1. Introduction
Among conservation agriculture practices, the introduction of perennials in crop rotations has been proposed as a viable opportunity to improve the long-term sustainability and productivity of systems thanks to the reduction in tillage, the protection of the soil surface, and the decrease in erosion and runoff. As a consequence, a considerable improvement in soil organic matter and nutrient cycling, as well as the overall physical and biological health of the soil, can be achieved. In this context, perennial medicinal and aromatic plants (MAPs) may represent interesting environmentally friendly crops for Mediterranean countries. In recent years, the attraction of MAPs as worthy farm crops has grown due to the demand created by consumer interest for these plants for culinary, medicinal, and other anthropogenic applications. Among MAPs,
Passiflora incarnata could represent an interesting crop for Mediterranean systems, due to its perennial cycle and its potential agronomic benefits.
Passiflora is a genus belonging to Passifloraceae’s family, consisting of more than 500 species, which mostly live in tropical and subtropical regions, except for
P. incarnata, which is native to temperate North America (southeast of the USA) and it has been introduced into Australasia, Bermuda, Europe, and Hawaii [
1].
P. incarnata (maypop) is mainly cultivated for its pharmaceutical and cosmetic properties. It was historically used as a sedative and anxiolytic plant and for the treatment of insomnia in North America; as an analgesic, anti-spasmodic, anti-asthmatic, wormicide, and sedative in Brazil; as a sedative and narcotic in Iraq; in diseased conditions like dysmenorrhea, epilepsy, insomnia, neurosis, and neuralgia in Turkey; to cure hysteria and neurasthenia in Poland; and for morphine deaddiction in the traditional system of medicine in India [
2,
3,
4,
5].
P. incarnata contains, in its leaves, flavonoids (mainly C-glycosides of apigenin and luteolin), which probably are responsible of the pharmacological effects, and alkaloids—based on the β-carboline ring system, namely harmane, harmol, harmine, harmalol, and harmaline. The main active ingredients include chrysin, vitexin and isovitexin, schaftoside, isoschaftoside, coumerin, and umbelliferone, with considerable variation in qualitative and quantitative composition according to the source [
6,
7,
8,
9]. The presence of gynocardin (a cyanogenic glycoside) and essential oil in traces, comprising more than 150 components, have been also revealed [
6,
7,
8]. Various other constituents have been identified, including γ-benzo-pyrone derivative maltol, carbohydrates such as raffinose, sucrose, D-glucose, and D-fructose [
10].
P. incarnata is successfully cultivated in Florida, Guatemala, and Italy. In Italy,
P. incarnata is grown mostly in the central regions of the country, where it behaves as perennial spring–summer crop with a stand duration of 5–7 years [
11]. In winter, the aerial part of the plant dies and, at the beginning of springtime, there is the vegetative upturn from the bottom of the plant.
P. incarnata is one of the most important medicinal plants in Italy, where it is cultivated on a total area of approximately 150–180 hectares, of which 50 ha operate under organic farming conditions, with a production of 800–1000 tons/year. The main problem in its large-scale cultivation is the poor seed quality, with erratic and low seed germination, due to its apparent pronounced seed dormancy. This makes it difficult to grow
P. incarnata crops from seeds, so the nursery reproduction is generally carried out by cuttings, with a substantial increase in the cultivation costs. Little is known about the seed germination behavior of
Passiflora species, and no information is reported in the “International Rules for Seed Testing” [
12] regarding minimum germination requirements or optimal conditions for germination. Dormancy and germination rates are factors that can control the number of progenies a plant can make, and they are variable both in space and time, with large environmental effects [
13]. Several studies seem to have confirmed that
Passiflora spp. have exogenous dormancy due to a combination of both mechanical and chemical factors. De Oliveira et al. [
14] and Torres [
15] highlighted that the semi-domesticated passionfruit have strong dormancy effects. However, the presence of other kinds of dormancy cannot be excluded, depending on the species, such as a physical dormancy due to the impermeable seed coat or even a physiological dormancy. Although for some
Passiflora species, a combination of physical and physiological dormancy has been highlighted, studies regarding
P. incarnata are very limited and not conclusive. In order to remove seed dormancy in
P. incarnata, mechanical scarification was the widely used method; nevertheless, it never gave satisfactory results with potential risk of embryo damaging [
16]. On the contrary, chemical scarification appeared to be more effective in overpassing dormancy in this species, but an extended period of soaking could reduce seed viability and germination rate [
17]. Furthermore, among pre-germination treatments tested on
P. incarnata seeds, different light and temperature conditions have been tested [
18,
19], highlighting that the seed negative photoblasty of this species increased at suboptimal temperature. To the best of our knowledge, pre-chilling treatment on
Passiflora spp. has been never tested before, even though it is a well-known procedure for some medicinal and aromatic plants [
20].
