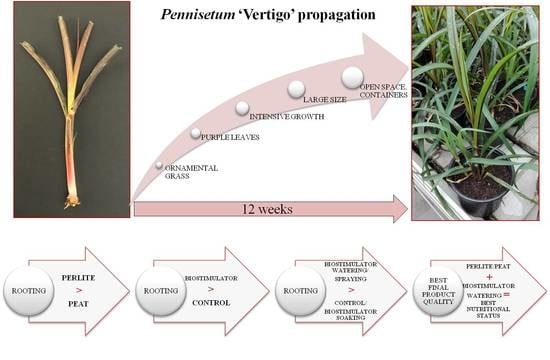
Rooting Media and Biostimulator Goteo Treatment Effect the Adventitious Root Formation of Pennisetum ‘Vertigo’ Cuttings and the Quality of the Final Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
- soaking the 2 cm cuttings base for 20 min in Goteo before the cutting were stuck (soaking),
- watering the cuttings with Goteo 5 times every 5 days (watering),
- applying foliar Goteo spraying 5 times every 5 days (spraying),
- for each Goteo treatment, a control was set up in which the cuttings were treated as follows: soaking with water, watering with water and spraying with water. An absolute control trial was also set up in which the cuttings were not treated with either the Goteo or the water; the results obtained for the absolute control were not statistically different from those obtained with the 3 control methods treated with water. On this basis, only the absolute control was selected for further statistical analyzes (control).
2.2. Subsequent Cultivation to the Final Product
2.3. Nutritional Status of Plants
2.4. Statistical Analysis
3. Results
3.1. Rooting of Cuttings
3.2. Subsequent Cultivation to the Final Product
3.3. Nutritional Status of Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darke, R. The Encyclopedia of Grasses for Livable Landscapes; Timber Press: Portland, OR, USA; London, UK, 2007; pp. 13–32. [Google Scholar]
- Kapczyńska, A. Effect of seed age and fertilisation on the growth and decorative quality of selected ornamental grasses. Folia Hortic. 2012, 24, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yuan, X.; Teng, W.; Chen, C.; Liu, H.; Wu, J. Karyotype diversity analysis and nuclear genome size estimation for Pennisetum Rich. (Poaceae) ornamental grasses reveal genetic relationship and chromosomal evolution. Sci. Hortic. 2015, 193, 22–31. [Google Scholar] [CrossRef]
- Contreras, R.N.; Owen, J.; Hanna, W.; Schwartz, B. Evaluation of Seven Complex Pennisetum Hybrids for Container and Landscape Performance in the Pacific Northwestern United States. Hort. Technol. 2013, 23, 525–528. [Google Scholar] [CrossRef]
- Hanna, W.W.; Braman, S.K.; Schwartz, B.M. Registration of Tift 8 Trispecific Ornamental Pennisetum. J. Plant. Regist. 2011, 5, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Hanna, W. Tift 15, Tift 26, Tift 114, Tift 118, and Tift 125 ornamental Pennisetums. HortScience 2016, 51, 444–447. [Google Scholar] [CrossRef] [Green Version]
- Ramanayake, S.M.S.D.; Meemaduma, V.N.; Weerawardene, T.E. In vitro shoot proliferation and enhancement of rooting for the large-scale propagation of yellow bamoo (Bambusa vulgaris Striat.). Sci. Hortic. 2006, 110, 109–113. [Google Scholar] [CrossRef]
