Effect of Raising Dairy Heifers on Their Performance and Reproduction after 12 Months
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Diet Management after Weaning
2.3. Live Body Weight Growth
2.4. Health and Reproduction
2.5. Statistical Calculations
3. Results
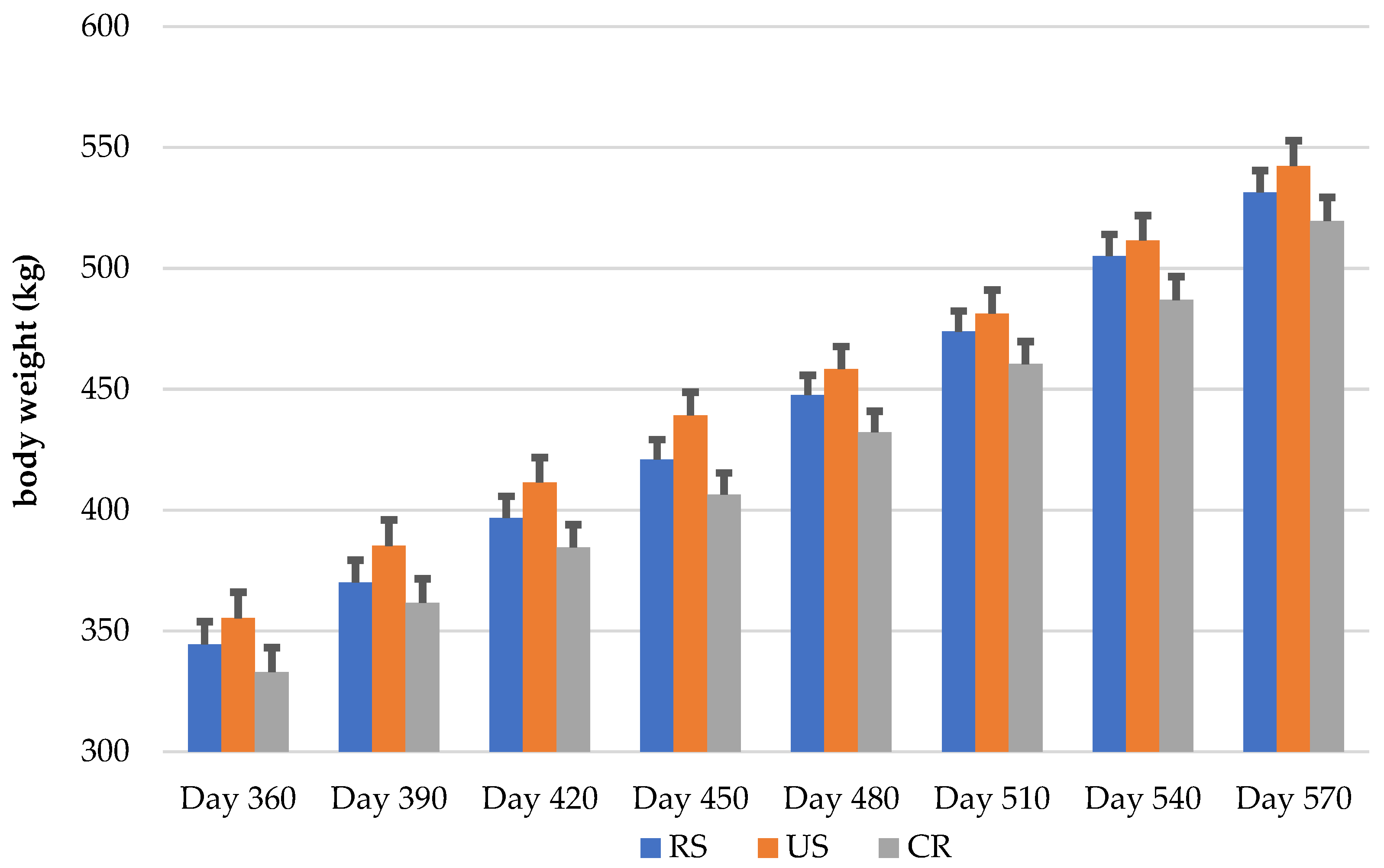
3.1. Growth
3.1.1. Factor Treatment Rearing (T)
3.1.2. Season of Birth and Father’s Lineage Factors (SB and F)
3.2. Health and Reproduction
4. Discussion
4.1. Growth
4.1.1. Factor Treatment Rearing (T)
4.1.2. Season of Birth and Father’s Lineage Factors (SB, F)
4.2. Health and Reproduction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albright, J.L.; Arave, C.W. The Behaviour of Cattle; CAB International: Wallingford, UK, 1997; p. 306. [Google Scholar]
- Flower, F.C.; Weary, D.M. The effects of early separation on the dairy cow and calf. Anim. Welf. 2003, 12, 339–348. [Google Scholar] [CrossRef]
- Jasper, J.; Weary, D.M. Effects of ad libitum milk intake on dairy calves. J. Dairy Sci. 2002, 85, 3054–3058. [Google Scholar] [CrossRef]
- Wagenaar, J.P.T.M.; Langhout, J. Practical implications of increasing ‘natural living’ through suckling systems in organic dairy calf rearing. NJAS Wagening. J. Life Sci. 2007, 54, 375–386. [Google Scholar] [CrossRef]
- Khattak, A.H.K.; Wasay, A.; Ali, T.; Iqbal, M.; Kalim, K.; Hassan, M.F.; Mobashar, M.; Ahmad, N.; Iqbal, A.; Islam, M.N. Influence of different weaning ages on growth performance of achai crossed jersey calves. Pak. J. Zool. 2018, 50, 2159–2163. [Google Scholar] [CrossRef]
- Meagher, R.K.; Beaver, A.; Weary, D.M.; Von Keyserlingk, M.A.G. A systematic review of the effects of prolonged cow–calf contact on behavior, welfare, and productivity. J. Dairy Sci. 2019, 102, 5765–5783. [Google Scholar] [CrossRef]
- Loberg, J.; Lidfors, L. Effect of stage of lactation and breed on dairy cows acceptance of foster calves. Appl. Anim. Behav. Sci. 2001, 74, 97–108. [Google Scholar] [CrossRef]
- Loberg, J.M.; Hernandez, C.E.; Thierfelder, T.; Jensen, M.B.; Berg, C.; Lidfors, L. Weaning and separation in two steps—A way to decrease stress in dairy calves suckled by foster cows. Appl. Anim. Behav. Sci. 2008, 111, 222–234. [Google Scholar] [CrossRef]
- Krohn, C.C. Effects of different suckling systems on milk production, udder health, reproduction, calf growth and some behavioural aspects in high producing dairy cows—A review. Appl. Anim. Behav. Sci. 2001, 72, 271–280. [Google Scholar] [CrossRef]
- Johnsen, J.F.; Zipp, K.A.; Kalber, T.; de Passille, A.M.; Knierim, U.; Barth, K.; Mejdell, C.M. Is rearing calves with the dam a feasible option for dairy farms?—Current and future research. Appl. Anim. Behav. Sci. 2016, 181, 1–11. [Google Scholar] [CrossRef]
- De Passillé, A.M.; Marnet, P.G.; Lapierre, H.; Rushen, J. Effects of twice-daily nursing on milk ejection and milk yield during nursing and milking in dairy cows. J. Dairy Sci. 2008, 91, 1416–1422. [Google Scholar] [CrossRef]
