PPMaP: Reproducible and Extensible Open-Source Software for Plant Phenological Phase Duration Prediction and Mapping in Sub-Saharan Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. PPMaP input Datasets
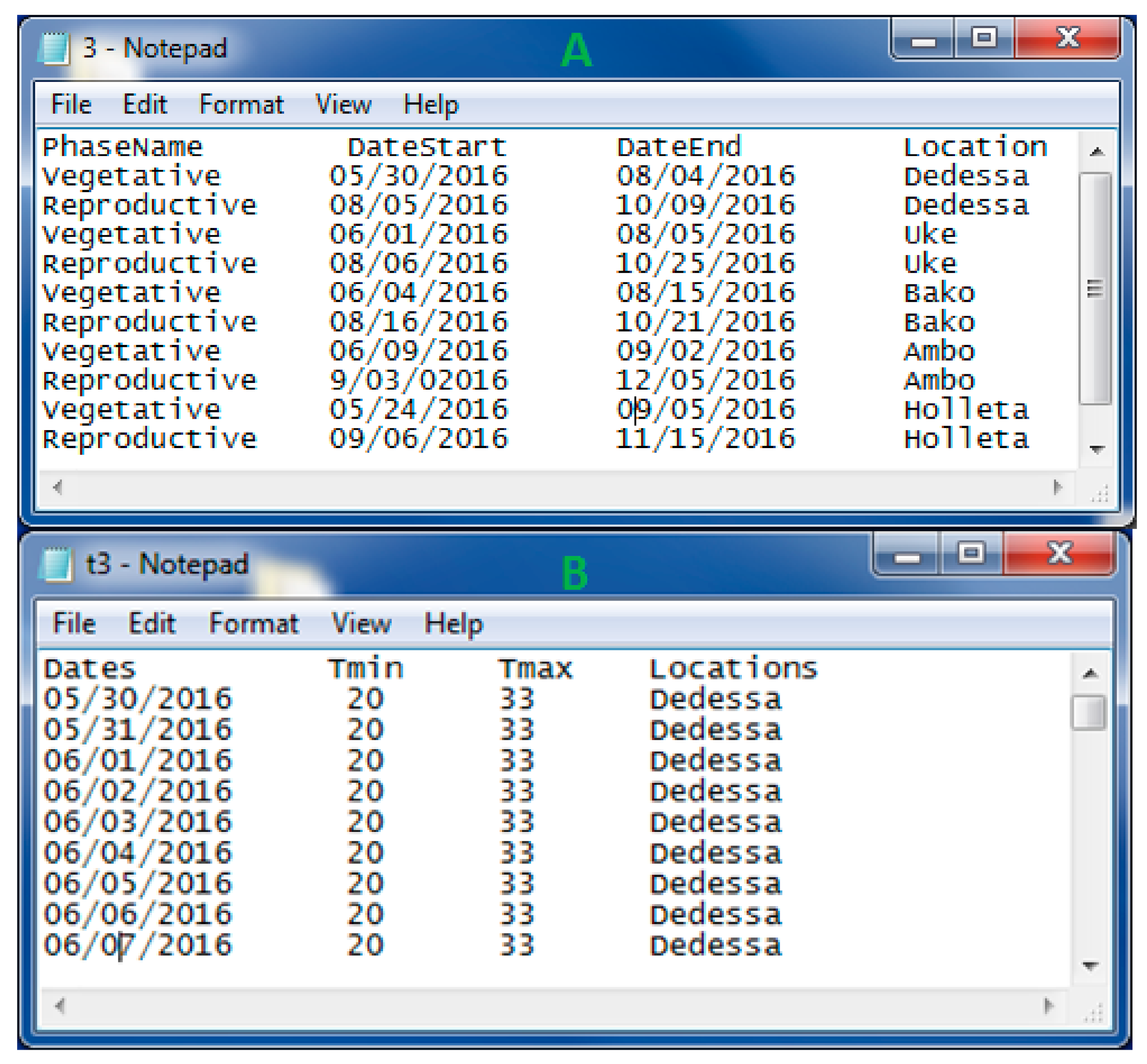
2.1.1. Plant Phenology Datasets
2.1.2. Temperature Datasets
2.2. PPMaP Software Components and Data Processing Mechanisms
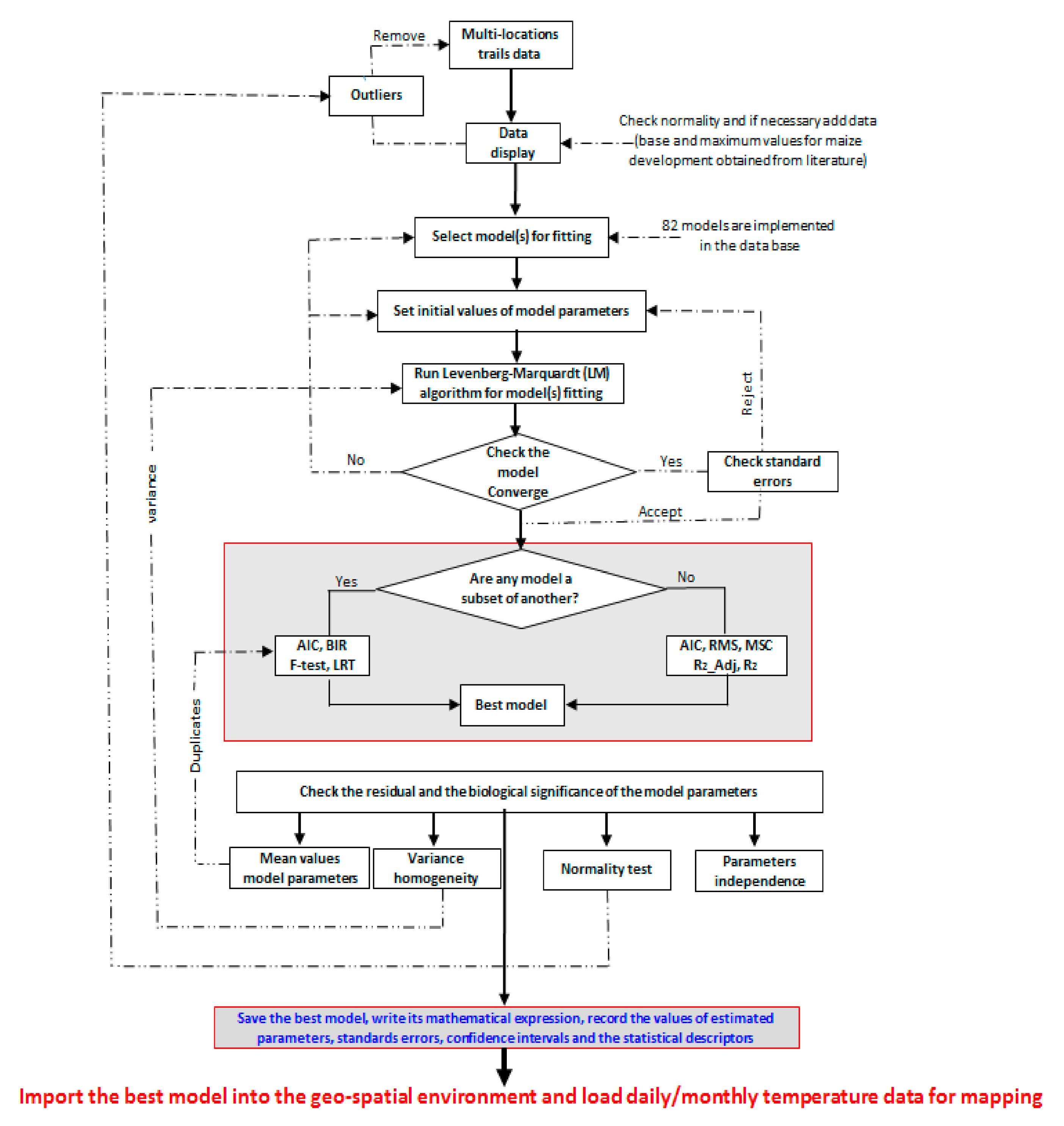
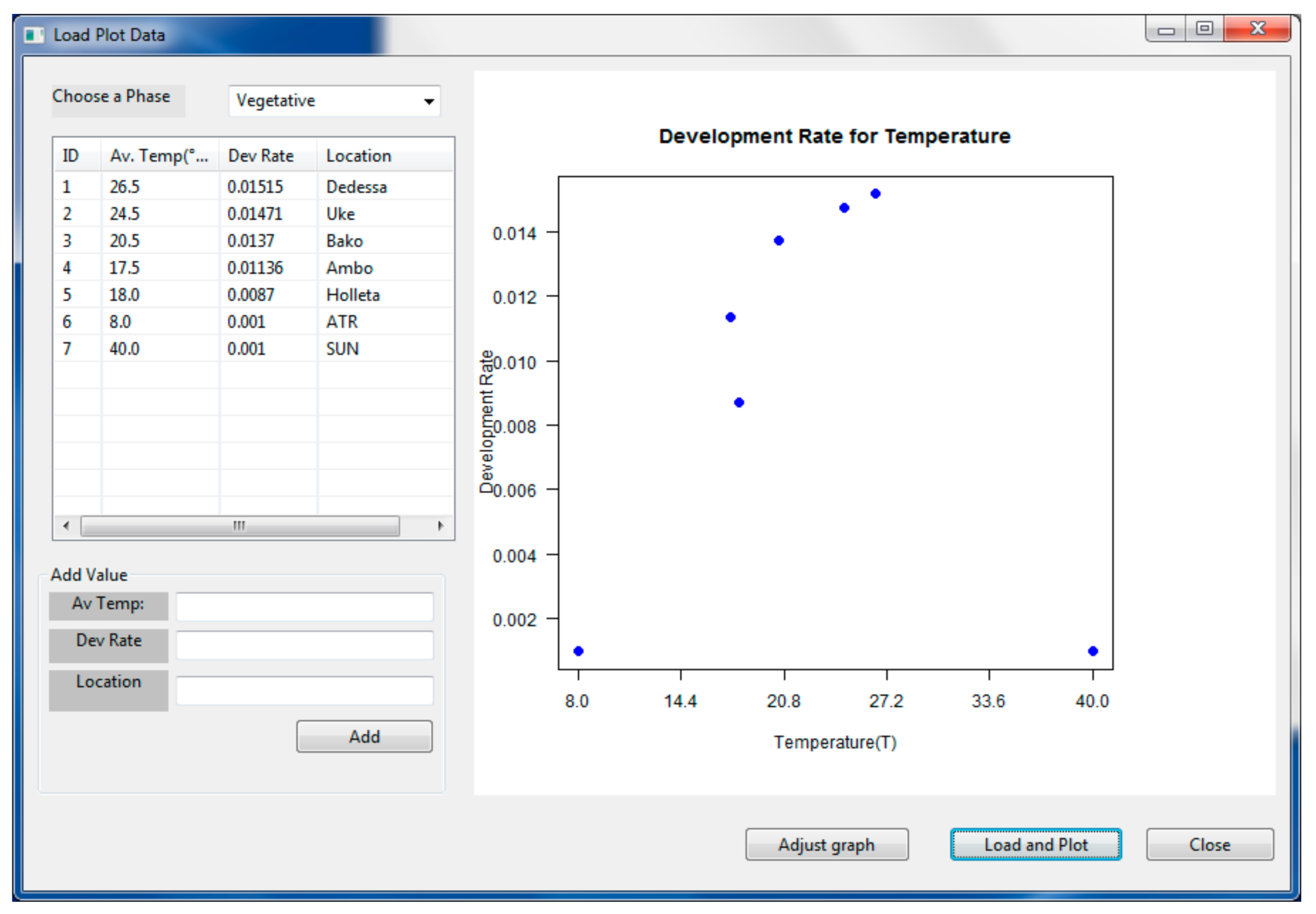
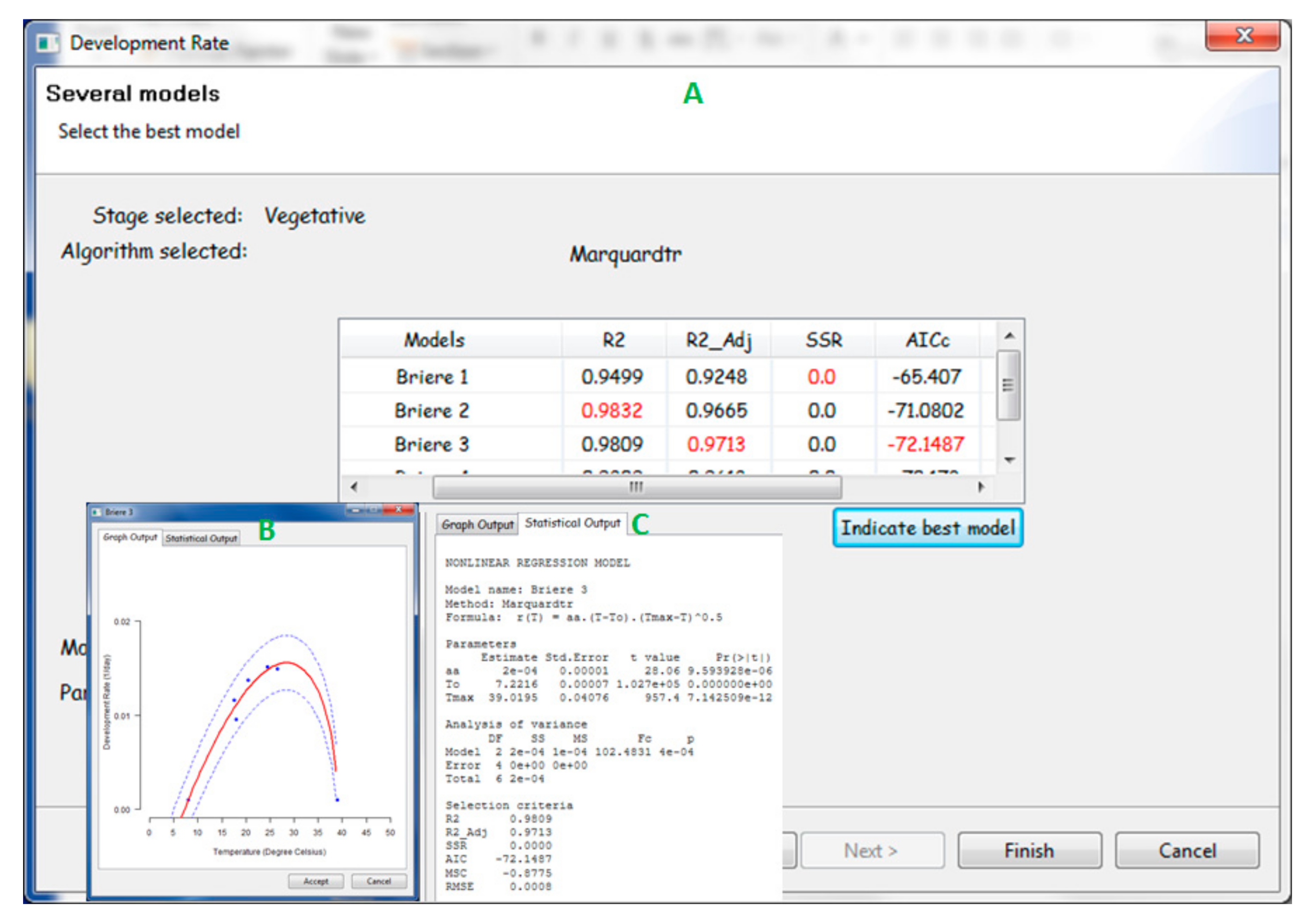
2.2.1. PPMaP Modeling Component
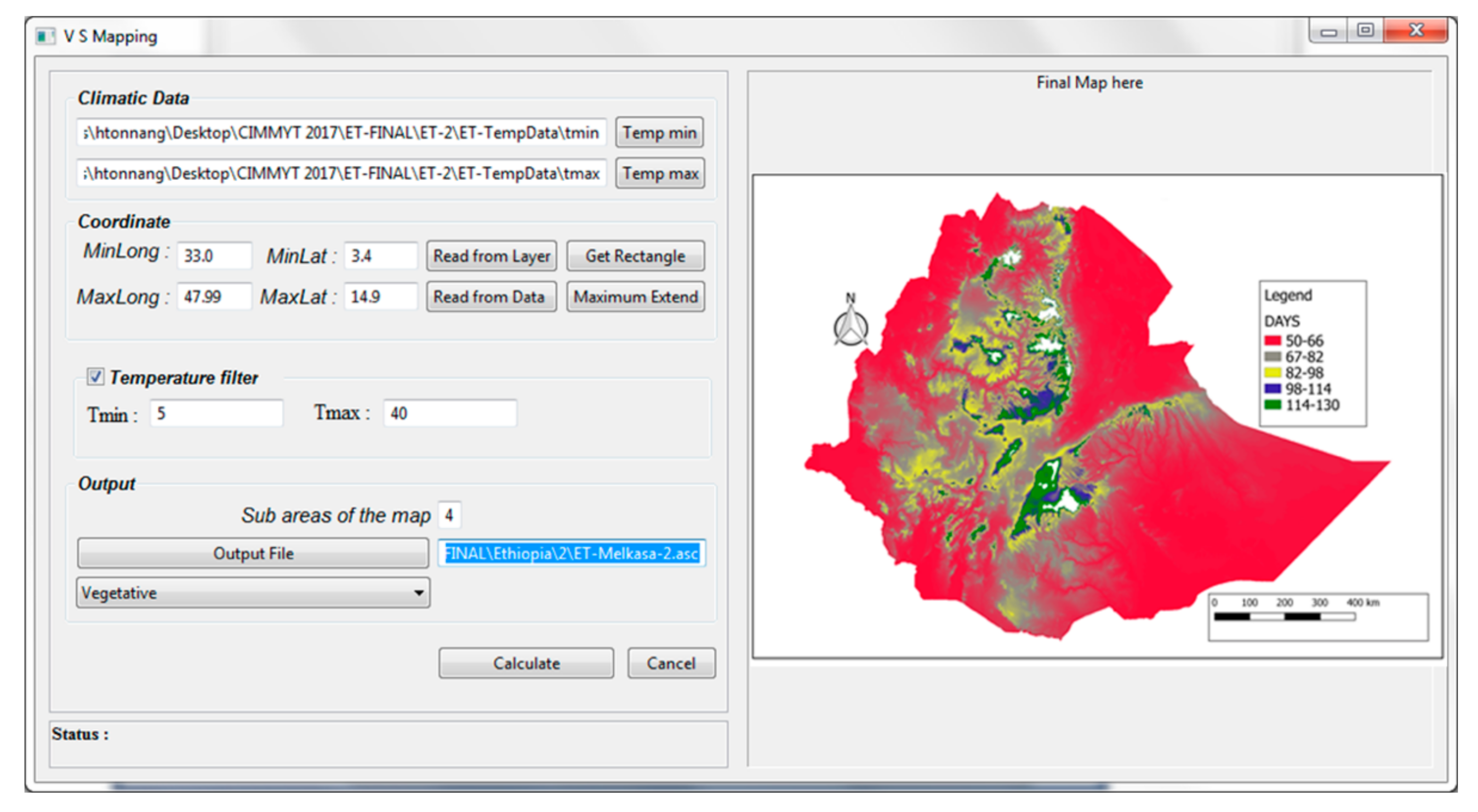
2.2.2. PPMaP Mapping Component
2.3. PPMaP Software Implementation
2.4. PPMaP Model Validation and Performance at Scale
3. PPMaP Software Usefulness and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Saiz-Rubio, V.; Rovira-Más, F. From smart farming towards agriculture 5.0: A review on crop data management. Agronomy 2020, 10, 207. [Google Scholar] [CrossRef]
- Mendes, J.; Pinho, T.M.; Neves dos Santos, F.; Sousa, J.J.; Peres, E.; Boaventura-Cunha, J.; Cunha, M.; Morais, R. Smartphone Applications Targeting Precision Agriculture Practices-A Systematic Review. Agronomy 2020, 10, 855. [Google Scholar] [CrossRef]
