Soil Health Impacts of Rubber Farming: The Implication of Conversion of Degraded Natural Forests into Monoculture Plantations
Abstract
1. Introduction
2. Methods
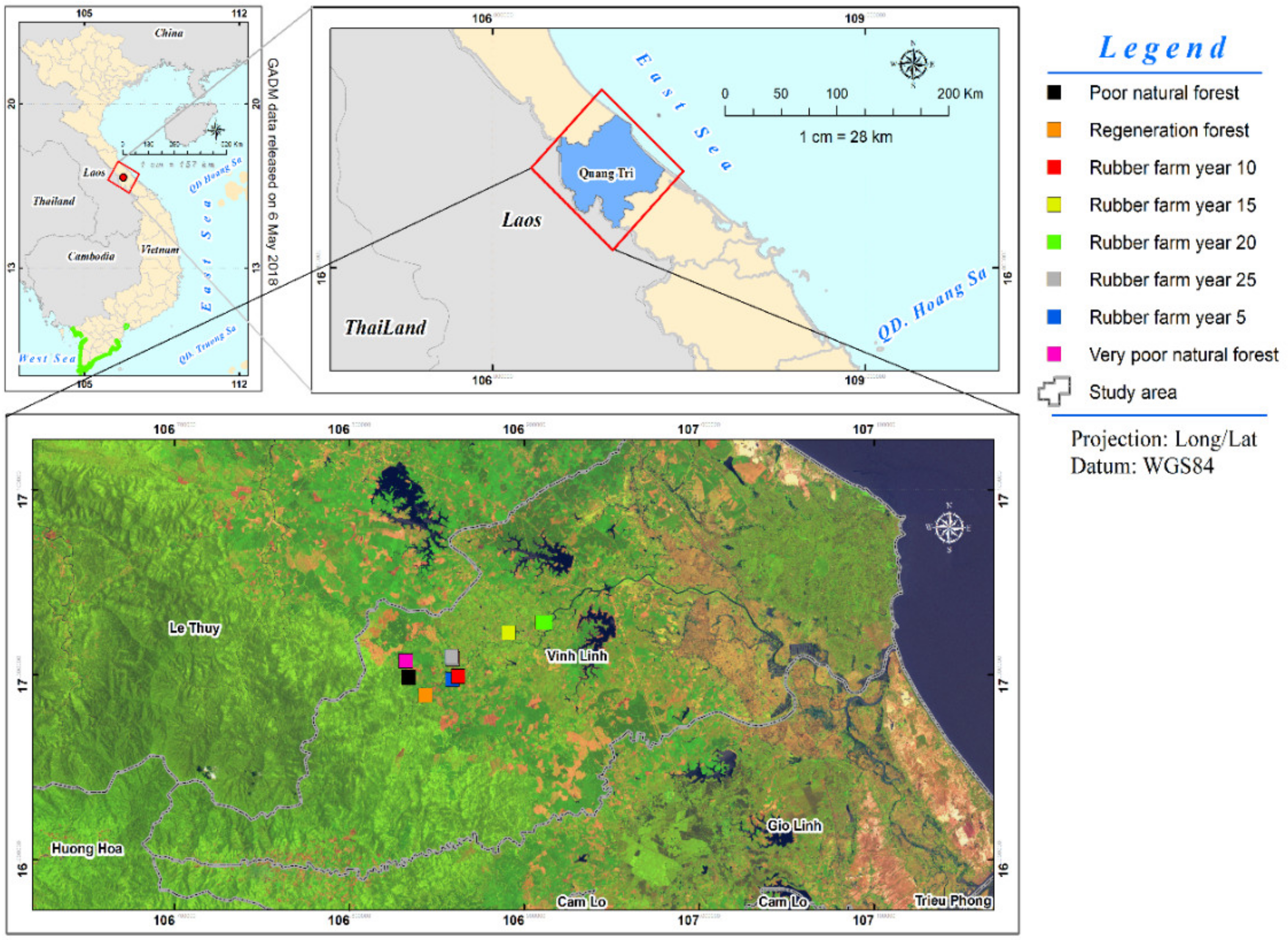
2.1. Study Area
2.2. Field Data Collection and Measurement of Soil Parameters
2.3. Statistical Analyses
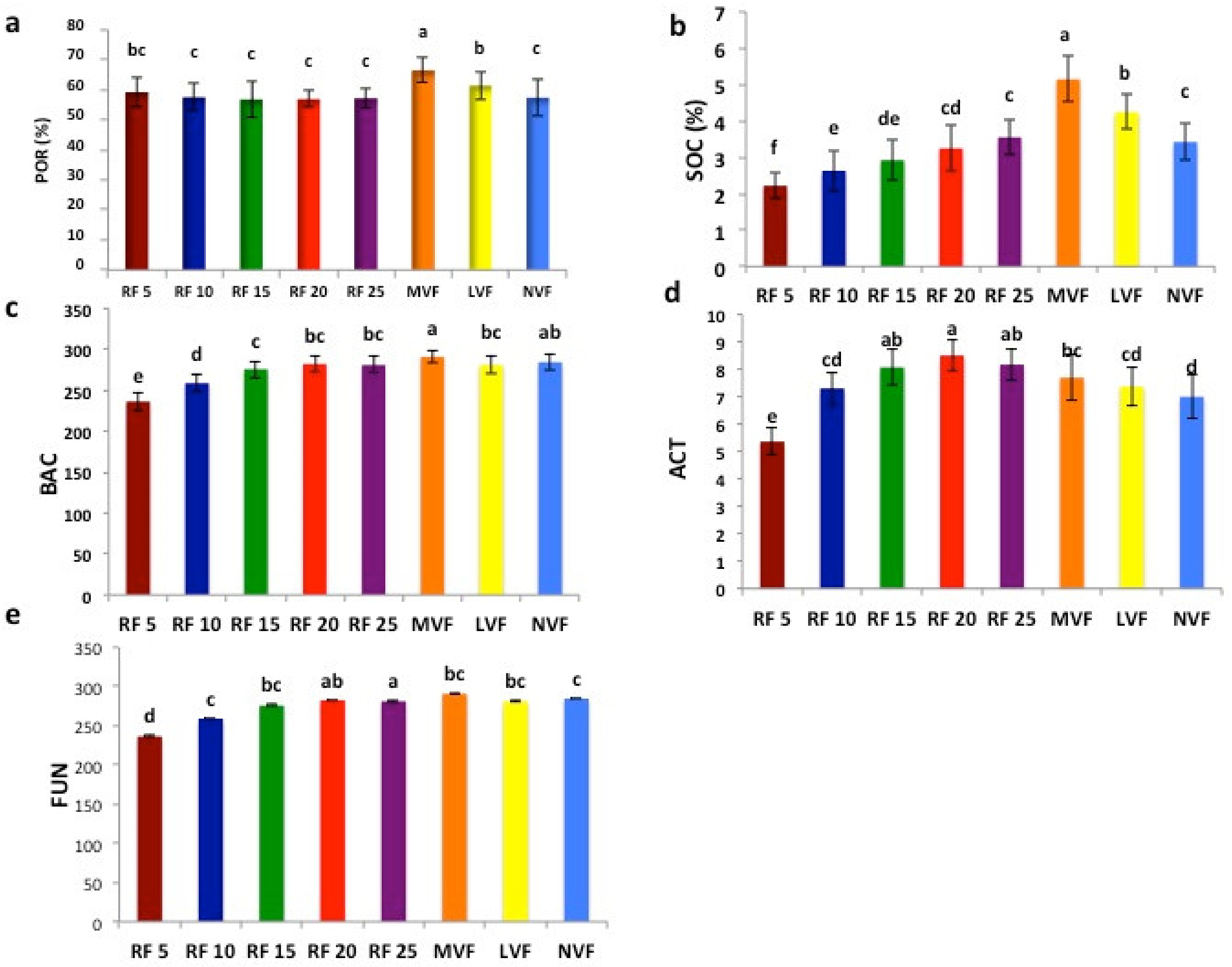
3. Results
4. Discussion
4.1. Variation in Soil Health Parameters by Vegetation Types
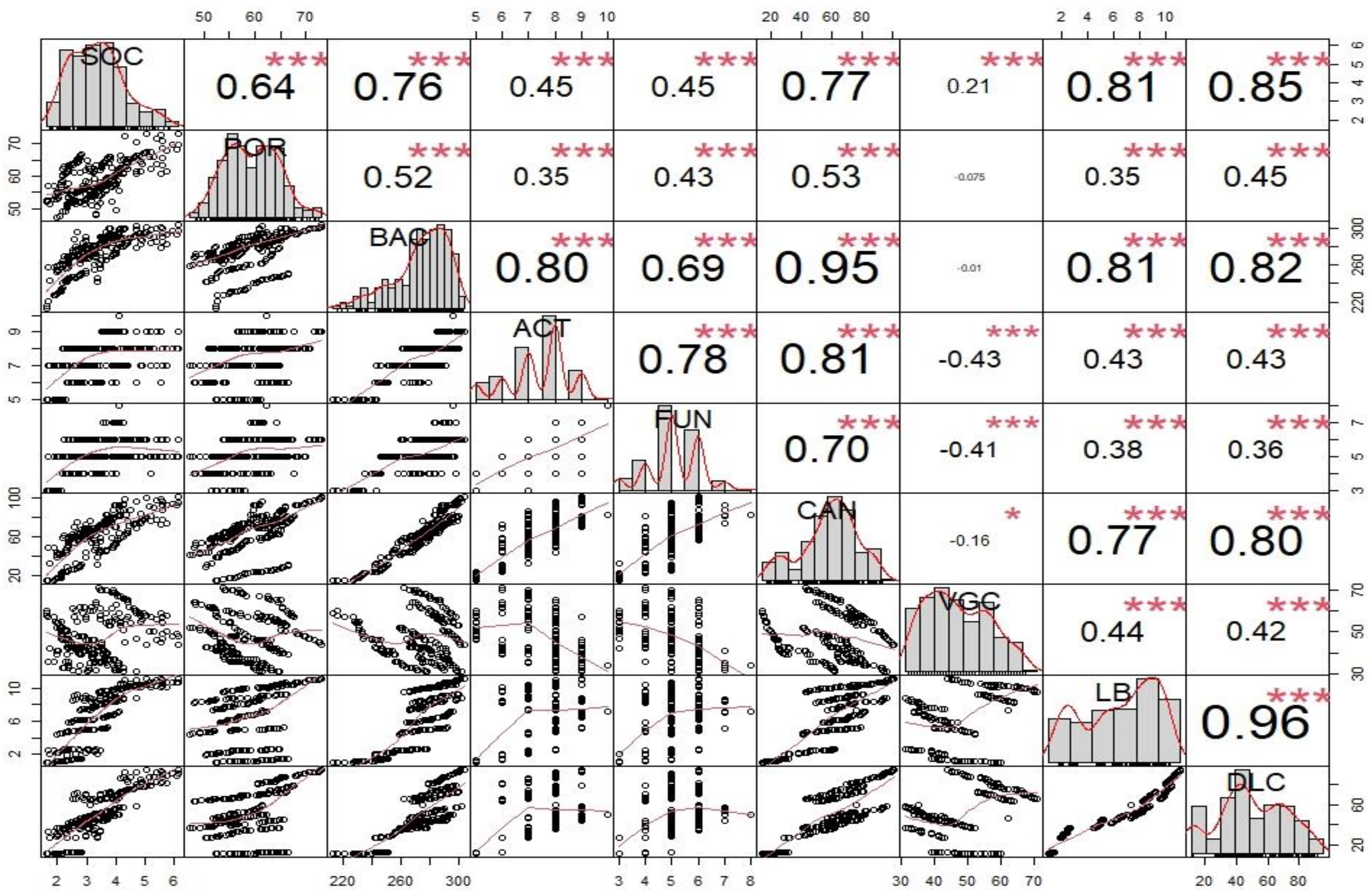
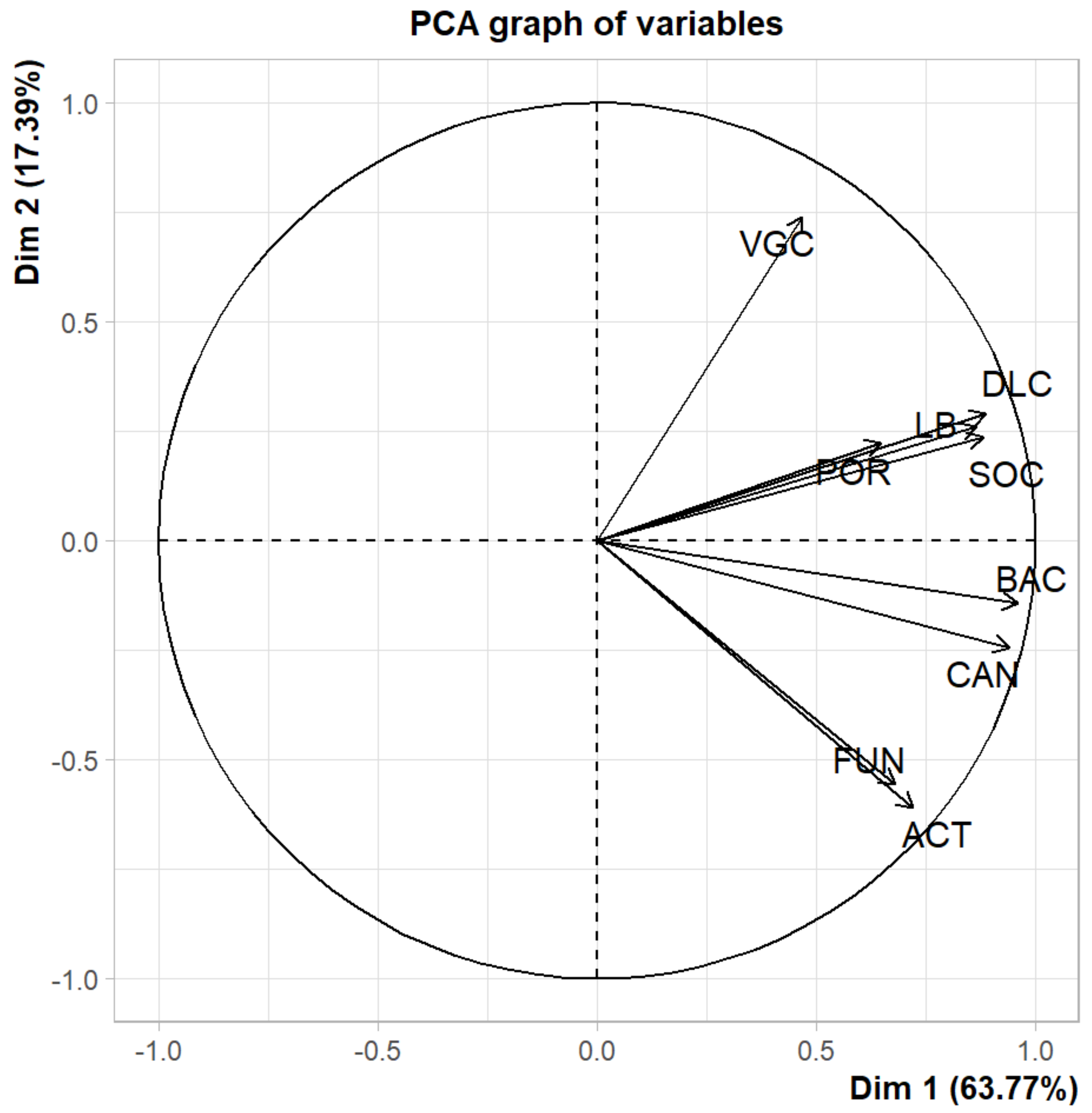
4.2. Correlations between Soil Health and Vegetation Structure Parameters
4.3. Implications of Land Conversion Policy and Land Management Practices
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arjunan, T.R.J.; Radha, G.K.; Karumankandathil, R.; Sankaran, S.; Parukuttiyamma, K.J.; Sreedharan, S.K.; Alikunju, S.; Perumal, V. Rubber Tree. In Compendium of Transgenic Crop Plants; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2019; pp. 117–139. [Google Scholar]
- Guo, Z.; Zhang, Y.; Deegen, P.; Uibrig, H. Economic Analyses of Rubber and Tea Plantations and Rubber-tea Intercropping in Hainan, China. Agrofor. Syst. 2006, 66, 117–127. [Google Scholar] [CrossRef]
- Antje, A.P.; Hollingsworth, M.H.; Alan, D.Z.; Jefferson, M.F.; Huafang, C.; Yufang, S.; Jianchu, X. Current trends of rubber plantation expansion may threaten biodiversity and livelihoods. Glob. Environ. Chang. 2015, 34, 48–58. [Google Scholar]