Therefore, with the objective to improve knowledge about seed germination and dormancy mechanisms of P. incarnata for enhancing nursery production, this work aimed to discover better and appropriate pre-germination treatments for overpassing seed dormancy and enhancing seed germination rates. For this purpose, the responses of seed lots of three P. incarnata accessions grown in 2016 in Central Italy with different treatments (pre-chilling, GA3, leaching, scarification, non-treated control), different light or darkness exposure, and different temperature conditions (25, 30, and 35 °C constant temperatures and 20–30 °C alternating temperatures) have been examined.
2. Materials and Methods
2.1. Plant Materials
The experiments were carried out at the Seed Research and Testing Laboratory of the Department of Agriculture, Food, and Environment (DAFE) of the University of Pisa.
The seeds of three P. incarnata accessions, namely F2016, FF2016, and A2016, were kindly supplied by F.I.P.P.O. (Federazione Italiana Produttori Piante Officinali) and by Aboca s.r.l. company (Sansepolcro, Arezzo, Italy). Mature fruits were collected during 2016 from plants grown in an open field in Central Italy (Tuscan-Umbrian, Val Tiberina, Italy), under the same pedo-climatic conditions and with an organic management system. The planting had been carried out in 2014 by transplanting the seedlings on a clay loamy soil. The crop was carried out without irrigation since rainfall in the area was able to satisfy crop water requirements.
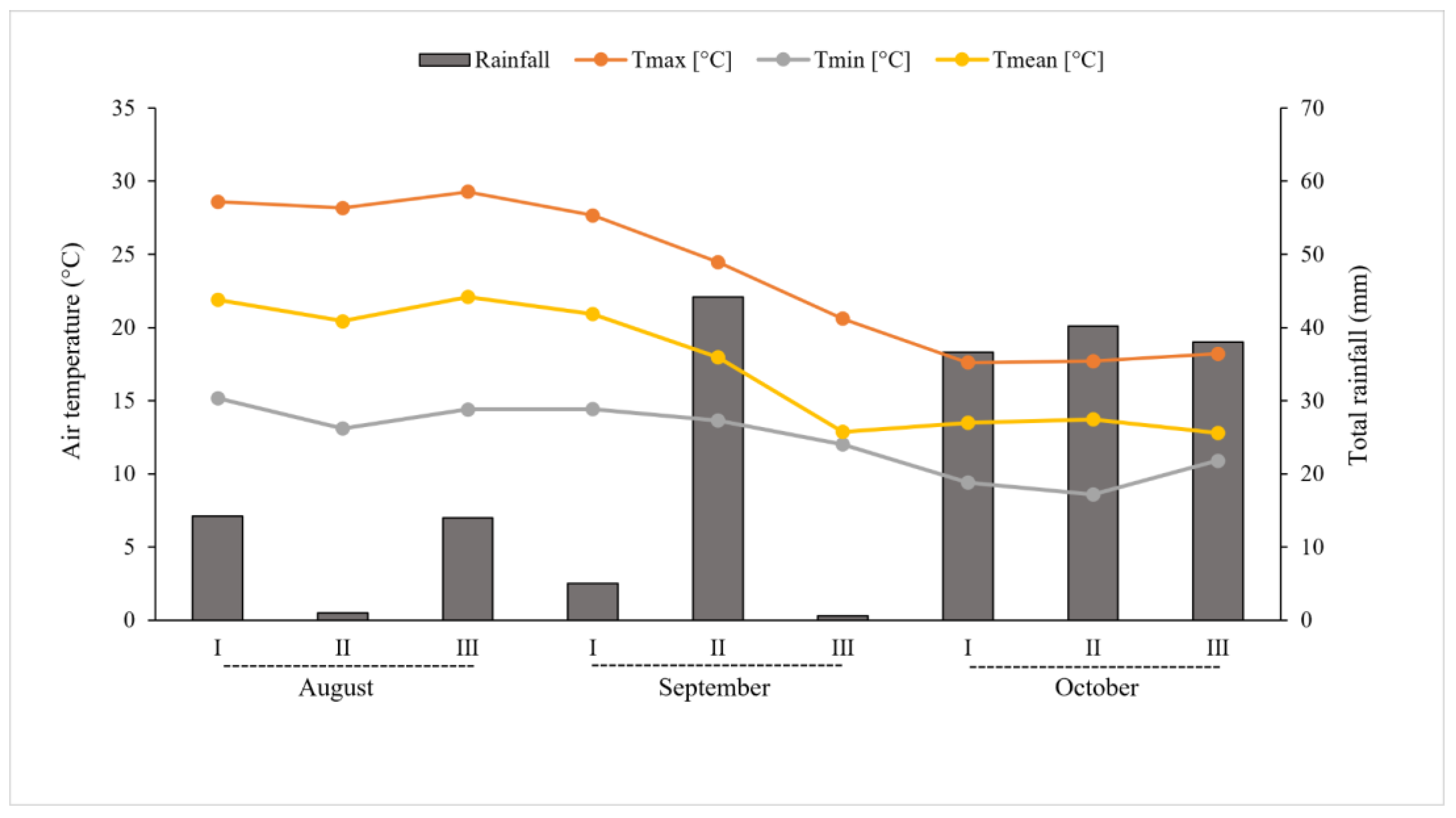
During the growing period, between the flowering and ripening stages, until fruit harvesting (from August to October 2016), rainfall and temperatures (maximum and minimum air temperature) were recorded using a weather station located nearby the cultivation area (
Figure 1).
2.2. Pre-Germination Treatments
Fruits were harvested and soaked in tap water for a couple of days until maceration occurred. At the end of maceration process, the seeds were separated from the pulp, washed with tap water at room temperature, air dried, and cleaned with sieves and flows of air (
Figure 2). Subsequently, seeds were stored in darkness at 4–5 °C and 60% relative humidity for 6 months.