- Gungula, D.T.; Kling, J.G.; Togun, A.O. CERES-Maize predictions of maize phenology under nitrogen-stressed conditions in Nigeria. Agron. J. 2003, 95, 892–899. [Google Scholar] [CrossRef]
- Han, W.; Yang, Z.; Di, L.; Mueller, R. CropScape: A Web service based application for exploring and disseminating US conterminous geospatial cropland data products for decision support. Comput. Electron. Agric. 2012, 84, 111–123. [Google Scholar] [CrossRef]
- Joly, A.; Goëau, H.; Bonnet, P.; Bakić, V.; Barbe, J.; Selmi, S.; Yahiaoui, I.; Carré, J.; Mouysset, E.; Molino, J.F.; et al. Interactive plant identification based on social image data. Ecol. Inform. 2014, 23, 22–34. [Google Scholar] [CrossRef]
- Bobryk, C.W.; Myers, D.B.; Kitchen, N.R.; Shanahan, J.F.; Sudduth, K.A.; Drummond, S.T.; Gunzenhauser, B.; Gomez Raboteaux, N.N. Validating a digital soil map with corn yield data for precision agriculture decision support. Agron. J. 2016, 108, 957–965. [Google Scholar] [CrossRef]
- Dia, M.; Wehner, T.C.; Arellano, C. Analysis of genotype × environment interaction (G×E) using SAS programming. Agron. J. 2016, 108, 1838–1852. [Google Scholar] [CrossRef]
