Living Mulch Management Spatially Localizes Nutrient Cycling in Organic Corn Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling
2.3. Permanganate Oxidizable Carbon
2.4. Particulate Organic Matter
2.5. Microbial Biomass
2.6. Statistical Analyses
3. Results and Discussion
3.1. Permanganate Oxidizable Carbon
3.2. Microbial Biomass and Particulate Organic Matter
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joyce, B.A.; Wallender, W.W.; Mitchell, J.P.; Huyck, L.M.; Temple, S.R.; Brostrom, P.N.; Hsiao, T.C. Infiltration and soil water storage under winter cover cropping in California’s Sacramento Valley. Trans. ASAE 2002, 45, 315–326. [Google Scholar] [CrossRef]
- Grabber, J.H.; Jokela, W.E. Off-season groundcover and runoff characteristics of perennial clover and annual grass companion crops for no-till corn fertilized with manure. J. Soil Water Conserv. 2013, 68, 411–418. [Google Scholar] [CrossRef]
- Bodner, G.; Himmelbauer, M.; Loiskandl, W.; Kaul, H.P. Improved evaluation of cover crop species by growth and root factors. Agron. Sustain. Dev. 2010, 30, 455–464. [Google Scholar] [CrossRef]
- Parr, M.; Grossman, J.M.; Reberg-Horton, S.C.; Brinton, C.; Crozier, C. Nitrogen Delivery from Legume Cover Crops in No-Till Organic Corn Production. Agron. J. 2011, 103, 1578–1590. [Google Scholar] [CrossRef]
- Wayman, S.; Cogger, C.; Benedict, C.; Burke, I.; Collins, D.; Bary, A. The influence of cover crop variety, termination timing and termination method on mulch, weed cover and soil nitrate in reduced-tillage organic systems. Renew. Agric. Food Syst. 2014, 30, 450–460. [Google Scholar] [CrossRef]
- Jani, A.D.; Grossman, J.; Smyth, T.J.; Hu, S. Winter legume cover-crop root decomposition and N release dynamics under disking and roller-crimping termination approaches. Renew. Agric. Food Syst. 2015, 31, 214–229. [Google Scholar] [CrossRef]