- Roth, B.A.; Barth, K.; Gygax, L.; Hillmann, E. Influence of artificial vs. mother-bonded rearing on sucking behaviour, health and weight gain in calves. Appl. Anim. Behav. Sci. 2009, 119, 143–150. [Google Scholar] [CrossRef]
- Fröberg, S.; Gratte, E.; Svennersten-Sjaunja, K.; Olsson, I.; Berg, C.; Orihuela, A.; Galina, C.S.; Garcia, B.; Lidfors, L. Effect of suckling (‘restricted suckling’) on dairy cows’ udder health and milk let-down and their calves’ weight gain, feed intake and behaviour. Appl. Anim. Behav. Sci. 2008, 113, 1–14. [Google Scholar] [CrossRef]
- Johnsen, J.F.; de Passillé, A.M.; Mejdell, C.M.; Boe, K.E.; Grondahl, A.M.; Beaver, A.; Rushen, J.; Weary, D.M. The effect of nursing on the cow-calf bond. Appl. Anim. Behav. Sci. 2015, 163, 50–57. [Google Scholar] [CrossRef]
- Appleby, M.C.; Weary, D.M.; Chua, B. Performance and feeding behaviour of calves on ad libitum milk from artificial teats. Appl. Anim. Behav. Sci. 2001, 74, 191–201. [Google Scholar] [CrossRef]
- Guler, O.; Yanr, M.; Bayram, B. Effect of different milk feeding schedules on the growth and feed conversion efficiencies in Holstein Friesian and Brown Swiss calves. Ind. J. Anim. Sci. 2003, 73, 1278–1280. [Google Scholar]
- Nielsen, P.P.; Jensen, M.B.; Lidfors, L. Milk allowance and weaning method affect the use of a computer controlled milk feeder and the development of cross-sucking in dairy calves. Appl. Anim. Behav. Sci. 2008, 109, 223–237. [Google Scholar] [CrossRef]
- Shamay, A.; Werner, D.; Moallem, U.; Barash, H.; Bruckental, I. Effect of nursing management and skeletal size at weaning on puberty, skeletal growth rate, and milk production during first lactation of dairy heifers. J. Dairy Sci. 2005, 88, 1460–1469. [Google Scholar] [CrossRef]
- Moallem, U.; Werner, D.; Lehrer, H.; Zachut, M.; Livshitz, L.; Yakoby, S.; Shamay, A. Long-term effects of ad libitum whole milk prior to weaning and prepubertal protein supplementation on skeletal growth rate and first-lactation milk production. J. Dairy Sci. 2010, 93, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Rushen, J.; De Passillé, A.M. Behaviour, welfare and productivity of dairy cattle. Can. J Anim. Sci. 1998, 78, 3–21. [Google Scholar]
- Grøndahl, A.M.; Skancke, E.M.; Mejdell, C.M.; Jansen, J.H. Growth rate, health and welfare in a dairy herd with natural suckling until 6–8 weeks of age: A case report. Acta Vet. Scand. 2007, 49, 16. [Google Scholar] [CrossRef]
- Paula Vieira de, A.; De Passillé, A.M.; Weary, D.M. Effects of the early social environment on behavioral responses of dairy calves to novel events. J. Dairy Sci. 2012, 95, 5149–5155. [Google Scholar] [CrossRef]
