Abstract
The identification of heterotic groups may provide an important advantage for hybrid eggplant (Solanum melongena) breeding. In this study, we evaluated the combining ability and heterotic patterns of eggplant lines in order to develop improved eggplant cultivars resistant to Fusarium oxysporum f. sp. melongenae (FOM). A set of 62 inbred lines was evaluated with 32 morphological descriptors and their relationships were analyzed through a multivariate cluster analysis. A subset of 39 inbred lines was selected and, together with 15 sister lines, they were crossed with two testers to investigate their general combining ability (GCA) and to establish heterotic groups. Twenty selected inbred lines with high GCA were intercrossed using a half-diallel mating design. Eighty-two hybrids were obtained and evaluated for yield and yield components. We found no association between morphological distance and membership to specific heterotic groups. However, heterosis for yield was found in hybrids among parents from different heterotic groups or that were included in all heterotic groups. Among the hybrids evaluated, some were found to be highly productive and resistant to FOM, being candidates for the registration of new cultivars with dramatically improved characteristics.
1. Introduction
Eggplant ranks among the top five vegetable crops in terms of production in Asian and Mediterranean basin countries. However, most of the available eggplant cultivars are susceptible to a wide range of diseases and pests that can cause significant reductions in yield and quality [1]. New eggplant F1 hybrid varieties, highly productive and tolerant to biotic and abiotic stress conditions, are needed to improve the sustainability of production [2]. Rootstocks tolerant to soil diseases and with better efficiency in the use of water and fertilizers, and conferring vigor to the scion are available in eggplant [3,4,5]. However, the grafting process increases the nursery costs and having varieties that combine high yield and quality with tolerance to stresses is a current eggplant breeding objective. Fusarium oxysporum f. sp. melongenae (FOM) is the causal agent of Fusarium wilt, one of the major phytopathological problems of eggplant, limiting its cultivation and causing important reductions in yield in both greenhouse and open-field production in Asia and Europe [6,7]. Controlling and eradicating FOM from eggplant fields with agrochemicals is expensive and largely ineffective [8]. Therefore, developing FOM resistant F1 varieties may be an efficient strategy for areas with a high prevalence of FOM infection [2]. Utilization of heterosis is important for developing superior hybrid varieties able to cope with the increasing demands of the global market in eggplant. Thus, heterosis can provide important advantages for breeding for yield, better growth and development, disease and pest resistance [9]. Assessment of the combining ability of parents and identification of their heterotic patterns can facilitate the selection of parents to be used for developing superior hybrids [10].
Establishing heterotic groups is a major step for hybrid breeding in many crops and this strategy has been applied successfully in several field crops, especially in maize [11]. In this way, highly heterotic hybrids can be obtained by crossing lines from different heterotic groups [12]. Recent theoretical developments have revealed that establishing heterotic groups provides many advantages in field crop breeding for developing hybrid cultivars [13]. However, its utilization in vegetable breeding has been limited [11,14].
In this study, we evaluated a set of eggplant lines and crossed them with two testers to identify materials with high general combining ability (GCA). A subset of selected lines was intercrossed and the hybrids evaluated. The aim is to identify heterotic groups in eggplant, to study the relationship of heterosis with morphological distances and general combining ability, and to assess the potential utility of establishing heterotic groups in eggplant. Some of the materials used are resistant to FOM, as one of the final objectives is to develop improved high-yielding and Fusarium wilt-resistant hybrid cultivars of eggplant.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
Sixty-two inbred lines of eggplant developed from diverse backgrounds and selfed for six generations were used for evaluation of morphoagronomic traits. Thirty (tdc1 to tdc30) were developed by a pedigree method in breeding programs aimed at selection for high yield and adaptation to low temperature conditions. The remaining 32 (tdc31 to tdc62) were developed through backcrossing in breeding programs performed for breeding for FOM resistance [15]. A subset of 39 lines plus 15 sister lines (i.e., sharing the same ancestors) for nine of the lines from the selected subset were crossed with two testers with known high combining ability and having different fruit shape (Tester 1: long-shaped; and Tester 2: oval-shaped), and 108 F1 hybrids were generated to perform a general combining ability GCA test. An additional round of selection was made among the lines for GCA and 12 of them, together with 8 sister lines, were selected and crossed using a half-diallel design, resulting in 82 hybrids. The experimental hybrids obtained were compared for yield performance with five commercial F1 hybrids: Brigitte (Rijk Zwaan Inc., De Lier, The Netherlands), Corsica (Semillas Fitó, Barcelona, Spain), Destan (Istanbul Seed Inc., Antalya, Turkey), Faselis (Antalya Tarim Inc., Antalya, Turkey), and Sicilia (Semillas Fitó, Barcelona, Spain).