- N’Dri, J.K.; Guéi, A.M.; Edoukou, E.F.; Yéo, J.G.; N’Guessan, K.; Lagerlöf, J. Can litter production and litter decomposition improve soil properties in the rubber plantations of different ages in Côte d’Ivoire? Nutr. Cycl. Agroecosyst. 2018, 111, 203–215. [Google Scholar] [CrossRef]
- Blagodatsky, S.; Xu, J.C.; Cadisch, G. Carbon balance of rubber (Hevea brasiliensis) plantations: A review of uncertainties at plot, landscape and production level. Agric. Ecosyst. Environ. 2016, 221, 8–19. [Google Scholar] [CrossRef]
- Aso, M. Rubber and the Making of Vietnam: An Ecological History, 1897–1975; The University of North Carolina Press: Chapel Hill, NC, USA, 2018; pp. 1–426. [Google Scholar]
- Phuc, T.X.; Nghi, T.H. Rubber Expansion and Forest Protection in Vietnam; Tropenbos International Viet Nam: Hue, Vietnam, 2014. [Google Scholar]
- Ministry of Agriculture and Rural Development (MARD). Circular 127/2008/TT-BNN Dated 31 December 2008 Providing Guidance on Planting Rubber Trees on Forestland; Ministry of Agriculture and Rural Development (MARD): Ha Noi, Vietnam, 2008.
- The Government of Vietnam (GOV). Decision No. 750/QD-TTg of June 3, 2009, Approving the Planning on Development of Rubber Tree up to 2015, with a Vision toward 2020; The Government of Vietnam, Ed.; The Government of Vietnam (GOV): Ha Noi, Vietnam, 2009.