- Gajewski, M.; Gos, K.; Bobruk, J. The influence of Goëmar Goteo biostimulator on yield and quality of two chinese cabbage cultivars. In Biostimulators in Modern Agriculture; Vegetable Crops; Dąbrowski, Z., Ed.; Editorial House Wieś Jutra: Warszawa, Poland, 2008; pp. 21–27. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant. Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Majkowska-Gadomska, J.; Francke, A.; Dobrowolski, A. The effect of selected biostimulants on seed germination of four plant species. Acta Agroph. 2017, 24, 591–599. [Google Scholar]
- Tarakhovskaya, E.R.; Maslov, Y.I.; Shishova, M.F. Phytohormones in algae. Russ. J. Plant. Physiol. 2007, 54, 163–170. [Google Scholar] [CrossRef]
- Rayorath, P.; Jithesh, M.N.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. Environ. Boil. Fishes 2007, 20, 423–429. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. The potential usefulness of a new generation of agro-products based on raw materials of biological origin. Acta Sci. Pol. Hortorum Cultus 2016, 15, 97–120. [Google Scholar]
- Pacholczak, A.; Nowakowska, K.; Mika, N.; Borkowska, M. The effect of the biostimulator Goteo on the rooting of ninebark stem cuttings. Folia Hortic. 2016, 28, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Nowakowska, K.; Pacholczak, A. Effect of the biopreparation "Goteo" on rooting of Hydrangea stem cuttings (Hydrangea paniculata siebold Limelight and Vanille Freise®Renhy). Propag. Ornam. Plants 2017, 17, 126–133. [Google Scholar]
- Salachna, P.; Zawadzinska, A.; Piechocki, R.; Wilas, J. Propagation of arabian star flower (Ornithogalum arabicum L.) by twin scales using seaweed extracts. Folia Pomeranae Univ. Technol. Stetin. 2014, 310, 105–112. [Google Scholar]
- Pacholczak, A.; Nowakowska, K. The Effect of Biostimulators and Indole-3-Butyric Acid on Rooting of Stem Cuttings of Two Ground Cover Roses. Acta Agrobot. 2020, 73. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.L.; Crespi, M.S.U. Plant root growth, architecture and function. Plant. Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Soethe, N.; Wilcke, W.; Homeier, J.; Lehmann, J.; Engels, C. Plant Growth Along the Altitudinal Gradient —Role of Plant Nutritional Status, Fine Root Activity, and Soil Properties. In Root Ecology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008; Volume 198, pp. 259–266. [Google Scholar]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant. Biol. 2015, 58, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Franco, J.A.; Martínez-Sánchez, J.J.; Fernández, J.A.; Bañón, S. Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J. Hortic. Sci. Biotechnol. 2006, 81, 3–17. [Google Scholar] [CrossRef]
- Pasławski, P.; Migaszewski, Z.M. The quality of element determinations in plant materials by instrumental methods. Pol. J. Environ. Stud. 2006, 15, 154–164. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Arlington, VA, USA, 1994. [Google Scholar]
- Cunliffe, B.A.; Meyer, M.H.; Ascher, P.D. Propagation of Pennisetum setaceum Rubrum from Cuttings. J. Environ. Hortic. 2001, 19, 1–3. [Google Scholar] [CrossRef]