Different pre-germination treatments in a completely random block design have been examined. In detail, the treatments were: (i) pre-chilling, (ii) Gibberellic acid (GA3), (iii) leaching, (iv) scarification, and (v) non-treated control. For each of these treatments, different light (or darkness) and temperature conditions (25, 30, and 35 °C constant temperatures and 20–30 °C alternating temperatures) were also examined. For each P. incarnata accession, four replications of 50 seeds each for every pre-germination treatment and for the control, were used.
The seeds were placed in 12 cm Petri dishes and incubated in climatic cabinets. Preliminary tetrazolium tests, according to the International Seed Testing Association (ISTA) [
12], were conducted to estimate the seed viability of each accession.
For pre-chilling treatment, seeds in groups of 25 were placed in Petri dishes between two sheets of filter paper and moistened with 5 mL of tap water, then they were put in a refrigerator at 4–5 °C for 4 days. The hormone treatment was conducted using GA3 (200 ppm or 0.2 g/L) prepared starting from gibberellic acid, 90% gibberellin A3 basis (TLC) (Fluka Biochemika, Buchs, Switzerland). Seeds were placed between two sheets of filter paper, moistened with 5 mL GA3 solution. The third treatment consisted of seed leaching in tap water for 8 h, which were then put in Petri dishes between two sheets of filter paper moistened with only 3 mL of tap water, because they were already partially imbibed. Finally, mechanical scarification was carried out by rubbing seeds manually on sandpaper to damage the hard outer layers, after which they were put in Petri dishes between two sheets of filter paper moistened with 5 mL of tap water. The dishes were put in climatized cabinets (Officine Meccaniche K.W. Mod. 1040, Siena, Italy) at different temperature and light conditions: 25, 30, and 35 °C, both in complete light or dark conditions or under 20–30 °C alternating temperature with a photoperiod of 16–8 h and 8–16 h. The light was provided by cool white-light fluorescent lamps Osram L18 W/20 (10 µmol photons s−1 m−2 photosynthetically active radiation) (Osram GmbH, München, Germany).