- Ministry of Agriculture and Rural Development (MARD). Emission Reductions Program Document (ER-PD); Ministry of Agriculture and Rural Development (MARD): Ha Noi, Vietnam, 2017.
- Schneider, D.; Engelhaupt, M.; Allen, K.; Kurniawan, S.; Heinemann, M.; Krashevska, V.; Nacke, H.; Wijayanti, M.; Meryandini, A.; Corre, M.D.; et al. Impact of Lowland Rainforest Transformation on Diversity and Composition of Soil Prokaryotic Communities in Sumatra (Indonesia). Front. Microbiol. 2015, 6, 1339. [Google Scholar] [CrossRef]
- Singh, D.; Ferry Slik, J.W.; Jeon, Y.S.; Tomlinson, K.W.; Yang, X.; Kerfahi, D.; Porazinska, D.L.; Adams, J.M. Tropical forest conversion to rubber plantation affects soil micro- & mesofaunal community & diversity. Sci. Rep. 2019, 9, 5893. [Google Scholar]
- Pauli, N.; Donough, C.R.; Oberthür, T.; Cock, J.H.; Verdooren, R.; Rahmadsyah; Abdurrohim, R.; Indrasuara, K.; Lubis, A.; Dolong, T.; et al. Changes in soil quality indicators under oil palm plantations following application of ‘best management practices’ in a four-year field trial. Agric. Ecosyst. Environ. 2014, 195, 98–111. [Google Scholar] [CrossRef]
- Velmourougane, K. Impact of Organic and Conventional Systems of Coffee Farming on Soil Properties and Culturable Microbial Diversity. Scientifica 2016, 2016, 3604026. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.M.; Cardoso, I.M.; Bianchi, F.J.J.A.; da Cruz Silva, A.; Jamme, D.; Peña-Claros, M. Linking vegetation and soil functions during secondary forest succession in the Atlantic forest. For. Ecol. Manag. 2020, 457, 117696. [Google Scholar] [CrossRef]