- Costa, J.H.C.; Meagher, R.K.; Von Keyserlingk, M.A.G.; Weary, D.M. Early pair housing increases solid feed intake and weight gains in dairy calves. J. Dairy Sci. 2015, 98, 6381–6386. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Weary, D.M.; von Keyserlingk, M.A.G. Effects of milk ration on solid feed intake, weaning, and performance in dairy heifers. J. Dairy Sci. 2011, 94, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Monteiro, A.P.A.; Thompson, I.M.; Hayen, M.J.; Dahl, G.E. Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J. Dairy Sci. 2012, 95, 7128–7136. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.P.A.; Tao, S.; Thompson, I.M.T.; Dahl, G.E. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 2016, 99, 8443–8450. [Google Scholar] [CrossRef]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2018, 9, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.E.; Tao, S.; Laporta, J. Heat stress impacts immune status in cows across the life cycle. Front. Vet. Sci. 2020, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Herbut, P.; Angrecka, S.; Walczak, J. Environmental parameters to assessing of heat stress in dairy cattle—A review. Int. J. Biometeorol. 2018, 62, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.M., Jr.; Edwards, M.J.; Edwards, M.J. Gestational effects of maternal hyperthermia due to febrile illnesses and resultant patterns of defects in humans. Teratology 1998, 58, 209–221. [Google Scholar] [CrossRef]
- Illek, J.; Kumprechtova, D.; Matejicek, M.; Vlcek, M. Metabolic profile in high-producing dairy cows in different phases of the calving-to-calving interval. Folia Veter. 2009, 53 (Suppl. 1), 73–74. [Google Scholar]
- Greenwood, P.L.; Thompson, A.N.; Ford, S.P. Postnatal consequences of the maternal environment and of growth during prenatal life for productivity of ruminants. In Managing the Prenatal Environment to Enhance Livestock Productivity; Greenwood, P.L., Bell, A.W., Vercoe, P.E., Viljoen, G.J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 3–36. [Google Scholar] [CrossRef]
- Tao, S.; Dahl, G.E. Heat stress effects during late gestation on dry cows and their calves. J. Dairy Sci. 2013, 96, 4079–4093. [Google Scholar] [CrossRef]
- Mellado, M.; Lopez, E.; Veliz, F.G.; De Santiago, M.H.; Macias-Cruz, U.; Avendaño-Reyes, L.; Garcia, J.E. Factors associated with neonatal dairy calf mortality in a hot-arid environment. Livest. Sci. 2014, 159, 149–155. [Google Scholar] [CrossRef]
- Collier, R.J.; Gebremedhin, K.G. Thermal biology of domestic animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef]
- Johnson, J.S.; Abuajamieh, M.; Sanz Fernandez, M.V.; Seibert, J.T.; Stoakes, S.K.; Nteeba, J.; Keating, A.F.; Ross, J.W.; Rhoads, R.P.; Baumgard, L. Thermal stress alters postabsorptive metabolism during pre- and postnatal development. In Climate Change Impact on Livestock: Adaptation and Mitigation, Chapter 5; Sejian, V., Gaughan, J., Baumgard, L., Prasad, C., Eds.; Springer: New Delhi, India, 2015; pp. 61–80. [Google Scholar] [CrossRef]
- Herbut, P.; Angrecka, S.; Godyń, D.; Hoffmann, G. The physiological and productivity effects of heat stress in cattle—A review. Ann. Anim. Sci. 2019, 19, 579–594. [Google Scholar] [CrossRef]