All the experiments were carried out throughout the years 2011–2015 in a glasshouse at the Department of Vegetable Crops and Ornamentals at Bati Akdeniz Agricultural Research Institute (BATEM, Antalya, Turkey). For each growing cycle, the plants were transplanted to a glasshouse in September. They were grown in double rows separated 100 cm apart from one another and spaced at 60 × 50 cm within double rows. Each plant was pruned to leave three basal branches. A plant bioactivator (Speedfol Flower&Fruit, Doktor Tarsa Agriculture Industry and Trade Inc., Antalya, Turkey) was applied to stimulate fruit setting from 15 November to 15 March. For each yield experiment, seven harvests were conducted. The experiments, both of lines and hybrids, were conducted in a randomized block design with two replicates. Five plants per replicate were used for the experiment of comparison of lines while, for experiments with hybrids, six plants per replicate were used.
2.2. Fusarium Resistance Screening of Inbred Lines
The responses of inbred lines to a highly virulent strain of FOM obtained from the vascular system of a diseased eggplant plant collected in the province of Antalya (Turkey) were evaluated by the classical root-dip method [15]. The isolate was cultured on potato dextrose agar (PDA, pH = 6.5) at 24 °C for 10 days. Disks taken from the PDA culture were placed in liquid medium and shaken at 50 rpm in an orbital shaker at 24 °C for eight days and spore density was adjusted to a concentration of 1 × 106 conidia/mL in the inoculum. For inoculation, seedlings that had two to four true leaves were removed from the seedling trays. Subsequently, the roots were washed with tap water and then wounded by trimming the tips and then submerged for 5 min in the inoculum suspension. The seedlings were subsequently transplanted and maintained in a greenhouse with controlled temperature. The experiment was fully randomized with the use of two replicates and 10 plants per replicate. The susceptible line NSFN-99 and the resistant accession LS1934 [15] were used as controls. Plants of both materials were also mock inoculated by immersion of the wounded roots in tap water as negative controls. After inoculations, seedlings were immediately transplanted into pots containing a mixture of sterile peat and perlite in a ratio of 1:1 (v/v) and maintained in a greenhouse. Twenty-eight days after inoculation, lines were classified as resistant or susceptible based on symptomatology. Plants were considered as susceptible if they displayed reduced growth with yellowing of leaves or heavy stunting, or died as a consequence of FOM infection [15]. If they had no symptoms or symptoms were mild, they were considered as resistant.
2.3. Traits Measured
The traits used for morphoagronomic characterization (Table 1) of the 62 lines were based on the European Cooperative Programme for Plant Genetic Resources (ECPGR) Solanaceae descriptors and the International Board for Plant Genetic Resource (IBPGR) descriptors for eggplant with some modifications. Phenotypic data on qualitative traits were collected per replication and quantitative traits related to fruit length, width, and weight were measured on 10 fruits per replication harvested at the commercial ripening stage (Table 1). Total yield of both lines and hybrids was calculated from replicate (i.e., five plants) averages.

Table 1.
Morphoagronomic descriptors used for characterization and evaluation of 62 eggplant inbred lines.
2.4. Multivariate Analysis of Morphological Relationships
The relationships among the 62 inbred lines based on morphological traits were analyzed using the NTSYS-PC version 2.2 software [16]. The standardization module was used. To determine the correlation coefficient, the correlation matrix, which was adjusted using the SIMINT module of NTSYS-PC, was performed. An unweighted pair group method with arithmetic mean (UPGMA) phenogram was obtained using the clustering technique via the SHAN module. The cophenetic correlation coefficient was calculated with a Mantel test [17].