- Peerawat, M.; Blaud, A.; Trap, J.; Chevallier, T.; Pascal, A.; Gay, F.; Thaler, P.; Spor, A.; Sebag, D.; Choosai, C.; et al. Rubber plantation ageing controls soil biodiversity after land conversion from cassava. Agric. Ecosyst. Environ. 2018, 257, 92–102. [Google Scholar] [CrossRef]
- Allen, K.; Corre, M.D.; Tjoa, A.; Veldkamp, E. Soil Nitrogen-Cycling Responses to Conversion of Lowland Forests to Oil Palm and Rubber Plantations in Sumatra, Indonesia. PLoS ONE 2015, 10, e0133325. [Google Scholar] [CrossRef]
- Kotowska, M.M.; Leuschner, C.; Triadiati, T.; Meriem, S.; Hertel, D. Quantifying above- and belowground biomass carbon loss with forest conversion in tropical lowlands of Sumatra (Indonesia). Glob. Chang. Biol. 2015, 21, 3620–3634. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, T.; Holtkamp, A.M.; Muhammad, D.; Brümmer, B.; Kuzyakov, Y. Soil degradation in oil palm and rubber plantations under land resource scarcity. Agric. Ecosyst. Environ. 2016, 232, 110–118. [Google Scholar] [CrossRef]
- Chandra, L.R.; Gupta, S.; Pande, V.; Singh, N. Impact of forest vegetation on soil characteristics: A correlation between soil biological and physico-chemical properties. 