- Monteiro, A.P.A.; Guo, J.R.; Weng, X.S.; Ahmed, B.M.; Hayen, M.J.; Dahl, G.E.; Bernard, J.K.; Tao, S. Effect of maternal heat stress during the dry period on growth and metabolism of calves. J. Dairy Sci. 2016, 99, 3896–3907. [Google Scholar] [CrossRef]
- Laporta, J.; Fabris, T.F.; Skibiel, A.L.; Powell, J.L.; Hayen, M.J.; Horvath, K.; Miller-Cushon, E.K.; Dahl, G.E. In utero exposure to heat stress during late gestation has prolonged effects on the activity patterns and growth of dairy calves. J. Dairy Sci. 2017, 100, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, P.J.; De Vries, A. Season of conception is associated with future survival, fertility, and milk yield of Holstein cows. J. Dairy Sci. 2017, 100, 6631–6639. [Google Scholar] [CrossRef] [PubMed]
- Recce, S.; Huber, E.; Notaro, U.S.; Rodríguez, F.M.; Ortega, H.H.; Rey, F.; Signorini, M.L.; Salvetti, N.R. Association between heat stress during intrauterine development and the calving-to-conception and calving-to-first-service intervals in Holstein cows. Theriogenology 2021, 162, 95–104. [Google Scholar] [CrossRef]
- Broucek, J.; Uhrincat, M.; Kisac, P.; Hanus, A. Effect of different rearing during the milk-feeding period on growth of dairy calves. Agriculture 2020, 10, 346. [Google Scholar] [CrossRef]
- De Passillé, A.M.; Rushen, J. Calves’ behaviour during nursing is affected by feeding motivation and milk availability. Appl. Anim. Behav. Sci. 2006, 101, 264–275. [Google Scholar] [CrossRef]
- Petrikovic, P.; Sommer, A. Requirement of Nutrients for Cattle, 2nd ed.; Publication of VUZV: Nitra, Slovakia, 2002; p. 62. [Google Scholar]
- McGuirk, S.M. Disease management of dairy calves and heifers. Vet. Clin. North Am. Food Anim. Pract. 2008, 24, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Slavik, P.; Illek, J.; Brix, M.; Musilova, L.; Rajmon, R.; Klabanova, P.; Jilek, F. Health status of beef cows and their calves in the Czech Republic. Acta Veter. Brno 2009, 78, 47–56. [Google Scholar] [CrossRef]
- Novak, P.; Vokralova, J.; Tittl, K.; Mala, G.; Illek, J. Selected aspects of welfare and prevention of disease in ruminants. Veterinarstvi 2010, 60, 25–27. [Google Scholar]
- Stevenson, J.S. Reproductive management of dairy cows in high milk-producing herds. J. Dairy Sci. 2001, 84 (Suppl. E), E128–E143. [Google Scholar] [CrossRef]
- Masello, M.; Perez, M.M.; Granados, G.E.; Stangaferro, M.L.; Ceglowski, B.; Thomas, M.J.; Giordano, J.O. Reproductive performance of replacement dairy heifers submitted to first service with programs that favor insemination at detected estrus, timed artificial insemination, or a combination of both. J. Dairy Sci. 2019, 102, 1671–1681. [Google Scholar] [CrossRef]
- Howlader, M.M.R.; Rahman, M.M.; Hossain, M.G.; Hai, M.A. Factors affecting conception rate of dairy cows following artificial insemination in selected area at sirajgonj district of Bangladesh. Biomed. J. Sci. Technol. Res. 2019, 13, 9907–9914. [Google Scholar]
- Moran, J.B. Rearing Young Stock on Tropical Dairy Farms in Asia; CSIRO Publishing: Clayton, Australia, 2012; p. 296. [Google Scholar]