2.5. General Combining Ability
The general combining ability (GCA) was calculated for yield, fruit length (cm), width (cm), weight (kg), and number of fruits. The general combining ability of inbred lines can be estimated by directly creating all combinations of all lines in a breeding program. However, as the number of inbred lines used for GCA testing increases, this method is not practical [18]. So, the line × tester method is considered as an economic and efficient alternative. Two testers (Tester 1 and Tester 2) identified as superior parents in previous work were used for GCA test.
Statistical evaluation of the data for CGA evaluation was performed by ANOVA test utilizing the Jump 5.0.1 software package (SAS Institute, Cary, NC, USA) for the GCA test. Pair-wise comparison of hybrids was performed with least significant difference (LSD) (p < 0.05) test. The inbred lines were grouped into heterotic categories to identify their potential for enhancement of high yielding hybrids [19]. For the establishment of heterotic groups, average yields of hybrids for all inbred lines were determined for each tester group. If hybrids with an inbred line had a yield above the average of both tester groups, the line was included in both heterotic groups. Conversely, if hybrids with an inbred line had yield below the average of the hybrids with both testers it was not included in any of the heterotic groups. If an inbred line gave a hybrid whose yield was lower than the average of hybrids for one tester, but it was above the average for the other tester, it was included in the heterotic group for the latter tester. In this situation, inbred lines were included in the group in which the yield of its hybrids was higher than the average of the hybrid with each tester [20]. Data on yield and the remaining traits were analyzed with the Jump 5.0.1 software. Differences among the mean values were evaluated with LSD tests at p < 0.05.
3. Results
3.1. Fusarium Resistance Screening
All inbred lines were checked for reaction against FOM. The Fusarium wilt resistance test results were as expected according to the pedigree of the lines and the phenotyping performed for FOM resistance during their development. Plants from the resistant lines survived and did not display symptoms or these were very mild, while those from the susceptible lines died or suffered severe disease symptoms (Figure 1). In this way, the 30 inbred lines (tdc1–tdc30) were found as susceptible, while the 32 inbred lines (tdc31–tdc62) previously selected for FOM resistance were confirmed as resistant.

Figure 1.
Fusarium resistant (left and center) and susceptible (right) eggplant lines at four weeks after the inoculation.
3.2. Morphoagronomic Characteristics and Relationships of the Set of Lines
The characterization of the 62 inbred lines with 32 morphological traits revealed a great diversity in the collection of lines. Twenty-five inbred lines displayed open growth habit, 28 semi-open growth habit, whereas nine had bushy growth habit. The plant height was found to be long for 17 lines, intermediate for 33, and short for 12, while the stem hairiness was dense in 10 lines, intermediate in 31, and sparse in 21. Considerable variation was also found for stem color, which varied from grayish green in three lines, green in 27, green-purple in 21, purple in five, and grayish purple in six. Leaf hairiness was dense in 11, intermediate in 26, and sparse in 25. Three lines had many prickles in the petiole, 26 had intermediate prickliness, 11 had few prickles, and 22 had none. Regarding flowering earliness, most of the lines (45) had intermediate flowering earliness, 11 were early, and six were late. The calyx size was small in five lines, intermediate in 49, and large in eight. Regarding fruit descriptors, a wide variation was found, and the distribution of lines for each of the states of the fruit descriptors is presented in Table 2. Most of the lines were long and with purple color, fruit pedicel length was mostly intermediate or long, and most of the lines had no or few prickles. The fruit tip shape was mostly rounded. Most of the lines had few seeds and no grooves with bright color. The fruit end button size was mostly medium sized. Most of the lines had fruits with a length over 18 cm, and with intermediate or small diameter. Fruit weight was distributed evenly among the three categories established (Table 2). An important percentage (58%) of the lines displayed parthenocarpic ability. Flesh color was mostly greenish cream. Fruit curvature was present in 52% of the lines (Table 2).