3 Biotech 2016, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- QuynhAnh, P.T.; Gomi, T.; MacDonald, L.; Mizugaki, S.; Van Khoa, P.; Furuichi, T. Linkages among land use, macronutrient levels, and soil erosion in northern Vietnam: A plot-scale study. Geoderma 2014, 232–234, 352–362. [Google Scholar]
- Trivedi, P.; Delgado-Baquerizo, M.; Anderson, I.C.; Singh, B.K. Response of Soil Properties and Microbial Communities to Agriculture: Implications for Primary Productivity and Soil Health Indicators. Front. Plant Sci. 2016, 7, 990. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Stratification of Soil Porosity and Organic Matter. In Encyclopedia of Agrophysics; Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 858–861. [Google Scholar]
- Dexter, A.R. Soil physical quality: Part I. Theory, effects of soil texture, density, and organic matter, and effects on root growth. Geoderma 2004, 120, 201–214. [Google Scholar]
- Biancalani, R.; Petri, M.; Bunning, S.; Salvatore, M.; Tubiello, F.N. The use of soil organic carbon as an indicator of soil degradation. Energ. Ambiente e Innov. 4 2012, 5, 73–78. [Google Scholar]
- Izquierdo, I.; Caravaca, F.; Alguacil, M.M.; Hernández, G.; Roldán, A. Use of Microbiological Indicators for Evaluating Success in Soil Restoration after Revegetation of a Mining Area under Subtropical Conditions. Appl. Soil Ecol. 2005, 30, 3–10. [Google Scholar] [CrossRef]