- Krohn, C.C.; Foldager, J.; Mogensen, L. Long-term effect of colostrum feeding methods on behaviour in female dairy calves. Acta Agric. Scand. Sect. A Anim. Sci. 1999, 49, 57–64. [Google Scholar] [CrossRef]
- Bar-Peled, U.; Robinzon, B.; Maltz, E.; Tagari, H.; Folman, Y.; Bruckental, I.; Voet, H.; Gacitua, H.; Lehrer, A.R. Increased weight gain and effects on production parameters of Holstein heifer calves that were allowed to suckle from birth to six weeks of age. J. Dairy Sci. 1997, 80, 2523–2528. [Google Scholar] [CrossRef]
- Soberon, F.; Raffrenato, E.; Everett, R.W.; Van Amburgh, M.E. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 2012, 95, 783–793. [Google Scholar] [CrossRef]
- Asheim, L.J.; Johnsen, J.F.; Havrevoll, Ø.; Mejdell, C.M.; Grøndahl, A.M. The economic effects of suckling and milk feeding to calves in dual purpose dairy and beef farming. Rev. Agric. Food Environ. Stud. 2016, 97, 225–236. [Google Scholar] [CrossRef]
- Davis, C.L.; Drackley, J.K. The Development, Nutrition, and Management of the Young Calf, 1st ed.; Iowa State University Press: Ames, IO, USA, 1998. [Google Scholar]
- Wójcik, J.; Pilarczyk, R.; Bilska, A.; Weiher, O.; Sanftleben, P. Performance and health of group-housed calves kept in Igloo calf hutches and calf barn. Pak. Vet. J. 2013, 33, 175–178. [Google Scholar]
- Jensen, M.B.; Duve, L.R.; Weary, D.M. Pair housing and enhanced milk allowance increase play behavior and improve performance in dairy calves. J. Dairy Sci. 2015, 98, 2568–2575. [Google Scholar] [CrossRef]
- Flower, F.C.; Weary, D.M. Effects of early separation on the dairy cow and calf: 2. separation at 1 day and 2 weeks after birth. Appl. Anim. Behav. Sci. 2001, 70, 275–284. [Google Scholar] [CrossRef]
- Kisac, P.; Broucek, J.; Uhrincat, M.; Hanus, A. Effect of weaning calves from mother at different ages on their growth and milk yield of mothers. Czech J. Anim. Sci. 2011, 56, 261–268. [Google Scholar] [CrossRef]
- Lee, H.J.; Khan, M.A.; Lee, W.S.; Yang, S.H.; Kim, S.B.; Kee, K.S.; Ha, J.K.; Choi, J.K. Influence of equalizing the gross composition of milk replacer to that of whole milk on the performance of Holstein calves. J. Anim. Sci. 2009, 87, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Miller-Cushon, E.K.; Bergeron, R.; Leslie, K.E.; DeVries, T.J. Effect of milk feeding level on development of feeding behavior in dairy calves. J. Dairy Sci. 2013, 96, 551–564. [Google Scholar] [CrossRef]
- Yavuz, E.N.; Todorov, N.; Ganchev, G.; Nedelkov, K. The effect of feeding different milk programs on dairy calf growth, health and development. Bulg. J. Agric. Sci. 2015, 21, 384–393. [Google Scholar]
- Johnsen, J.F.; Holmoy, I.H.; Nodtvedt, A.; Mejdell, C.M. A survey of pre-weaning calf management in Norwegian dairy herds. Acta. Vet. Scand. 2021, 63, 20. [Google Scholar] [CrossRef] [PubMed]