Table 2.
Fruit features of the 62 lines characterized and from which lines were used for hybrid development.
The relationships among the inbred lines were determined by using a multivariate UPGMA cluster analysis with the 32 basic morphological descriptors. The eigenvalue for the first five factors reached 91%. The correlation (r) for the morphological distance and cophenetic matrices of the phenogram was r = 0.79. The approximate two-way Mantel t-test value was t = 9.2508 (p < 0.0001). The coefficient of similarity of inbred lines ranged between 0.61 and 0.98. The UPGMA cluster analysis separated the lines into two major groups (Figure 1). The first cluster consisted of six inbred lines. This group was considerably distinct compared to all other inbred lines, particularly for fruit traits. The most important feature that distinguished them from the larger group was the fruit length. The fruit lengths of these six lines (between 11 and 15 cm) were shorter than for the other lines. In addition, these six lines shared the traits of purple fruit color and greenish-white fruit flesh. The remaining 56 inbred lines formed a second cluster and were divided into four different subclusters (Figure 2). The inbred lines tdc2 and tdc4, which belonged to the fourth subcluster, displayed the highest morphological similarity (Figure 2).
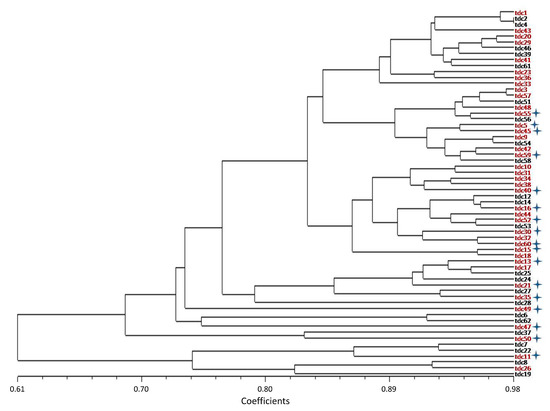
Figure 2.
Dendrogram based on morphological data of 62 inbred lines using the unweighted pair group method with arithmetic mean (UPGMA) cluster analysis using Dice (1945) similarity coefficients. Lines labeled in red, including three lines sister lines for tdc5, tdc15, and tdc30, were selected for general combining ability evaluation. Lines marked with a star were chosen for half diallel cross design.
3.3. General combining Ability and Heterotic Groups
The maximum, minimum, and average of fruit length (cm), fruit diameter (cm), fruit weight (kg), fruit number per plant, and fruit yield for per plant (kg) of the two testers are presented in Table 3. Tester 2 on average had broader and heavier fruits and higher yield than tester 1.

Table 3.
Average and standard deviation (SD) values for yield and yield components for the tester parents.
The general combining ability (GCA) and heterotic groups of inbred lines were determined using average yield values of hybrids obtained from crosses with testers. The ANOVA of data of the 108 hybrids of 54 lines with two testers demonstrated considerable differences (p < 0.001) among testers, lines, and line × tester hybrids (Table 4).

Table 4.
Mean square deviations of the analysis of variance for yield of 54 lines with two testers and 108 hybrids produced from their crosses.
Most of the hybrids with the two testers displayed a wide variation in the fruit characteristics measured. In this way, for 28 and 18 hybrids with tester 1 and tester 2, respectively, the fruit length was above the mean of their respective testers. The same occurred for fruit width for 27 and 19 hybrids, respectively. A considerable variation was also observed in fruit weight. For 29 hybrids with the first tester, the weight of fruits was over the mean of this tester (0.17 kg), and for 25 hybrids with the second tester, the fruit weight was over the mean of this second tester (0.19 kg). Thirty-two hybrids for each of the testers had higher number of fruits than their respective testers.