- Kang, D.-J.; Ishii, Y.; Nishiwaki, A. Effects of the shoot-cutting method on field propagation in napiergrass (Pennisetum purpureum Schum.). J. Crop. Sci. Biotechnol. 2011, 14, 139–142. [Google Scholar] [CrossRef]
- Meyer, M.H.; Hong, J. Miscanthus × giganteus Can be Propagated from Stem Cuttings. J. Environ. Hortic. 2011, 29, 193–196. [Google Scholar] [CrossRef]
- Cunliffe, B.A.; Meyer, M.H. Propagation Time Affects Winter Survival and Finishing Date for Ornamental Grasses. J. Environ. Hortic. 2002, 20, 201–203. [Google Scholar] [CrossRef]
- Harvey, M.P.; Brand, M.H. Division Size and Shade Density Influence Growth and Container Production of Hakonechloa macra Makino Aureola. HortScience 2002, 37, 196–199. [Google Scholar] [CrossRef]
- Xue, S.; Kalinina, O.; Lewandowski, I. Present and future options for Miscanthus propagation and establishment. Renew. Sustain. Energy Rev. 2015, 49, 1233–1246. [Google Scholar] [CrossRef]
- Ceotto, E.; Di Candilo, M. Shoot cuttings propagation of giant reed (Arundo donax L.) in water and moist soil: The path forward? Biomass Bioenergy 2010, 34, 1614–1623. [Google Scholar] [CrossRef]
- Scordia, D.; Zanetti, F.; Varga, S.S.; Alexopoulou, E.; Cavallaro, V.; Monti, A.; Copani, V.; Cosentino, S. New Insights into the Propagation Methods of Switchgrass, Miscanthus and Giant Reed. BioEnergy Res. 2015, 8, 1480–1491. [Google Scholar] [CrossRef]
- Haegele, T.; Arjharn, W. The effects of cultivation methods and planting season on biomass yield of Napier grass (Pennisetum purpureum Schumach.) under rainfed conditions in the northeast region of Thailand. Field Crop. Res. 2017, 214, 359–364. [Google Scholar] [CrossRef]
- Gebrehiwot, K.; Woldetensae, T.; Birhane, E.; Tewolde-Berhan, S. Propagation potential of the lowland bamboo through seed and culm cuttings. J. Drylands 2016, 6, 513–518. [Google Scholar]
- Owen, W.G.; Lopez, R.G. Propagation Daily Light Integral and Root-Zone Temperature Influence Rooting of Single-internode Pennisetum ×advena Culm. Cuttings. HortScience 2018, 53, 176–182. [Google Scholar] [CrossRef]
- Hawkins, S.M.; Robacker, C.D. Micropropagation of Little Bluestem (Schizachyrium scoparium L.). HortScience 2019, 54, 348–352. [Google Scholar] [CrossRef] [Green Version]
- Ilahi, W.F.F.; Ahmad, D. A study on the physical and hydraulic characteristics of cocopeat perlite mixture as a growing media in containerized plant production. Sains Malays. 2017, 46, 975–980. [Google Scholar] [CrossRef]
- Olle, M.; Ngouajio, M.; Siomos, A. Vegetable quality and productivity as influenced by growing medium: A review. Zemdirbyste 2012, 99, 399–408. [Google Scholar]
- Szmidt, R.; Hitchon, G.; Hall, D. Sterilisation of perlite growing substrates. Acta Hortic. 1989, 255, 197–204. [Google Scholar] [CrossRef]
- Agulló-Antón, M.Á.; Sánchez-Bravo, J.; Acosta, M.; Druege, U. Auxins or Sugars: What Makes the Difference in the Adventitious Rooting of Stored Carnation Cuttings? J. Plant. Growth Regul. 2011, 30, 100–113. [Google Scholar] [CrossRef]