- Liebman, A.M.; Grossman, J.; Brown, M.; Wells, M.S.; Reberg-Horton, S.C.; Shi, W. Legume cover crops and tillage impact nitrogen dynamics in organic corn production. Agron. J. 2018, 110, 1046–1057. [Google Scholar] [CrossRef]
- Ali, S.A.; Tedone, L.; Verdini, L.; Cazzato, E.; De Mastro, G. Wheat response to no-tillage and nitrogen fertilization in a long-term faba bean-based rotation. Agronomy 2019, 9, 50. [Google Scholar] [CrossRef]
- Ochsner, T.E.; Albrecht, K.A.; Schumacher, T.W.; Baker, J.M.; Berkevich, R.J. Water Balance and Nitrate Leaching under Corn in Kura Clover Living Mulch. Agron. J. 2010, 102, 1169–1178. [Google Scholar] [CrossRef]
- Goudriaan, J.; Groot, J.J.R.; Uithol, P.W.J. Productivity of Agro-ecosystems. In Terrestrial Global Productivity; Roy, J., Saugier, B., Mooney, H.A., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 301–313. [Google Scholar]
- Saugier, B.; Roy, J.; Mooney, H.A. Estimations of Global Terrestrial Productivity. In Terrestrial Global Productivity; Roy, J., Saugier, B., Mooney, H.A., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 543–557. [Google Scholar]
- Yacobi, Y.Z.; Erez, J.; Hadas, O. Primary production. In Aquatic Ecology; Whalen, J.K., Sampedro, L., Eds.; CAB International: Wallingford, UK, 2014; Volume 6, pp. 417–438. [Google Scholar]
- Duda, G.P.; Guerra, J.G.M.; Monteiro, M.T.; De-Polli, H.; Teixeira, M.G. Perennial herbaceous legumes as live soil mulches and their effects on C, N and P of the microbial biomass. Sci. Agric. 2003, 60, 139–147. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Dabney, S.M.; Delgado, J.A.; Reeves, D.W. Using winter cover crops to improve soil and water quality. Commun. Soil Sci. Plant. Anal. 2001, 32, 1221–1250. [Google Scholar] [CrossRef]
- Kabir, Z.; Koide, R.T. Effect of autumn and winter mycorrhizal cover crops on soil properties, nutrient uptake and yield of sweet corn in Pennsylvania, USA. Plant. Soil 2002, 238, 205–215. [Google Scholar] [CrossRef]