Estimates of general combining ability effects of parental lines for yield (g/plot) and heterotic groups are presented in Table 5. Maximum and minimum yield values of hybrids were 1.08 kg/plot and 7.56 kg/plot for tester 1, and 0.99 kg/plot and 9.01 kg/plot for tester 2. The mean fruit yield of hybrids was 4.10 kg/plot for tester 1 and 5.28 kg/plot for tester 2. While the inbred line tdc5/2 showed the highest positive GCA effect with 1.55 kg/plot, tdc30/1 showed the lowest negative GCA effect with −1.73 kg/plot. Twenty-eight out of the 54 inbred lines displayed positive GCA values for fruit yield. The highest positive GCA effect for yield for tester 1 was observed for line tdc21/20, which had a yield per plot of 7.56 kg with tester 1. It was followed by tdc13/9, tdc5/2, tdc45, tdc15/18, and tdc55 inbred lines. GCA values for lines with tester 2 were higher than those for tester 1. In this way, six lines gave higher value for GCA with tester 1, while 26 lines gave higher value with tester 2. For tester 2, line tdc5/12 displayed the highest positive GCA value, with a yield of 9005 g/plot, followed by lines tdc35, tdc45, tdc5/2, tdc48, and tdc21/20 (Table 5). In fact, one of the best heterosis rates was observed in hybrid combinations for which the parental lines were morphologically very similar (tdc45 × tdc21/20), and one of the worst heterosis rates was observed in hybrid combinations in which the parental lines were morphologically very distinct (tdc59 × tdc50).

Table 5.
Yields (kg/plot) of hybrids of lines with testers 1 and 2, estimates of general combining ability effects of parental lines for yield, and membership to heterotic groups. Lines containing a slash (/) indicate sister lines.
The 54 inbred lines were classified into heterotic groups for two testers. Ten lines were included in heterotic group 1, whereas 11 lines were included in heterotic group 2 (Table 5). Nine lines were not assigned to any of the heterotic groups. Twenty-four lines were included in both heterotic groups. Surprisingly, some sister lines were assigned into different heterotic groups (Table 5).
3.4. Selection of Lines and Half-Diallel Results
Estimated GCA values for the parental lines showed that the best lines (as general combiners) for each trait were as follows: tdc5/2 and tdc21/20 for fruits number in per plant, tdc48 and tdc60 for average fruit weight (kg), tdc11/13 and tdc11/15 for average fruit diameter (cm), and tdc13/5 and tdc20 for average fruit length. Based on high CGA values for yield and/or yield components, as well as FOM resistance and other traits of commercial interest, 20 inbred lines (tdc5/2, tdc5/3, tdc11/13, tdc13/5, tdc15/13, tdc15/18, tdc16, tdc21/20, tdc30/2, tdc30/1, tdc35, tdc40/1, tdc45, tdc47, tdc49, tdc50, tdc52, tdc55, tdc59, and tdc60) that were ascribed to different heterotic groups were selected for half-diallel crosses. In addition, fruit quality features that are desired attributes for eggplant, such as shape, color, brightness, and hardness of the flesh of the fruits, were considered during the selection.
Eighty-two hybrids obtained from the half-diallel crosses of parental lines and five commercial varieties were compared for yield. The analyses of variance of yield data revealed significant differences (p < 0.01) among the hybrids. Thirty-one hybrids had yields higher than the best performing commercial variety used as control. The highest and lowest yield of plants (kg/plant), number of fruits, weight of fruits, and fruit length ranged from 4.8 to 1.5 kg, 9 to 26, 0.13 to 0.25 kg, and 14 to 28 cm, respectively. The highest specific combining ability effects were observed in the following combinations having highest yield values: tdc21/20 × tdc49, with about 4.8 kg/plant; tdc30/1 × tdc47, with 4.7 kg/plant; and tdc49 × tdc5/2, with 4.4 kg/plant. The same combinations produced the highest numbers of fruits. The largest fruit weights were obtained in hybrids tdc11/13 × tdc55 and tdc49 × tdc47 (both with 0.25 kg). The longest fruits were those of tdc15/13 × tdc55 and tdc13/5 × tdc59, while those with broadest diameter were observed in tdc49 × tdc47, tdc55 × tdc49, and tdc11/13 × tdc55. The worst negative special combining ability effect was detected in the tdc47 × tdc60 combination (Table 6). Most of the best hybrids were obtained by crossing parents from different heterotic groups.

Table 6.