2.3. Germination Test and Measurements
Prior to the germination test, thousand seed weight, for each accession, was assessed according to ISTA [
12]. Germination was monitored every two or three days up to 30 days as a function of temperature. Germination ended with the appearance of cotyledons. Germinated seeds were counted, and germination counts were stopped when final germination percentages were reached.
Germination percentage (G %) and mean of germination time (MGT) were calculated according to following equations:
- G (%) = SNG/SNO × 100; where SNG is the number of germinated seeds and SNO is the number of experimental seeds with viability, respectively.
- MGT = Σ (n × d)/N, where n = number of germinated seeds per day; d = number of days needed for germination, and N = total number of germinated seeds.
2.4. Statistical Analyses
Data of both seed germination and MGT tests were subjected to an analysis of variance (ANOVA) using the statistical software Costat Cohort V6.201 (CoHort Software, Monterey, CA, USA). For each accession, the effect of pre-treatments (A, i.e., prechilling, GA
3, leaching, scarification, and control) and the temperature/light treatments (B, i.e., different constant and alternating temperatures and light/dark conditions) and their reciprocal interactions (A × B) were analyzed by two-way ANOVA. Means were separated on the basis of a least significant difference (LSD) test only when the ANOVA F-test per treatment was significant at the 0.05 probability level [
21]. For germination percentage, data arcsin transformation [(x + 0.5)/n]
1/2 was performed before variance analysis.
3. Results
The 1000 seeds weights (TSW), evaluated before starting the experiment, are reported in
Table 1. TSW significantly varied depending on accession, with the highest value reached by F2016, followed by A2016 and, finally, FF2016.
The main results of the two-way ANOVA, performed to assess the effects of pre-germination treatments (A), temperature/light (T&L) exposure (B), and A × B interaction on germination percentage and MGT, are reported in
Table 2. The results showed that germination percentage was significantly affected by pre-germination treatments and T&L exposure, as well as by their reciprocal interactions, in all
P. incarnata accessions. On the other hand, pre-germination treatments did not show any significant effect on MGT values of any accession. T&L exposure and the interaction between the two variability factors, instead, played a key role in affecting mean germination time in all three accessions.
In
Table 3,
Table 4 and
Table 5, the differences in germination percentage, separately for each
P. incarnata accession, are reported. Regarding the effect of temperature and light/dark (L/D) conditions, no germination was achieved for the control at 25 °C both in light and dark conditions, confirming that this temperature value represents, for maypop, the below threshold for germination. In F2016 accession (
Table 3), the highest germination rate was achieved at 35 °C/D, while the lowest one was observed at 25 °C/L. Considering the effect of pre-germination treatments, significantly higher germination percentages were registered with prechilling, GA
3, and leaching in comparison with the control. Scarification worsened germination rate, with values equal to control. Taking into account the effect of AxB interaction, interestingly, the best conditions were found at 35 °C/D in control and after prechilling, GA
3, and leaching. It is important to note that, in all other conditions (25 °C/D; 30 °C/D&L; 35 °C/L), the pre-germination treatments significantly increased the germination rates in comparison with the control, except for scarification.
For FF2016 accession (
Table 4), the highest germination was obtained under both 35 °C/D and 30 °C/D, while, as observed for F2016, the lowest germination occurred by adopting the 25 °C/L condition. Differently to what was observed for F2016 accession, all the pre-germination treatments significantly enhanced the germination percentage in FF2016 seeds. Considering AxB interaction, a significant improvement of germination, going from the control to pre-chilling under alternating temperatures of 20/30 °C (both photoperiods), as well as under 35 °C/L, was observed (
Table 4). Furthermore, the combination between leaching and 35 °C/D conditions provided the highest germination percentage, followed by the seeds subjected to GA
3 and scarification treatments under the same T&L conditions. For A2016 accession (
Table 5), a similar trend as described for FF2016 was detected. In fact, under 35 °C/D, the best germination conditions occurred, followed by 30 °C both in light and dark. On the contrary, as observed for the other accessions, under 25 °C (light and dark), the worst germination values were recorded. Once again, considering the AxB interaction, the best conditions able to enhance germination percentage were due to the combination of 20/30 °C 16/8 h and prechilling, and the combination of 35 °C/D and leaching.