- Ghestem, M.; Sidle, R.C.; Stokes, A. The Influence of Plant Root Systems on Subsurface Flow: Implications for Slope Stability. Bioscience 2011, 61, 869–879. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Marten, G.C. Kura clover forage yield, forage quality, and stand dynamics. Can. J. Plant. Sci. 1991, 71, 1169–1172. [Google Scholar] [CrossRef]
- Seguin, P.; Russelle, M.P.; Sheaffer, C.C.; Ehlke, N.J.; Graham, P.H. Dinitrogen fixation in kura clover and birdsfoot trefoil. Agron. J. 2000, 92, 1216–1220. [Google Scholar] [CrossRef]
- Qi, Z.; Helmers, M.J.; Kaleita, A.L. Soil water dynamics under various agricultural land covers on a subsurface drained field in north-central Iowa, USA. Agric. Water Manag. 2011, 98, 665–674. [Google Scholar] [CrossRef]
- Siller, A.R.S.; Albrecht, K.A.; Jokela, W.E. Soil Erosion and Nutrient Runoff in Corn Silage Production with Kura Clover Living Mulch and Winter Rye. Agron. J. 2016, 108, 989–999. [Google Scholar] [CrossRef]
- Ginakes, P.; Grossman, J.M.; Baker, J.M.; Dobbratz, M.; Sooksa-nguan, T. Soil carbon and nitrogen dynamics under zone tillage of varying intensities in a kura clover living mulch system. Soil Tillage Res. 2018, 184, 310–316. [Google Scholar] [CrossRef]
- Ginakes, P.; Grossman, J.; Baker, J.; Sooksa-nguan, T. Tillage intensity influences nitrogen cycling in organic kura clover living mulch. Nutr. Cycl. Agroecosystems 2020, 116, 71–82. [Google Scholar] [CrossRef]
- Alexander, J.R.; Venterea, R.T.; Baker, J.M.; Coulter, J.A. Kura clover living mulch: Spring management effects on nitrogen. Agronomy 2019, 9, 69. [Google Scholar] [CrossRef]
- Dobbratz, M.; Baker, J.M.; Grossman, J.; Wells, M.S.; Ginakes, P. Rotary zone tillage improves corn establishment in a kura clover living mulch. Soil Tillage Res. 2019, 189, 229–235. [Google Scholar] [CrossRef]
- Alvarez, R. A review of nitrogen fertilizer and conservation tillage effects on soil organic carbon storage. Soil Use Manag. 2005, 21, 38–52. [Google Scholar] [CrossRef]