- Atkinson, C.J. Establishing perennial grass energy crops in the UK: A review of current propagation options for Miscanthus. Biomass Bioenergy 2009, 33, 752–759. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Institute of Plant Nutrition, University of Hohenheim: Stuttgart, Germany, 1995; pp. 229–299. [Google Scholar]
- Chouliaras, V.; Tasioula, M.; Chatzissavvidis, C.; Therios, I.; Tsabolatidou, E. The effects of a seaweed extract in addition to nitrogen and boron fertilization on productivity, fruit maturation, leaf nutritional status and oil quality of the olive (Olea europaea L.) cultivar Koroneiki. J. Sci. Food Agric. 2009, 89, 984–988. [Google Scholar] [CrossRef]
- Rathore, S.; Chaudhary, D.R.; Boricha, G.; Ghosh, A.; Bhatt, B.; Zodape, S.; Patolia, J. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. South. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Dudaš, S.; Šola, I.; Sladonja, B.; Erhatić, R.; Ban, D.; Poljuha, D. The Effect of Biostimulant and Fertilizer on “Low Input” Lettuce Production. Acta Bot. Croat. 2016, 75, 253–259. [Google Scholar] [CrossRef] [Green Version]


| Medium | Goteo | Rooting (%) | Roots (no.) | Root Length (cm) | Root FW (g) | Root DW (%) |
|---|---|---|---|---|---|---|
| Peat | Control | 83.7 ± 5.5 c | 2.3 ± 0.5 c | 5.7 ± 1.1 e | 0.7 ± 0.1 b | 9.2 ± 0.5 a |
| Soaking | 83.3 ± 5.5 c | 2.5 ± 0.2 bc | 6.7 ± 0.7 de | 0.8 ± 0.1 b | 8.5 ± 0.1 ab | |
| Spraying | 94.3 ± 5.5 ab | 2.4 ± 0.4 bc | 7.2 ± 0.6 de | 0.7 ± 0.1 b | 8.4 ± 0.4 ab | |
| Watering | 100.0 ± 0.0 a | 2.6 ± 0.2 bc | 8.3 ± 0.8 c | 0.8 ± 0.1 b | 8.1 ± 0.3 b | |
| Perlite | Control | 94.0 ± 5.5 bab | 3.1 ± 0.2 ab | 8.3 ± 1.0 cd | 1.0 ± 0.1 ab | 8.3 ± 1.1 ab |
| Soaking | 89.0 ± 0.0 bc | 3.0 ± 0.3 ab | 9.6 ± 1.0 bc | 1.2 ± 0.2 a | 8.6 ± 0.2 ab | |
| Spraying | 100.0 ± 0.0 a | 3.2 ± 0.4 a | 10.1 ± 1.3 b | 1.0 ± 0.4 ab | 9.0 ± 0.1 ab | |
| Watering | 100.0 ± 0.0 a | 3.2 ± 1.1 a | 11.9 ± 0.6 a | 1.0 ± 0.1 ab | 8.2 ± 0.4 b | |
| Peat | 90.3 ± 8.4 b | 2.5 ± 0.3 b | 7.0 ± 1.2 b | 0.7 ± 0.1 b | 8.6 ± 0.5 | |
| Perlite | 95.8 ± 5.3 a | 3.3 ± 0.5 a | 10.0 ± 1.6 a | 1.1 ± 0.2 a | 8.5 ± 0.6 | |
| Control | 89.0 ± 7.6 b | 2.7 ± 0.7 | 7.0 ± 1.7 c | 0.8 ± 0.2 | 8.8 ± 0.9 | |
| Soaking | 86.2 ± 4.7 b | 3.0 ± 0.6 | 8.2 ± 1.8 b | 1.0 ± 0.3 | 8.6 ± 0.2 | |
| Spraying | 97.2 ± 4.7 a | 2.8 ± 0.6 | 8.7 ± 1.8 b | 0.9 ± 0.3 | 8.7 ± 0.4 | |
| Watering | 100.0 ± 0.0 a | 2.9 ± 0.8 | 10.1 ± 2.1 a | 0.9 ± 0.1 | 8.2 ± 0.3 | |
| Main Effects | ||||||
| Medium × Goteo | * | * | * | * | * | |
| Medium | * | * | * | * | NS | |
| Goteo | * | NS | * | NS | NS | |
| Medium | Goteo | Plant Height (cm) | Stems (no.) | Overground Part FW (g) |
|---|---|---|---|---|
| Peat | Control | 91.0 ± 2.5 c | 3.2 ± 0.1 bc | 47.9 ± 6.6 ab |
| Soaking | 109.6 ± 7.8 b | 2.8 ± 0.3 bc | 47.4 ± 2.2 ab | |
| Spraying | 105.3 ± 8.6 b | 3.0 ± 0.5 bc | 42.4 ± 5.7 bc | |