Yield and yield component values of 82 experimental hybrids generated from half-diallel cross having high GCA compared with five commercial controls. Experimental hybrids and commercial hybrids used as controls are ranked according to total yield.
4. Discussion
The phenotypic descriptors used for eggplant characterization were highly effective for revealing a high variation among the inbred lines used in this study. This finding is consistent with other published works on eggplant line diversity [21,22,23]. These works showed that morphological dissimilarities could be efficient in the identification of genetic diversity among the cultivars.
Eggplant has a formidable potential for exploitation of heterosis for yield and quality traits, and for resistance to biotic stresses [9,24]. In addition, there is evidence that selecting parents that are genetically distant may be useful for obtaining heterotic hybrids in terms of yield [25,26,27]. In fact, in eggplant it has been found that there is a correlation between the yield and genetic distance based on molecular markers among parents [27]. Similarly to the findings of these authors, we did not find a relationship between the distance obtained with morphological data and the heterotic performance of hybrids. In this way, some of the most heterotic combinations were between materials morphologically very similar. In maize, Benchimol et al. [28] found that while the correlations of parental genetic distances and heterosis of the hybrids from the same heterotic group cross were high for grain yield, when parents were from different heterotic groups the correlations were low. In our case, we found that by crossing lines from different heterotic groups, or that belonged to both heterotic groups, high yields were generally obtained. However, high yields are not enough for a successful eggplant variety having high marketable value. There is a need for other characteristics, such as high fruit quality, long shelf-life and other quality attributes for F1 varieties, in addition to the yield [2]. However, the large number of hybrids that we obtained with yield superior to the commercial F1 hybrid cultivars and the fact that many of them include one or both parental lines with resistance to FOM indicates that some of them may be promising as candidates for registration of new varieties. In fact, given that resistance to FOM present in the lines evaluated is dominant [15], hybrids for which one or both parent lines are resistant to FOM are expected to be resistant to this pathogen.
Mohammadi and Prasanna [19] stated that the identifying parental combination and assigning lines into specific heterotic groups can be a useful and practical way to select promising hybrids. Thanks to the establishment of heterotic groups, we have found that it may be possible to obtain superior hybrids of eggplant by testing a reduced number of hybrid combinations. A similar pattern of results was reported by Melchinger and Gumber [12]. Similarly, Miranda et al. [29] provided information on the enhancement of improved lines and cultivars, and their favorable outcomes were based on the selection of parents and their distribution to specific heterotic groups. Another interesting result revealed in our study is that sister lines, despite their genetic similarity, can belong to different heterotic groups, suggesting that residual genetic variation among them may have important implications in the expression of heterosis. It was evident in our study that the evaluation of the GCA of lines and assigning them into heterotic group greatly facilitated the election of parents.
5. Conclusions
Our work is the first study aimed at establishing heterotic groups in eggplant. We found that there is no relationship between morphological relationships and GCA or membership of a specific heterotic group. In order to better understand the true combining ability of inbred lines, it is necessary to use more than one tester for establishing heterotic groups. Thus, outperforming hybrid combinations can be obtained with a small number of crosses between selected parents from heterotic groups. The combination of high general combining ability and establishment of heterotic groups allows the selection of the best combinations of parents in eggplant breeding programs. As a result of our study, we also obtained hybrids with dramatically increased yield that are superior to present commercial cultivars. Some of these hybrids are also resistant to FOM, providing an added value to their inherent high yield.
Author Contributions
Conceptualization, H.F.B. and A.U.; methodology, H.F.B., E.G., and J.P.; resources, H.F.B., A.U. and J.P.; formal analysis, H.F.B., M.O. and M.P.; investigation, H.F.B., E.G., A.U., and M.O.; writing—original draft preparation, H.F.B. and M.O.; writing—review and editing, H.F.B., J.P. and M.P.; project administration, H.F.B. and A.U.; funding acquisition, H.F.B. and A.U. All authors have read and agreed to the published version of the manuscript.