- Masto, R.E.; Chhinkar, P.K.; Singh, D.; Patra, A.K. Changes in soil quality indicators under long-term sewage irrigation in a sub-tropical environment. Environ. Geol. 2009, 56, 1237–1243. [Google Scholar] [CrossRef]
- Bennett, L.T.; Mele, P.M.; Annett, S.; Kassel, S. Examining links between soil management, soil health, and public benefits in agricultural landscapes: An Australian perspective. Agric. Ecosyst. Environ. 2010, 139, 1–12. [Google Scholar]
- Rodrigues, J.L.M.; Pellizari, V.H.; Mueller, R.; Baek, K.; da C Jesus, E.; Paula, F.S.; Paula, F.S.; Hamaoui, B.M., Jr.; Tsai, S.M.; Feigl, B.; et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar]
- Angers, D.A.; Caron, J. Plant-Induced Changes in Soil Structure: Processes and Feedbacks. In Plant-Induced Soil Changes: Processes and Feedbacks; Van Breemen, N., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 55–72. [Google Scholar]
- Lal, R. Soil Carbon Sequestration Impacts on Global Climate Change and Food Security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Quang Tri online. Vinh Linh: Rubber Production in 2018 Reached Nearly 8.000 tons 2019. Available online: http://baoquangtri.vn/Th%C6%A1%CC%80i-s%C6%B0%CC%A3/modid/445/ItemID/136590 (accessed on 1 May 2020).
- Ministry of Agriculture and Rural Development (MARD). Circular 34/2009/TT-BNNPTNT Dated 10 June 2009 on Criteria of Forest Classification and Identification; Ministry of Agriculture and Rural Development (MARD): Ha Noi, Vietnam, 2009.