| Watering | 107.7 ± 1.7 b | 4.0 ± 0.7 ab | 52.0 ± 6.8 a | |
| Perlite | Control | 82.9 ± 1.6 c | 2.2 ± 0.2 c | 39.9 ± 6.2 bc |
| Soaking | 106.5 ± 6.5 b | 2.2 ± 0.1 c | 36.8 ± 3.9 c | |
| Spraying | 112.8 ± 6.4 ab | 2.2 ± 0.1 c | 42.7 ± 0.4 bc | |
| Watering | 120.8 ± 2.7 a | 4.8 ± 0.2 a | 50.7 ± 4.6 a | |
| Peat | - | 103.4 ± 9.0 | 3.2 ± 0.5 | 47.4 ± 6.0 a |
| Perlite | 105.8 ± 15.3 | 2.8 ± 0.6 | 42.5 ± 6.5 b | |
| Control | 87.0 ± 4.8 b | 2.8 ± 0.3 b | 44.0 ± 7.3 b | |
| Soaking | 108.1 ± 6.7 a | 2.6 ± 0.3 b | 42.1 ± 6.5 b | |
| Spraying | 109.1 ± 7.9 a | 2.6 ± 0.4 b | 42.6 ± 3.6 b | |
| Watering | 114.2 ± 7.5 a | 4.4 ± 0.5 a | 51.4 ± 5.3 a | |
| Main Effects | ||||
| Medium × Goteo | * | * | * | |
| Medium | NS | NS | * | |
| Goteo | * | * | * | |
| Root Length (cm) | Root FW (g) | Root DW (%) | Plant Height (cm) | Stems (no.) | Aboveground Part FW (g) | |
|---|---|---|---|---|---|---|
| Roots (no.) | 0.556 * | 0.539 * | NS | NS | NS | NS |
| Root length (cm) | - | 0.594 * | NS | 0.545 * | NS | NS |
| Root FW (g) | - | - | NS | NS | NS | NS |
| Root DW (%) | - | - | - | NS | NS | NS |
| Plant height (cm) | - | - | - | - | NS | NS |
| Stems (no.) | - | - | - | - | - | 0.421 * |
| Medium | Goteo | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|---|
| Peat | Control | 1.38 ± 0.04 a | 0.48 ± 0.03 c | 2.42 ± 0.06 b | 0.33 ± 0.03 | 0.40 ± 0.05 | 0.15 ± 0.03 |
| Soaking | 0.99 ± 0.06 ac | 0.40 ± 0.02 c | 2.23 ± 0.12 b | 0.20 ± 0.02 | 0.29 ± 0.04 | 0.11 ± 0.01 | |
| Spraying | 1.33 ± 0.18 ab | 0.55 ± 0.01 bc | 2.78 ± 0.03 b | 0.26 ± 0.03 | 0.42 ± 0.09 | 0.16 ± 0.02 | |
| Watering | 1.07 ± 0.05 ac | 0.81 ± 0.11 a | 2.72 ± 0.37 b | 0.24 ± 0.04 | 0.44 ± 0.02 | 0.17 ± 0.05 | |
| Perlite | Control | 1.13 ± 0.04ac | 0.45 ± 0.01c | 2.19 ± 0.04b | 0.22 ± 0.03 | 0.26 ± 0.02 | 0.75 ± 0.03 |
| Soaking | 0.94 ± 0.02 bc | 0.61 ± 0.02 ac | 3.68 ± 0.16 a | 0.25 ± 0.02 | 0.39 ± 0.02 | 0.14 ± 0.00 | |
| Spraying | 0.87 ± 0.09 c | 0.45 ± 0.01 c | 2.40 ± 0.05 b | 0.21 ± 0.01 | 0.39 ± 0.02 | 0.13 ± 0.00 | |
| Watering | 1.39 ± 0.08 a | 0.75 ± 0.02 ab | 2.40 ± 0.12 b | 0.26 ± 0.02 | 0.35 ± 0.01 | 0.14 ± 0.01 | |
| Peat | - | 1.19 ± 0.07 | 0.56 ± 0.05 | 2.54 ± 0.11 | 0.26 ± 0.02 | 0.37 ± 0.03 | 0.15 ± 0.01 |
| Perlite | - | 1.08 ± 0.07 | 0.57 ± 0.04 | 2.67 ± 0.18 | 0.24 ± 0.01 | 0.39 ± 0.02 | 0.12 ± 0.01 |
| Control | 1.25 ± 0.06 a | 0.47 ± 0.01 b | 2.31 ± 0.06 b | 0.27 ± 0.03 | 0.33 ± 0.04 | 0.11 ± 0.03 | |
| Soaking | 0.97 ± 0.03 b | 0.51 ± 0.05 b | 2.96 ± 0.34 a | 0.23 ± 0.02 | 0.34 ± 0.03 | 0.13 ± 0.01 | |
| Spraying | 1.10 ± 0.14 ab | 0.50 ± 0.02 b | 2.59 ± 0.08 ab | 0.24 ± 0.02 | 0.40 ± 0.01 | 0.15 ± 0.01 | |
| Watering | 1.23 ± 0.08 a | 0.78 ± 0.05 a | 2.56 ± 0.19 ab | 0.25 ± 0.02 | 0.39 ± 0.05 | 0.16 ± 0.02 | |
| Main effects | |||||||
| Medium × Goteo | * | * | * | NS | NS | NS | |
| Medium | NS | NS | NS | NS | NS | NS | |
| Goteo | * | * | * | NS | NS | NS | |
| Medium | Goteo | Fe | B | Cu | Mo | Zn | Mn |