Funding
Scientific and Technological Research Council of Turkey financially supported this study with Project No: 107G027. Mariola Plazas gratefully acknowledges financial support from Generalitat Valenciana and Fondo Social Europeo for a post-doctoral grant (APOSTD/2018/014).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chapman, M.A. Introduction: The Importance of Eggplant. In The Eggplant Genome, 1st ed.; Chapman, M.A., Ed.; Springer International Publishing: New York, NY, USA, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Taher, D.; Solberg, S.Ø.; Prohens, J.; Chou, Y.Y.; Rakha, M.; Wu, T.H. World Vegetable Center eggplant collection: Origin, composition, seed dissemination and utilization in breeding. Front. Plant. Sci. 2017, 8, 1484. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, C.; Prohens, J.; Raigón, M.D.; Stommel, J.R.; Nuez, F. Eggplant relatives as sources of variation for developing new rootstocks. Sci. Hort. 2011, 128, 14–22. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; D’Anna, F.; Palazzolo, E.; Mennella, G.; Rotino, G.L. Hybrids and allied species as potential rootstocks for eggplant: Effect of grafting on vigour, yield and overall fruit quality traits. Sci. Hort. 2018, 228, 81–90. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Rotino, G.L.; Palazzolo, E.; Mennella, G.; D’Anna, F. Solanum aethiopicum gr. gilo and its interspecific hybrids with S. melongena as alternative rootstocks for eggplant: Effects on vigor, yield, and fruit physicochemical properties of cultivar ‘Scarlatti’. Agronomy 2019, 9, 223. [Google Scholar] [CrossRef]
- Barchi, L.; Toppino, L.; Valentino, D.; Bassolino, L.; Portis, E.; Lanteri, S.; Rotino, G.L. QTL analysis reveals new eggplant loci involved in resistance to fungal wilts. Euphytica 2018, 214, 220. [Google Scholar] [CrossRef]
- Altinok, H.H.; Can, C.; Altinok, M.A. Characterization of Fusarium oxysporum f. sp. melongenae isolates from Turkey with ISSR markers and DNA sequence analyses. Eur. J. Plant. Pathol. 2018, 150, 609–621. [Google Scholar] [CrossRef]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases—Review and future prospects. Biol. Control. 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, V.; Jain, B.T.; Kaushik, P. Heterosis breeding in eggplant (Solanum melongena L.): Gains and provocations. Plants 2020, 9, 403. [Google Scholar] [CrossRef]
- Kaushik, P.; Plazas, M.; Prohens, J.; Vilanova, S.; Gramazio, P. Diallel genetic analysis for multiple traits in eggplant and assessment of genetic distances for predicting hybrids performance. PLoS ONE 2018, 13, e0199943. [Google Scholar] [CrossRef]
- Xing, L.; Hailong, Y.; Zhiyuan, L.; Xiaoping, L.; Zhiyuan, F.; Yumei, L.; Limei, Y.; Mu, X.; Honghao, L.; Zhang, Y. Heterotic group classification of 63 inbred lines and hybrid purity identification by using SSR markers in winter cabbage (Brassica oleracea L. var. capitata). Hortic. Plant. J. 2018, 4, 158–164. [Google Scholar] [CrossRef]
- Melchinger, A.E.; Gumber, R.K. Overview of Heterosis and Heterotic Groups in Agronomic Crops. In Concepts and Breeding of Heterosis in Crop Plants; Larnkey, K.R., Staub, J.E., Eds.; Crop Science Society of America, Inc.: Madison, WI, USA, 1998; Volume 25, pp. 29–44. [Google Scholar] [CrossRef]
- Larièpe, A.; Moreau, L.; Laborde, J.; Bauland, C.; Mezmouk, S.; Décousset, L.; Mary-Huard, T.; Fièvet, J.B.; Gallais, A.; Dubreuil, P.; et al. General and specific combining abilities in a maize (Zea mays L.) test-cross hybrid panel: Relative importance of population structure and genetic divergence between parents. Theor. Appl. Genet. 2017, 130, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhao, L.; Wang, Y.; Zhou, R.; Song, L.; Xu, L.; Cui, X.; Li, R.; Yu, W.; Zhao, T. Genetic diversity of 324 cultivated tomato germplasm resources using agronomic traits and InDel markers. Euphytica 2019, 215, 69. [Google Scholar] [CrossRef]