- Lemmon, P.E. A Spherical Densiometer For Estimating Forest Overstory Density. For. Sci. 1956, 2, 314–320. [Google Scholar]
- Canfield, R.H. Application of the Line Interception Method in Sampling Range Vegetation. J. For. 1941, 39, 388–394. [Google Scholar]
- Grossman, R.B.; Reinsch, T.G. 2.1 Bulk Density and Linear Extensibility. In Methods of Soil Analysis; Soil Science Society of America: Madison, WI, USA, 2018; pp. 201–228. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Susan, I.; Jennings, D. Microbial Culture; Bios Scientific: London, UK, 1995. [Google Scholar]
- Wotling, G.; Bouvier, C.; Danloux, J.; Fritsch, J.-M. Regionalization of extreme precipitation distribution using the principal components of the topographical environment. J. Hydrol. 2000, 233, 86–101. [Google Scholar] [CrossRef]
- Team, R.C. R: A language and Environment for Statistical Computing Computer Program, version 3.6.1; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Soong, J.L.; Janssens, I.A.; Grau, O.; Margalef, O.; Stahl, C.; Van Langenhove, L.; Urbina, I.; Chave, J.; Dourdain, A.; Ferry, B.; et al. Soil properties explain tree growth and mortality, but not biomass, across phosphorus-depleted tropical forests. Sci. Rep. 2020, 10, 2302. [Google Scholar] [CrossRef]
- Tardy, V.; Spor, A.; Mathieu, O.; Eque, J.; Terrat, S.; Plassart, P.; Regnier, T.; Bardgett, R.; Putten, W.; Roggero, P.P.; et al. Shifts in microbial diversity through land use intensity as drivers of carbon mineralization in soil. Soil Biol. Biochem. 2015, 90, 204–213. [Google Scholar] [CrossRef]
- Bouchez, T.; Blieux, A.L.; Dequiedt, S.; Domaizon, I.; Dufresne, A.; Ferreira, S.; Godon, J.J.; Hellal, J.; Joulian, C.; Quaiser, A.; et al. Molecular microbiology methods for environmental diagnosis. Environ. Chem. Lett. 2016, 14, 423–441. [Google Scholar] [CrossRef]
- Hill, P.; Krištůfek, V.; Dijkhuizen, L.; Boddy, C.; Kroetsch, D.; Van Elsas, J.D. Land use intensity controls actinobacterial community structure. Microb. Ecol. 2011, 61, 286–302. [Google Scholar] [CrossRef] [PubMed]
- Shange, R.S.; Ankumah, R.O.; Ibekwe, A.M.; Zabawa, R.; Dowd, S.E. Distinct soil bacterial communities revealed under a diversely managed agroecosystem. PLoS ONE 2012, 7, e40338. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, W.-B.; Luo, X.; Wu, X. Variations in rhizosphere microbial communities of rubber plantations in Hainan island China. J. Rubber Res. 2013, 16, 243–256. [Google Scholar]
- de Blécourt, M.; Hänsel, V.M.; Brumme, R.; Corre, M.D.; Veldkamp, E. Soil redistribution by terracing alleviates soil organic carbon losses caused by forest conversion to rubber plantation. For. Ecol. Manag. 2014, 313, 26–33. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Li, J.-H.; Ross Friedman, C.; Wang, H. Variation of Soil Bacterial Communities in a Chronosequence of Rubber Tree (Hevea brasiliensis) Plantations. Front. Plant Sci. 2017, 8, 849. [Google Scholar] [CrossRef] [PubMed]
- Umami, I.M.; Kamarudin, K.N.; Abe, S.; Hermansah, H. Does Soil Fertility Decline under Smallholder Rubber Farming? The Case of a West Sumatran Lowland in Indonesia. Jpn. Agric. Res. Q. 2019, 3, 279–287. [Google Scholar] [CrossRef]
- Aweto, A.O. Physical and nutrient status of soils under rubber (Hevea brasiliensis) of different ages in south-western Nigeria. Agric. Syst. 1987, 23, 63–72. [Google Scholar] [CrossRef]