- Van Eetvelde, M.; Kamal, M.M.; Vandaele, L.; Opsomer, G. Season of birth is associated with first-lactation milk yield in Holstein Friesian cattle. Animal 2017, 11, 2252–2259. [Google Scholar] [CrossRef]
- Kamal, M.M.; Van Eetvelde, M.; Depreester, E.; Hostens, M.; Vandaele, L.; Opsomer, G. Age at calving in heifers and level of milk production during gestation in cows are associated with the birth size of Holstein calves. J. Dairy Sci. 2014, 97, 5448–5458. [Google Scholar] [CrossRef]
- Tao, S.; Monteiro, A.P.A.; Hayen, M.J.; Dahl, G.E. Short communication: Maternal heat stress during the dry period alters postnatal whole-body insulin response of calves. J. Dairy Sci. 2014, 97, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Place, N.T.; Heinrichs, A.J.; Erb, H.N. The effects of disease, management, and nutrition on average daily gain of dairy heifers from birth to four months. J. Dairy Sci. 1998, 81, 1004–1009. [Google Scholar] [CrossRef]
- Broucek, J.; Kisac, P.; Uhrincat, M.; Hanus, A.; Benc, F. Effect of high temperature on growth performance of calves maintained in outdoor hutches. J. Anim. Feed Sci. 2008, 17, 139–146. [Google Scholar] [CrossRef][Green Version]
- Chester-Jones, H.; Heins, B.J.; Ziegler, D.; Schimek, D.; Schuling, S.; Ziegler, B.; De Ondarza, M.B.; Sniffen, C.J.; Broadwater, N. Relationships between early-life growth, intake, and birth season with first-lactation performance of Holstein dairy cows. J. Dairy Sci. 2017, 100, 3697–3704. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Aboujaoude, C.; Penagaricano, F.; Santos, J.E.P.; DeVries, T.J.; McBride, B.W. Associations between maternal characteristics and health, survival, and performance of dairy heifers from birth through first lactation. J. Dairy Sci. 2020, 103, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Broucek, J.; Kisac, P.; Uhrincat, M. Effect of hot temperatures on the hematological parameters, health and performance of calves. Int. J. Biometeorol. 2009, 53, 201–208. [Google Scholar] [CrossRef]
- Illek, J.; Kumprechtova, D.; Kudrna, V.; Matejicek, M.; Vlcek, M. Epidemiology of production diseases in high producing dairy cows on Czech dairy farms. Veterinarstvi 2010, 60, 20–24. [Google Scholar]
- Strong, R.A.; Silva, E.B.; Cheng, H.W.; Eicher, S.D. Acute brief heat stress in late gestation alters neonatal calf innate immune functions. J. Dairy Sci. 2015, 98, 7771–7783. [Google Scholar] [CrossRef]
- Collier, R.J.; Renquist, B.J.; Xiao, Y. Stress physiology including heat stress. J. Dairy Sci. 2017, 100, 10367–10380. [Google Scholar] [CrossRef]
- Novak, P.; Mala, G.; Jarolimkova, A. Animal, housing and nutrition as prerequisite for health, reproduction and production in dairy cattle. In Proceedings of the XIXth Congress International Society of Animal Hygiene, Wroclaw, Poland, 8–12 September 2019; pp. 49–51. [Google Scholar]
- Bakony, M.; Jurkovich, V. Heat stress in dairy calves from birth to weaning. J. Dairy Res. 2020, 87, 53–59. [Google Scholar] [CrossRef]