- Mikha, M.M.; Vigil, M.F.; Benjamin, J.G. Long-Term Tillage Impacts on Soil Aggregation and Carbon Dynamics under Wheat-Fallow in the Central Great Plains. Soil Sci. Soc. Am. J. 2013, 77, 594–605. [Google Scholar] [CrossRef]
- Ladd, J.N.; Oades, J.M.; Amato, M. Microbial biomass formed from 14 C, 15 N-labelled plant material decomposing in soils in the field. Soil Biol. Biochem. 1981, 13, 119–126. [Google Scholar] [CrossRef]
- Zhu, B.; Gutknecht, J.L.M.; Herman, D.J.; Keck, D.C.; Firestone, M.K.; Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol. Biochem. 2014, 76, 183–192. [Google Scholar] [CrossRef]
- Larsen, E.; Grossman, J.; Edgell, J.; Hoyt, G.; Osmond, D.; Hu, S. Soil biological properties, soil losses and corn yield in long-term organic and conventional farming systems. Soil Tillage Res. 2014, 139, 37–45. [Google Scholar] [CrossRef]
- Plaza, C.; Courtier-Murias, D.; Fernández, J.M.; Polo, A.; Simpson, A.J. Physical, chemical, and biochemical mechanisms of soil organic matter stabilization under conservation tillage systems: A central role for microbes and microbial by-products in C sequestration. Soil Biol. Biochem. 2013, 57, 124–134. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Janzen, H.H. Storage of soil carbon in the light fraction and macroorganic matter. In Structure and organic matter storage in agricultural soils; Carter, M.R., Stewart, M.A., Eds.; CRC Press: New York, NY, USA, 1996; pp. 167–190. [Google Scholar]
- Christensen, B.T. Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur. J. Soil Sci. 2001, 52, 345–353. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Morrow, J.G.; Huggins, D.R.; Carpenter-Boggs, L.A.; Reganold, J.P. Evaluating measures to assess soil health in long-term agroecosystem trials. Soil Sci. Soc. Am. J. 2016, 80, 450–462. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Awale, R.; Chatterjee, A.; Franzen, D. Tillage and N-fertilizer influences on selected organic carbon fractions in a North Dakota silty clay soil. Soil Tillage Res. 2013, 134, 213–222. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Variations in microbial community composition through two soil depth profiles. Soil Biol. Biochem. 2003, 35, 167–176. [Google Scholar] [CrossRef]