|---|---|---|---|---|---|---|---|
| Peat | Control | 28.37 ± 2.46 b | 5.08 ± 0.67 | 5.67 ± 0.35 b | 2.37 ± 0.27 | 31.51 ± 9.86 | 39.60 ± 3.80 |
| Soaking | 23.70 ± 2.30 b | 4.19 ± 0.07 | 7.80 ± 0.34 ab | 1.09 ± 0.05 | 21.86 ± 1.50 | 38.44 ± 2.46 | |
| Spraying | 29.42 ± 2.25 b | 5.52 ± 0.44 | 5.82 ± 0.38 b | 1.82 ± 0.15 | 28.17 ± 5.36 | 33.57 ± 5.03 | |
| Watering | 31.65 ± 5.98 ab | 4.12 ± 1.10 | 9.45 ± 1.53 a | 1.48 ± 0.29 | 40.28 ± 10.16 | 52.41 ± 6.30 | |
| Perlite | Control | 37.70 ± 3.77 ab | 4.43 ± 0.40 | 7.49 ± 0.32 ab | 1.42 ± 0.11 | 22.99 ± 2.16 | 39.35 ± 4.28 |
| Soaking | 46.07 ± 1.94 a | 4.66 ± 0.42 | 9.17 ± 0.21 a | 2.03 ± 0.10 | 30.67 ± 2.66 | 43.18 ± 3.10 | |
| Spraying | 26.28 ± 0.78 b | 4.31 ± 0.28 | 9.29 ± 0.22 a | 1.42 ± 0.08 | 28.26 ± 0.46 | 40.10 ± 1.90 | |
| Watering | 30.70 ± 2.04 b | 4.75 ± 0.18 | 8.19 ± 0.16 ab | 1.42 ± 0.08 | 27.20 ± 0.67 | 33.252.08 | |
| Peat | - | 28.20 ± 1.77 b | 4.73 ± 0.34 | 7.18 ± 0.57 b | 1.69 ± 0.17 | 30.46 ± 3.81 | 41.00 ± 2.88 |
| Perlite | - | 35.19 ± 2.48 a | 4.54 ± 0.15 | 8.35 ± 0.24 a | 1.57 ± 0.09 | 27.28 ± 1.12 | 38.97 ± 1.68 |
| Control | 32.87 ± 2.95 | 4.57 ± 0.38 | 6.58 ± 0.46 b | 1.90 ± 0.25 | 27.25 ± 4.90 | 39.47 ± 2.56 | |
| Soaking | 34.88 ± 5.18 | 4.43 ± 0.22 | 8.48 ± 0.36 a | 1.56 ± 0.21 | 26.26 ± 2.40 | 40.81 ± 2.06 | |
| Spraying | 27.85 ± 1.28 | 4.91 ± 0.57 | 7.55 ± 0.80 a | 1.62 ± 0.16 | 28.21 ± 2.40 | 36.84 ± 2.81 | |
| Watering | 31.17 ± 2.83 | 4.43 ± 0.27 | 8.82 ± 0.75 a | 1.45 ± 0.08 | 33.74 ± 5.41 | 42.83 ± 5.21 | |
| Main effects | |||||||
| Medium × Goteo | * | NS | * | NS | NS | NS | |
| Medium | * | NS | * | NS | NS | NS | |
| Goteo | NS | NS | * | NS | NS | NS | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapczyńska, A.; Kowalska, I.; Prokopiuk, B.; Pawłowska, B. Rooting Media and Biostimulator Goteo Treatment Effect the Adventitious Root Formation of Pennisetum ‘Vertigo’ Cuttings and the Quality of the Final Product. Agriculture 2020, 10, 570. https://doi.org/10.3390/agriculture10110570
Kapczyńska A, Kowalska I, Prokopiuk B, Pawłowska B. Rooting Media and Biostimulator Goteo Treatment Effect the Adventitious Root Formation of Pennisetum ‘Vertigo’ Cuttings and the Quality of the Final Product. Agriculture. 2020; 10(11):570. https://doi.org/10.3390/agriculture10110570
Chicago/Turabian StyleKapczyńska, Anna, Iwona Kowalska, Barbara Prokopiuk, and Bożena Pawłowska. 2020. "Rooting Media and Biostimulator Goteo Treatment Effect the Adventitious Root Formation of Pennisetum ‘Vertigo’ Cuttings and the Quality of the Final Product" Agriculture 10, no. 11: 570. https://doi.org/10.3390/agriculture10110570
APA StyleKapczyńska, A., Kowalska, I., Prokopiuk, B., & Pawłowska, B. (2020). Rooting Media and Biostimulator Goteo Treatment Effect the Adventitious Root Formation of Pennisetum ‘Vertigo’ Cuttings and the Quality of the Final Product. Agriculture, 10(11), 570. https://doi.org/10.3390/agriculture10110570