- Heinrichs, A.J.; Wells, S.J.; Hurd, H.S.; Hill, G.W.; Dargatz, D.A. The National dairy heifer evaluation project: A profile of heifer management practices in the United States. J. Dairy Sci. 1994, 77, 1548–1555. [Google Scholar] [CrossRef]
- Drackley, J.K. Early growth effects on subsequent health and performance of dairy heifers. In Calf and Heifer Rearing; Garnsworthy, P.C., Ed.; Nottingham University Press: Nottingham, UK, 2005; Volume 12, pp. 213–235. [Google Scholar]
- Svensson, C.; Hultgren, J.; Oltenacu, P.A. Morbidity in Swedish dairy calves from 3 to 7 months of age, and risk factors for diarrhea and respiratory disease. Prev. Vet. Med. 2006, 74, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Beaver, A.; Meagher, R.K.; Von Keyserlingk, M.A.G.; Weary, D.M. A systematic review of the effects of early separation on dairy cow and calf health. J. Dairy Sci. 2019, 102, 5784–5810. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, L.A.; Bonilla-Jaime, H.; Orozco-Gregorio, H.; Tarazona-Morales, A.M.; Ballesteros-Rodea, G.; Roldán-Santiago, P.; Waytula, M.; Vargas-Romero, J. Effects of weaning on the stress responses and productivity of water buffalo in different breeding systems: A review. Livest. Sci. 2019, 226, 73–81. [Google Scholar] [CrossRef]
- Banos, G.; Brotherstone, S.; Coffey, M.P. Prenatal maternal effects on body condition score, female fertility, and milk yield of dairy cows. J. Dairy Sci. 2007, 90, 3490–3499. [Google Scholar] [CrossRef]
- Rincker, L.D.; VandeHaar, M.J.; Wolf, C.A.; Liesman, J.S.; Chapin, L.T.; Nielsen, M.W. Effect of intensified feeding of heifer calves on growth, pubertal age, calving age, milk yield, and economics. J. Dairy Sci. 2011, 94, 3554–3567. [Google Scholar] [CrossRef]
- Wathes, D.C.; Pollott, G.E.; Johnson, K.F.; Richardson, H.; Cooke, J.S. Heifer fertility and carry over consequences for life time production in dairy and beef cattle. Animal 2014, 8, 91–104. [Google Scholar] [CrossRef] [PubMed]
| Group | N | Mean | SE | p Value/ Significance | Mean | SE | p Value/ Significance |
|---|---|---|---|---|---|---|---|
| Day 360 | Day 390 | ||||||
| SB1 | 21 | 329.2 | 9.1 | 0.0031 ** | 366.5 | 6.9 | 0.1958 |
| SB2 | 8 | 368.1 | 10.5 | SB2: SB1, SB4 * | 383.0 | 20.3 | |
| SB3 | 5 | 364.5 | 14.2 | 387.4 | 16.2 | ||
| SB4 | 8 | 315.1 | 12.8 | 355.9 | 9.2 | ||
| Day 420 | Day 450 | ||||||
| SB1 | 21 | 394.1 | 6.4 | 0.1224 | 420.9 | 6.2 | 0.0772 |
| SB2 | 8 | 412.5 | 20.6 | 438.7 | 19.0 | ||
| SB3 | 5 | 405.6 | 14.8 | 424.4 | 14.7 | ||
| SB4 | 8 | 383.6 | 8.6 | 408.1 | 8.1 | ||
| Day 480 | Day 510 | ||||||
| SB1 | 21 | 449.3 | 6.5 | 0.1075 | 474.6 | 6.6 | 0.3547 |
| SB2 | 8 | 461.0 | 17.0 | 482.4 | 15.9 | ||
| SB3 | 5 | 446.0 | 13.1 | 474.4 | 12.9 | ||
| SB4 | 8 | 431.9 | 7.4 | 461.7 | 10.9 | ||
| Day 540 | Day 570 | ||||||
| SB1 | 21 | 508.6 | 7.9 | 0.3982 | 535.0 | 7.9 | 0.6746 |
| SB2 | 8 | 507.4 | 13.8 | 534.2 | 12.9 | ||
| SB3 | 5 | 497.8 | 14.6 | 528.8 | 16.4 | ||
| SB4 | 8 | 497.7 | 13.1 | 533.1 | 14.2 | ||