All these findings revealed that, among accessions, the untreated/control seeds of F2016 had the highest germination rate (germination percentage up to 90%) when exposed to 35 °C under dark conditions. This behavior confirmed that, for
P. incarnata, the optimal germination can be achieved at 35 °C in the dark. In such conditions, the untreated seeds of FF2016 and A2016 achieved lower germination percentages (around to 60%) than F2016. The higher values observed for control seeds of F2016 were expected on the basis of 1000-seed weight (
Table 1). In FF2016 and A2016 accessions, pre-germination treatments were absolutely necessary in order to improve the germination process.
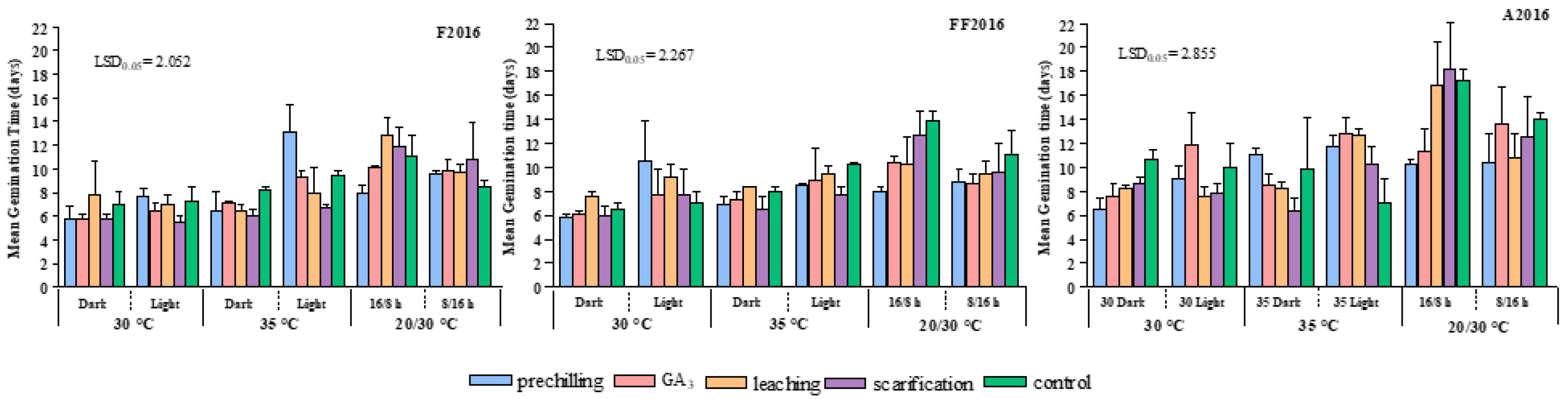
MGT values showed that germination peaks usually occurred within two weeks. Beyond this time, seeds sporadically sprouted. As a general trend, the time required for germination (
Figure 3) decreased progressively from light to dark conditions, depending on pre-germination treatments, accession, and temperature. In F2016, this behavior was particularly evident under 35 °C, while no differences were observed between light and dark at 30 °C. Conversely, in FF2016 and A2016, a strong decrease in MGT was observed from light to dark, both at 30 °C and 35 °C. MGT lasted roughly ten days in light conditions (as mean value among accessions and pre-germination treatments) but fell to about one week in dark conditions.
On the contrary, alternating temperature (20/30 °C) did not improve germination energy, except when combined with prechilling. In fact, the interaction “pre-chilling × 20/30 °C (16/8 h)” conditions resulted in MGT reduction in comparison with the control (−200%). Alternating 20/30 °C temperatures, not associated with pre-chilling, gave the longer germination times, as well as 35 °C under light conditions. Furthermore, in all accessions, the adoption of an 8/16 h photoperiod significantly decreased MGT only in the control and in seeds subjected to scarification and leaching in comparison with a 16/8 h photoperiod.
Finally, considering the average values over the three accessions, all the pre-germination treatments generally stimulated a faster germination compared to control. Among the tested treatments, scarification seemed to lead to a quick germination process, even if germination rate was not elevated.