- Tonnang, H.E.Z.; Balemi, T.; Masuki, K.F.; Mohammed, I.; Adewopo, J.; Adnan, A.A.; Mudereri, B.T.; Vanlauwe, B.; Craufurd, P. Rapid acquisition, management, and analysis of spatial Maize (Zea mays L.) phenological data—Towards ‘Big Data’ for agronomy transformation in Africa. Agronomy 2020, 10, 1363. [Google Scholar] [CrossRef]
- Magarey, R.D.; Fowler, G.A.; Borchert, D.M.; Sutton, T.B.; Colunga-Garcia, M.; Simpson, J.A. NAPPFAST: An internet system for the weather-based mapping of plant pathogens. Plant Dis. 2007, 91, 336–345. [Google Scholar] [CrossRef]
- Inostroza, L.; Acuña, H.; Munoz, P.; Vásquez, C.; Ibáñez, J.; Tapia, G.; Pino, M.T.; Aguilera, H. Using aerial images and canopy spectral reflectance for high-throughput phenotyping of white clover. Crop. Sci. 2016, 56, 2629–2637. [Google Scholar] [CrossRef]
- Ritchie, J.T.; Nesmith, D.S. Temperature and Crop Development. In Modeling Plant and Soil Systems-Agronomy Monograph No. 31; American Society of Agronomy, Inc.; Crop Science Society of America, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 1991; pp. 5–29. [Google Scholar]
