
| F test | 1056.66 | 873.83 | 114.07 | 7815.42 |
| p.val | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Ov Varieties | Essential Oil Content, % in: | Polyphenols Content, mg/gg | Raw Materials Production, kg/ha | |
|---|---|---|---|---|
| Fresh Raw Matter | Standard Humidity | |||
| Ov_N15 * | 0.09 ± 0.01 a | 0.11 ± 0 a | 61.38 ± 0.19 b | 7819.33 ± 21.01 b |
| Ov_N16 | 0.09 ± 0 a | 0.11 ± 0 a | 51.67 ± 0.08 d | 6329.33 ± 35.85 d |
| Ov_N17 | 0.06 ± 0 b | 0.07 ± 0 b | 65.9 ± 0.22 f | 3638.33 ± 29.26 f |
| Ov_N18 | 0.06 ± 0 b | 0.07 ± 0 bc | 66.14 ± 1.19 c | 7715.33 ± 16.17 c |
| Ov_N19 | 0.04 ± 0 bc | 0.05 ± 0 cd | 66.76 ± 0.13 e | 4235 ± 11.79 e |
| Ov_N20 | 0.04 ± 0.02 bc | 0.05 ± 0.02 bc | 56.59 ± 1.03 g | 2544 ± 6 g |
| Ov_N21 | 0.02 ± 0 c | 0.03 ± 0 d | 68.5 ± 1.36 a | 9732.67 ± 12.22 a |
| F test | 183.25 | 55.93 | 183.25 | 45,210.09 |
| p.val | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonceariuc, M.; Muntean, M.V.; Butnaraş, V.; Duda, M.M.; Benea, A.; Jelezneac, T.; Vornicu, Z.; Cotelea, L.; Botnarenco, P. Quality Variation of the Moldovan Origanum vulgare L. ssp. vulgare L. and Origanum vulgare L. ssp. hirtum (Link) Ietsw. Varieties in Drought Conditions. Agriculture 2021, 11, 1211. https://doi.org/10.3390/agriculture11121211
Gonceariuc M, Muntean MV, Butnaraş V, Duda MM, Benea A, Jelezneac T, Vornicu Z, Cotelea L, Botnarenco P. Quality Variation of the Moldovan Origanum vulgare L. ssp. vulgare L. and Origanum vulgare L. ssp. hirtum (Link) Ietsw. Varieties in Drought Conditions. Agriculture. 2021; 11(12):1211. https://doi.org/10.3390/agriculture11121211
Chicago/Turabian StyleGonceariuc, Maria, Mircea Valentin Muntean, Violeta Butnaraş, Marcel Matei Duda, Anna Benea, Tamara Jelezneac, Zinaida Vornicu, Ludmila Cotelea, and Pantelimon Botnarenco. 2021. "Quality Variation of the Moldovan Origanum vulgare L. ssp. vulgare L. and Origanum vulgare L. ssp. hirtum (Link) Ietsw. Varieties in Drought Conditions" Agriculture 11, no. 12: 1211. https://doi.org/10.3390/agriculture11121211
APA StyleGonceariuc, M., Muntean, M. V., Butnaraş, V., Duda, M. M., Benea, A., Jelezneac, T., Vornicu, Z., Cotelea, L., & Botnarenco, P. (2021). Quality Variation of the Moldovan Origanum vulgare L. ssp. vulgare L. and Origanum vulgare L. ssp. hirtum (Link) Ietsw. Varieties in Drought Conditions. Agriculture, 11(12), 1211. https://doi.org/10.3390/agriculture11121211