4. Discussion
In the effort to improve and promote the cultivation of
P. incarnata, the effects of temperature, dark/light conditions, and pre-germination treatments on the germination percentage and MGT of its seeds were investigated. The 1000 seeds weight (TSW) was also evaluated as it represents one of the most important components in determining seed quality. In fact, it is generally reported that seed germinability is positively related to seed mass. Larger seeds germinated to a higher percentage thanks to their greater reserves, which enable a greater tolerance to a range of hazards, including shade, drought, and physical damage [
22,
23,
24].
Definitively, the obtained results confirmed that
P. incarnata seeds are photoblastically negative and have pronounced heat requirements for germination. Optimal germination percentages, in fact, were achieved with 35 °C in darkness, for each accession. In such conditions, a significant and strong decline in MGT was also detected, confirming the tropical origins of this species [
25]. On the contrary, very low values were observed at 25 °C, more pronounced under light conditions, for each pre-germination treatment and seed accession. Data showed a significant interaction between complete light/dark exposition and temperatures, underlining the fact that the light exposition has an inhibitory effect on the germination of
P. incarnata seeds. Among pre-germination treatments, pre-chilling, GA
3, and leaching appeared to be the most effective in enhancing normal seedling germination. Only for A2016, scarification gave similar results to pre-chilling, GA
3, and leaching treatments. On the contrary, in the other two accessions, under scarification, the dead seeds percentage considerably increased, probably due to embryo damaging. A significant interaction between pre-chilling and temperature was observed with significantly higher germination values than control (+330%) at 20/30 °C (16/8 h).
Previous studies investigated the effect of different combinations of light and different temperature regimes with the aim to improve seed germination in
P. incarnata. In this regard, Benvenuti et al. [
18] tested combinations of white light (or darkness) and temperature (20, 25, 30, 35, and 40 °C), or subjected
P. incarnata seeds to different sequences of light treatments (succession of red and far-red light, with 5 min each one) after 12 h of dark incubation at 30 °C. These authors found that the germination threshold was surprisingly high, both in darkness and light conditions (25.4 °C and 23.9 °C, respectively), while no germination was observed at 20 °C. In addition, these authors observed that a suboptimal temperature (lower than 35 °C) and far/far-red light both produced extremely low levels of germination (around 5%). Zucarelli et al. [
19] investigated the effect of alternate temperatures of 20–30 °C and 30–20 °C for periods of 16 and 8 h, simulating the photoperiod and highlighting that alternating temperatures of 30–20 °C promoted the highest germination rates. The results of these studies also demonstrated that high temperatures progressively decreased the time required for germination, independently from light conditions. Similarly, in our study, MGT decreased as result of the combination of high temperatures (35 °C) and dark conditions, regardless of the pre-germination treatment.
In the literature, several studies have underlined the presence of dormancy in
Passiflora spp. [
16,
26] and, specifically in
P. incarnata, a combination of physical and physiological dormancy has been detected [
27]. In general, all seed pre-germination treatments led to enhanced water and oxygen exchange across seed coat layers. However, scarification did not have the best result, as was supposed to be the case with physical dormancy. Otherwise, results obtained with GA
3 and leaching seem to confirm a possible physiological dormancy in
P. incarnata, as hormones and inhibitors were stimulated/removed, respectively.
Interestingly, our study, for the first time, pointed out that pre-chilling is an efficient treatment to improve germination in
P. incarnata seeds. Pre-chilling enhanced germination as well, probably because this pre-treatment stimulated a variation in abscisic acid/gibberellic acid rate (ABA/GA
3) and free gibberellins biosynthesis [
28]. Macchia et al. [
29] found that prechilling for 7–15 days in light or in darkness hardly affected percentage germination of
Echinacea angustifolia seeds, but significantly increased the rate of germination. On the contrary, GA
3 treatment was not useful for this species.
The time conclusively required for seed germination decreased progressively with increasing temperatures, but only under dark conditions, while in complete light conditions, no variation was observed and MGT values remained almost constant with increasing temperatures. Optimal germination times were achieved at 35 °C in dark conditions. Similarly to germination percentages, even in MGT, alternating temperature (20/30 °C) did not improve germination energy, except when combined with pre-chilling treatment.