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. 2016. [Google Scholar]
- Panettieri, M.; Knicker, H.; Berns, A.E.; Murillo, J.M.; Madejón, E. Moldboard plowing effects on soil aggregation and soil organic matter quality assessed by 13C CPMAS NMR and biochemical analyses. Agric. Ecosyst. Environ. 2013, 177, 48–57. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Álvaro-Fuentes, J.; Cantero-Martínez, C. Identifying soil organic carbon fractions sensitive to agricultural management practices. Soil Tillage Res. 2014, 139, 19–22. [Google Scholar] [CrossRef]
- Melero, S.; López-Garrido, R.; Madejón, E.; Murillo, J.M.; Vanderlinden, K.; Ordóñez, R.; Moreno, F. Long-term effects of conservation tillage on organic fractions in two soils in southwest of Spain. Agric. Ecosyst. Environ. 2009, 133, 68–74. [Google Scholar] [CrossRef]
- Lewis, D.B.; Kaye, J.P.; Jabbour, R.; Barbercheck, M.E. Labile carbon and other soil quality indicators in two tillage systems during transition to organic agriculture. Renew. Agric. Food Syst. 2011, 26, 342–353. [Google Scholar] [CrossRef]
- Spargo, J.T.; Cavigelli, M.A.; Mirsky, S.B.; Maul, J.E.; Meisinger, J.J. Mineralizable soil nitrogen and labile soil organic matter in diverse long-term cropping systems. Nutr. Cycl. Agroecosystems 2011, 90, 253–266. [Google Scholar] [CrossRef]
- Mirsky, S.B.; Lanyon, L.E.; Needelman, B.A. Evaluating Soil Management Using Particulate and Chemically Labile Soil Organic Matter Fractions. Soil Sci. Soc. Am. J. 2008, 72, 180–185. [Google Scholar] [CrossRef]
- De Neergaard, A.; Gorissen, A. Carbon allocation to roots, rhizodeposits and soil after pulse labelling: A comparison of white clover (Trifolium repens L.) and perennial ryegrass (Lolium perenne L.). Biol. Fertil. Soils 2004, 39, 228–234. [Google Scholar] [CrossRef]