| Group | N | Mean | SE | p Value/ Significance | Mean | SE | p Value/ Significance |
|---|---|---|---|---|---|---|---|
| From the 360th day to the 390th day | From the 390th day to the 420th day | ||||||
| SB1 | 21 | 1.26 | 0.22 | 0.1250 | 0.95 | 0.06 | 0.0440 * |
| SB2 | 8 | 0.30 | 0.36 | 0.94 | 0.07 | SB1:SB3 * | |
| SB3 | 5 | 0.16 | 0.34 | 0.62 | 0.10 | ||
| SB4 | 8 | 1.48 | 0.40 | 0.86 | 0.09 | ||
| From the 420th day to the 450th day | From the 450th day to the 480th day | ||||||
| SB1 | 21 | 0.89 | 0.08 | 0.3774 | 0.94 | 0.06 | 0.1528 |
| SB2 | 8 | 0.87 | 0.10 | 0.74 | 0.11 | ||
| SB3 | 5 | 0.63 | 0.05 | 0.72 | 0.08 | ||
| SB4 | 8 | 0.82 | 0.12 | 0.79 | 0.06 | ||
| From the 480th day to the 510th day | From the 510th day to the 540th day | ||||||
| SB1 | 21 | 0.84 | 0.04 | 0.1177 | 1.13 | 0.13 | 0.1934 |
| SB2 | 8 | 0.71 | 0.07 | 0.83 | 0.09 | ||
| SB3 | 5 | 0.94 | 0.08 | 0.78 | 0.07 | ||
| SB4 | 8 | 0.99 | 0.15 | 1.20 | 0.10 | ||
| From the 540th day to the 570th day | From the 360th day to the 570th day | ||||||
| SB1 | 21 | 0.89 | 0.06 | 0.0245 * | 0.98 | 0.04 | 0.0002 *** |
| SB2 | 8 | 0.87 | 0.07 | SB2:SB4 * | 0.81 | 0.04 | SB2:SB1,SB4 * |
| SB3 | 5 | 1.04 | 0.09 | 0.73 | 0.06 | SB3: SB1,SB4 ** | |
| SB4 | 8 | 1.18 | 0.09 | 1.03 | 0.05 | ||
| Group | N | Mean | SE | p Value/ Significance |
|---|---|---|---|---|
| Age of the first insemination (days) | ||||
| RS | 18 | 427.3 | 3.9 | 0.0386 * |
| US | 16 | 412.7 | 5.3 | US:CR * |
| CR | 17 | 448.2 | 4.7 | |
| Live body weight at the first insemination (kg) | ||||
| RS | 18 | 402.4 | 6.9 | 0.8324 |
| US | 16 | 405.3 | 7.9 | |
| CR | 17 | 405.5 | 9.2 | |
| Age of the conception (days) | ||||
| RS | 18 | 452.1 | 5.9 | 0.0420 * |
| US | 16 | 441.3 | 8.8 | US:CR * |
| CR | 17 | 472.4 | 7.3 | |
| Live body weight at the conception (kg) | ||||
| RS | 18 | 422.7 | 11.2 | 0.6369 |
| US | 16 | 433.1 | 9.2 | |
| CR | 17 | 426.4 | 10.7 | |
| Number of services per conception | ||||
| RS | 18 | 1.39 | 0.15 | 0.8790 |
| US | 16 | 1.51 | 0.17 | |
| CR | 17 | 1.43 | 0.16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhrincat, M.; Broucek, J.; Hanus, A.; Kisac, P. Effect of Raising Dairy Heifers on Their Performance and Reproduction after 12 Months. Agriculture 2021, 11, 973. https://doi.org/10.3390/agriculture11100973
Uhrincat M, Broucek J, Hanus A, Kisac P. Effect of Raising Dairy Heifers on Their Performance and Reproduction after 12 Months. Agriculture. 2021; 11(10):973. https://doi.org/10.3390/agriculture11100973
Chicago/Turabian StyleUhrincat, Michal, Jan Broucek, Anton Hanus, and Peter Kisac. 2021. "Effect of Raising Dairy Heifers on Their Performance and Reproduction after 12 Months" Agriculture 11, no. 10: 973. https://doi.org/10.3390/agriculture11100973
APA StyleUhrincat, M., Broucek, J., Hanus, A., & Kisac, P. (2021). Effect of Raising Dairy Heifers on Their Performance and Reproduction after 12 Months. Agriculture, 11(10), 973. https://doi.org/10.3390/agriculture11100973