- Liu, C.; Jin, Y.; Liu, C.; Tang, J.; Wang, Q.; Xu, M. Phosphorous fractions in soils of rubber-based agroforestry systems: Influence of season, management and stand age. Sci. Total Environ. 2017, 616-617, 1576–1588. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, R.; Jiang, J. Variation of soil fertility and carbon sequestration by planting Hevea brasiliensis in Hainan Island, China. J. Environ. Sci. 2007, 19, 348–352. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Ogunkunle, C.; Awotoye, O. Soil Fertility Status under Different Tree Cropping System in a Southwestern Zone of Nigeria. Not. Sci. Biol. 2011, 3, 123–128. [Google Scholar] [CrossRef]
- Food Agricuture Organization (FAO). The Rubber Tree; Better Farming Series; Food Agricuture Organization (FAO): Rome, Italy, 1977; pp. 1–31. [Google Scholar]
- Raj, A. Role of Trees and Woody Vegetation in Soil Fertility Enrichment and Food Security in Dryland Agroforestry as a Climate-Smart Agriculture Strategy. Int. J. Trop. Agric. 2017, 35, 1147–1161. [Google Scholar]
- Barrios, E.; Sileshi, G.W.; Shepherd, K.D.; Sinclair, F. Agroforestry and Soil Health: Linking Trees, Soil Biota, and Ecosystem Services. In Soil Ecology and Ecosystem Services; Oxford University Press (OUP): Oxford, UK, 2012; pp. 315–330. [Google Scholar]
- Palm, C.A. Contribution of agroforestry trees to nutrient requirements of intercropped plants. Agrofor. Syst. 1995, 30, 105–124. [Google Scholar] [CrossRef]
| Chi Square | Significant Level (p Value) | |
|---|---|---|
| POR | 68.889 | <3.45 × 10−5 |
| SOC | 170.5 | <2.2 × 10−16 |
| BAC | 142.71 | <2.2 × 10−16 |
| ACT | 137.7 | <2.2 × 10−16 |
| FUN | 79.621 | 1.65 × 10−14 |
| Rotated Component Matrix | ||
|---|---|---|
| Component | ||
| 1 | 2 | |
| POR | 0.647 | 0.223 |
| SOC | 0.881 | 0.237 |
| BAC | 0.959 | −0.143 |
| ACT | 0.722 | −0.609 |
| FUN | 0.680 | −0.557 |
| CAN | 0.940 | −0.244 |
| VGC | 0.469 | 0.739 |
| LB | 0.866 | 0.261 |
| DLC | 0.888 | 0.291 |
| Eigenvalues | 5.739 | 1.565 |
| Variance (%) | 63.76 | 17.39 |
| Cumulative variance (%) | 63.76 | 81.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Do, T.T.; Harper, R.; Pham, T.T.; Linh, T.V.K.; Le, T.S.; Thanh, L.B.; Giap, N.X. Soil Health Impacts of Rubber Farming: The Implication of Conversion of Degraded Natural Forests into Monoculture Plantations. Agriculture 2020, 10, 357. https://doi.org/10.3390/agriculture10080357
Nguyen TT, Do TT, Harper R, Pham TT, Linh TVK, Le TS, Thanh LB, Giap NX. Soil Health Impacts of Rubber Farming: The Implication of Conversion of Degraded Natural Forests into Monoculture Plantations. Agriculture. 2020; 10(8):357. https://doi.org/10.3390/agriculture10080357
Chicago/Turabian StyleNguyen, Thu Thi, Truong Tat Do, Richard Harper, Trang Thanh Pham, Tran Vu Khanh Linh, Thai Son Le, Le Bao Thanh, and Nguyen Xuan Giap. 2020. "Soil Health Impacts of Rubber Farming: The Implication of Conversion of Degraded Natural Forests into Monoculture Plantations" Agriculture 10, no. 8: 357. https://doi.org/10.3390/agriculture10080357
APA StyleNguyen, T. T., Do, T. T., Harper, R., Pham, T. T., Linh, T. V. K., Le, T. S., Thanh, L. B., & Giap, N. X. (2020). Soil Health Impacts of Rubber Farming: The Implication of Conversion of Degraded Natural Forests into Monoculture Plantations. Agriculture, 10(8), 357. https://doi.org/10.3390/agriculture10080357