- Diochon, A.; Gillespie, A.W.; Ellert, B.H.; Janzen, H.H.; Gregorich, E.G. Recovery and dynamics of decomposing plant residue in soil: An evaluation of three fractionation methods. Eur. J. Soil Sci. 2016, 67, 196–205. [Google Scholar] [CrossRef]
- St. Luce, M.; Whalen, J.K.; Ziadi, N.; Zebarth, B.J. Net nitrogen mineralization enhanced with the addition of nitrogen-rich particulate organic matter. Geoderma 2016, 262, 112–118. [Google Scholar] [CrossRef]
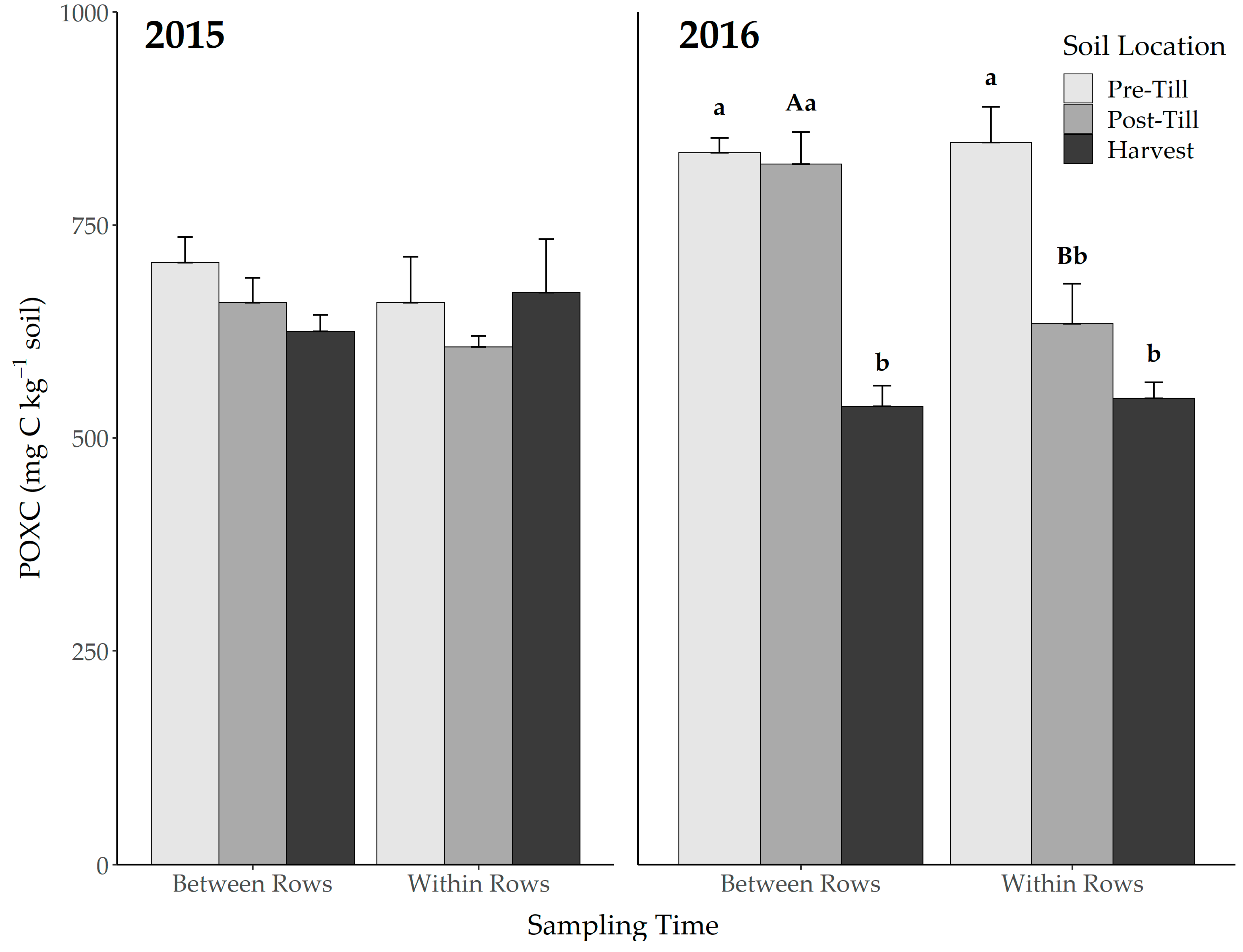
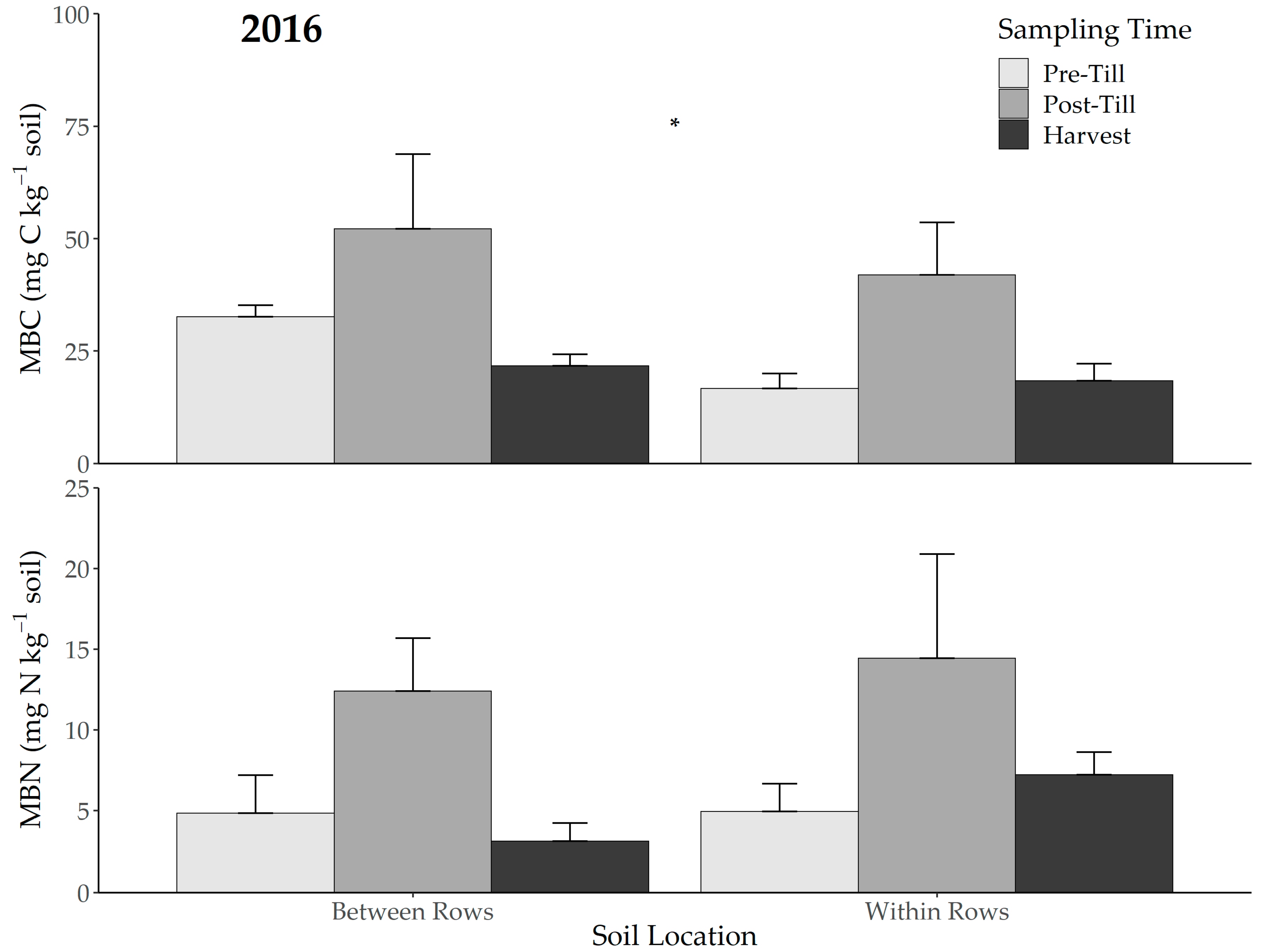
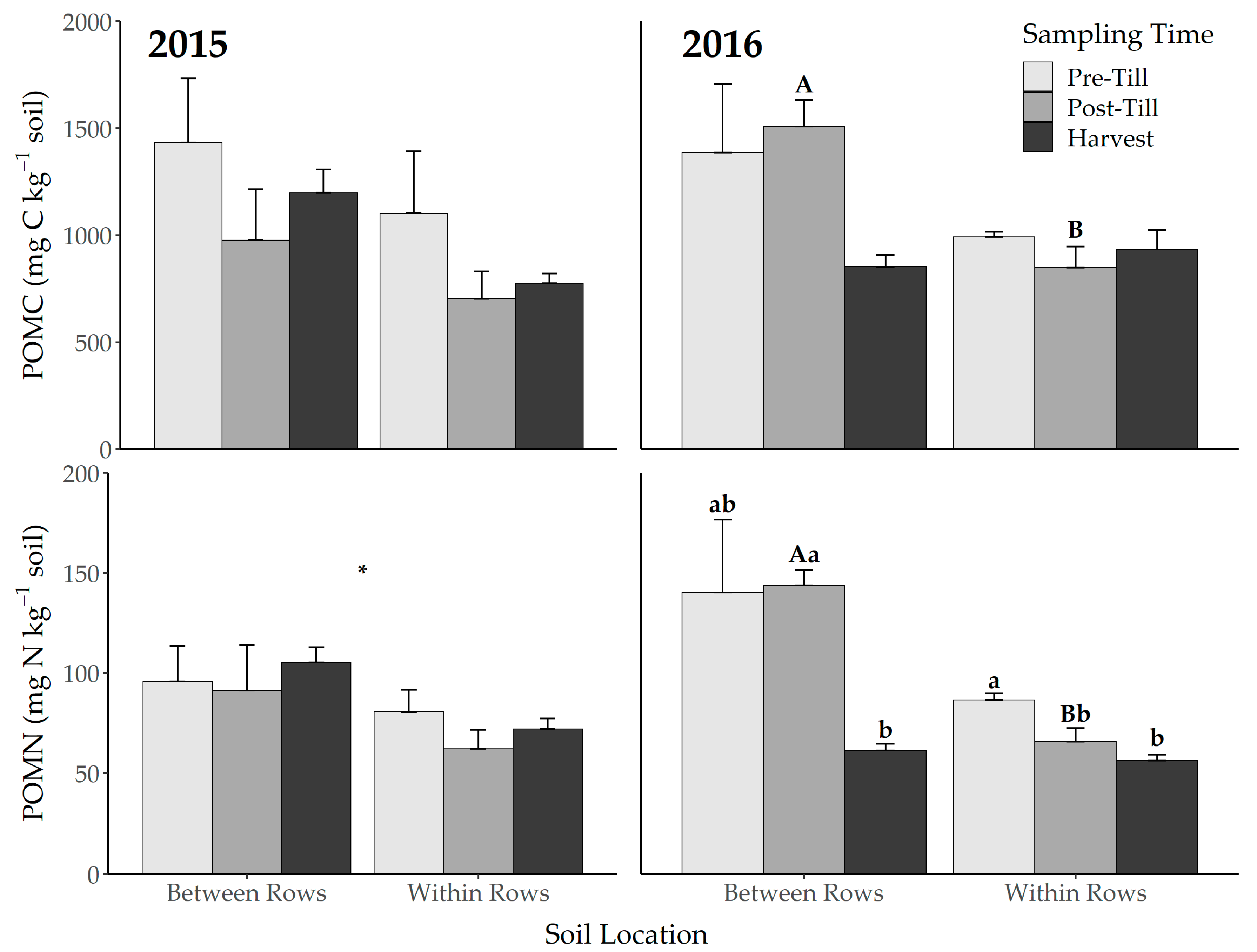
| 2015 | 2016 | |||||||
|---|---|---|---|---|---|---|---|---|
| POXC 1 | POMC | POMN | POXC | POMC | POMN | MBC | MBN | |
| Fixed Effect | p-Value | |||||||
| Location | 0.549 | 0.14 | 0.084 | 0.153 | 0.027 | 0.004 | 0.005 | 0.741 |
| Time | 0.283 | 0.0392 | 0.034 | <0.001 | 0.108 | <0.001 | 0.209 | 0.056 |
| Location × Time | 0.243 | 0.578 | 0.231 | 0.06 | 0.05 | 0.024 | 0.553 | 0.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginakes, P.; Grossman, J.M.; Baker, J.M.; Sooksa-nguan, T. Living Mulch Management Spatially Localizes Nutrient Cycling in Organic Corn Production. Agriculture 2020, 10, 243. https://doi.org/10.3390/agriculture10060243
Ginakes P, Grossman JM, Baker JM, Sooksa-nguan T. Living Mulch Management Spatially Localizes Nutrient Cycling in Organic Corn Production. Agriculture. 2020; 10(6):243. https://doi.org/10.3390/agriculture10060243
Chicago/Turabian StyleGinakes, Peyton, Julie M. Grossman, John M. Baker, and Thanwalee Sooksa-nguan. 2020. "Living Mulch Management Spatially Localizes Nutrient Cycling in Organic Corn Production" Agriculture 10, no. 6: 243. https://doi.org/10.3390/agriculture10060243
APA StyleGinakes, P., Grossman, J. M., Baker, J. M., & Sooksa-nguan, T. (2020). Living Mulch Management Spatially Localizes Nutrient Cycling in Organic Corn Production. Agriculture, 10(6), 243. https://doi.org/10.3390/agriculture10060243