- Boyacı, H.F.; Ünlü, A.; Abak, K. Genetic analysis of resistance to wilt caused by Fusarium (Fusarium oxysporum melongenae) in eggplant (Solanum melongena). Indian J. Agric. Sci. 2011, 81, 812–815. [Google Scholar]
- Rohlf, F.J. NTSYS-pc version 2.0. Numerical Taxonomy and Multivariate Analysis System, Exeter Software; Applied Biostatistics Inc.: Setauket, NY, USA, 1998. [Google Scholar]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Longin, C.F.-H.; Utz, H.F.; Melchinger, A.E.; Reif, J.C. Hybrid maize breeding with doubled haploids: II. Optimum type and number of testers in two-stage selection for general combining ability. Theor. Appl. Genet. 2007, 114, 393–402. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Prasanna, B.M. Analysis of genetic diversity in crop plants-salient statistical tools and considerations. Crop. Sci. 2003, 43, 1235–1248. [Google Scholar] [CrossRef]
- Menkir, A.; Badu-Apruka, B.; The, C.; Adepoju, A. Evaluation of heterotic patterns of IITA’s lowland white maize lines. Maydica 2003, 48, 161–170. [Google Scholar]
- Muñoz-Falcón, J.E.; Prohens, J.; Vilanova, S.; Nuez, F. Diversity in commercial varieties and landraces of black eggplants and implications for broadening the breeders’ gene pool. Ann. Appl. Biol. 2009, 154, 453–465. [Google Scholar] [CrossRef]
- Cericola, F.; Portis, E.; Toppino, L.; Barchi, L.; Acciarri, N.; Ciriaci, T.; Sala, T.; Rotino, G.L.; Lanteri, S. The population structure and diversity of eggplant from Asia and the Mediterranean Basin. PLoS ONE 2013, 8, e73702. [Google Scholar] [CrossRef]
- Kaushik, P.; Prohens, J.; Vilanova, S.; Gramazio, P.; Plazas, M. Phenotyping of eggplant wild relatives and interspecific hybrids with conventional and phenomics descriptors provides insight for their potential utilization in breeding. Front. Plant. Sci. 2016, 7, 677. [Google Scholar] [CrossRef]
- Swarup, V. Genetic resources and breeding of aubergine (Solanum melongena L.). Acta Hort. 1995, 412, 71–79. [Google Scholar] [CrossRef]
- Kalloo, G. 43 - Eggplant: Solanum melongena L. In Genetic Improvement of Vegetable Crops; Kalloo, G., Bergh, B.O., Eds.; Pergamon: Oxford, UK, 1993; pp. 587–604. [Google Scholar] [CrossRef]
- Qian, W.; Sass, O.; Meng, J.; Li, M.; Frauen, M.; Jung, C. Heterotic patterns in rapeseed (Brassica napus L.): I. Crosses between spring and Chinese semi-winter lines. Theor. Appl. Genet. 2007, 115, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Burruezo, A.; Prohens, J.; Nuez, F. Performance of hybrids between local varieties of eggplant (Solanum melongena) and its relation to the mean of parents and to morphological and genetic distances among parents. Eur. J. Hortic. Sci. 2008, 73, 76–83. [Google Scholar]
- Benchimol, L.L.; de Souza, C.L., Jr.; Garcia, A.A.F.; Kono, P.M.S.; Mangolin, C.A.; Barbosa, M.M.A.; Coelho, A.S.G.; de Souza, A.P. Genetic diversity in tropical maize inbred lines: Heterotic group assignment and hybrid performance determined by RFLP markers. Plant. Breed. 2000, 119, 491–496. [Google Scholar] [CrossRef]
- Miranda, G.V.; De Souza, L.V.; Galvão, J.C.C.; Guimarães, L.J.M.; De Melo, A.V.; Dos Santos, I.C. Genetic variability and heterotic groups of Brazilian popcorn populations. Euphytica 2008, 162, 431–440. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).