- Schröder, W.; Schmidt, G.; Schönrock, S. Modelling and mapping of plant phenological stages as bio-meteorological indicators for climate change. Environ. Sci. Eur. 2014, 26, 5. [Google Scholar] [CrossRef][Green Version]
- Wu, L.; Feng, L.; Zhang, Y.; Gao, J.; Wang, J. Comparison of five wheat models simulating phenology under different sowing dates and varieties. Agron. J. 2017, 109, 1280–1293. [Google Scholar] [CrossRef]
- Tonnang, H.E.Z.; Makumbi, D.; Craufurd, P. Methodological approach for predicting and mapping the phenological adaptation of tropical maize (Zea mays L.) using multi-environment trials. Plant Methods 2018, 14, 108. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, R.; Hou, P.; Li, S.; Zhang, H.; Ming, B.; Long, H.; Liang, S. Phenological responses of maize to changes in environment when grown at different latitudes in China. Field Crop. Res. 2013, 144, 192–199. [Google Scholar] [CrossRef]
- Sacks, W.J.; Kucharik, C.J. Crop management and phenology trends in the U.S. Corn Belt: Impacts on yields, evapotranspiration and energy balance. Agric. For. Meteorol. 2011, 151, 882–894. [Google Scholar] [CrossRef]
- Lobell, D.B.; Bänziger, M.; Magorokosho, C.; Vivek, B. Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat. Clim. Chang. 2011, 1, 42–45. [Google Scholar] [CrossRef]
- Hopkins, W.G. Plant Development; Infobase Publishing: New York, NY, USA, 2006; ISBN 0791085627. [Google Scholar]
- McMaster, G.S.; Green, T.R.; Erskine, R.H.; Edmunds, D.A.; Ascough, J.C. Spatial interrelationships between wheat phenology, thermal time, and terrain attributes. Agron. J. 2012, 104, 1110–1121. [Google Scholar] [CrossRef]
- Archontoulis, S.V.; Miguez, F.E. Nonlinear regression models and applications in agricultural research. Agron. J. 2015, 107, 786–798. [Google Scholar] [CrossRef]
- Chang, K.H.; Warland, J.S.; Bartlett, P.A.; Arain, A.M.; Yuan, F. A simple crop phenology algorithm in the land surface model CN-CLASS. Agron. J. 2014, 106, 297–308. [Google Scholar] [CrossRef]
- Yin, X.; Goudriaan, J.; Lantinga, E.A.; Vos, J.; Spiertz, H.J. A flexible sigmoid function of determinate growth. Ann. Bot. 2003, 91, 361–371. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A. An equation for modelling the temperature response of plants using only the cardinal temperatures. Ann. Bot. 1999, 607–614. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A powerful new tool for measuring fractional green canopy cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- Mudereri, B.T.; Abdel-Rahman, E.; Dube, T.; Landmann, T.; Niassy, S.; Tonnang, H.E.Z.; Khan, Z.R. Potential of resampled multispectral data for detecting Desmodium-Brachiaria intercropped with maize in a “Push-Pull” system. ISPRS Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2020, XLIII-B3, 1017–1022. [Google Scholar] [CrossRef]
- Gage, J.L.; Miller, N.D.; Spalding, E.P.; Kaeppler, S.M.; de Leon, N. TIPS: A system for automated image-based phenotyping of maize tassels. Plant Methods 2017, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Crain, J.; Mondal, S.; Rutkoski, J.; Singh, R.P.; Poland, J. Combining High-Throughput Phenotyping and Genomic Information to Increase Prediction and Selection Accuracy in Wheat Breeding. Plant Genome 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Singh, P.; Boote, K.J.; Kadiyala, M.D.M.; Nedumaran, S.; Gupta, S.K.; Srinivas, K.; Bantilan, M.C.S. An assessment of yield gains under climate change due to genetic modification of pearl millet. Sci. Total Environ. 2017, 601–602, 1226–1237. [Google Scholar] [CrossRef]
- Smale, M.; Byerlee, D.; Jayne, T. Maize revolutions in Sub-Saharan Africa. In An African Green Revolution Finding Ways to Boost Productivity on Small Farms; Springer: Dordrecht, The Netherlands, 2013; pp. 165–195. ISBN 978-94-007-5760-8. [Google Scholar]
- Mudereri, B.T.; Abdel-Rahman, E.M.; Dube, T.; Landmann, T.; Khan, Z.; Kimathi, E.; Owino, R.; Niassy, S. Multi-source spatial data-based invasion risk modeling of Striga (Striga asiatica) in Zimbabwe. GIScience Remote Sens. 2020, 57, 553–571. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Bonhomme, R. Predicting maize phenology. Predict. Crop Phenol. 1991, 11, 115–131. [Google Scholar]
- Tonnang, H.E.Z.; Juarez, H.; Carhuapoma, P.; Gonzales, J.C.; Mendoza, D.; Sporleder, M.; Simon, R.; Kroschel, J. ILCYM—Insect life cycle modeling: Software for developing temperature-based insect phenology models with applications for regional and global pest risk assessments and mapping. In Potential Invasive Pests of Agricultural Crops; International Potato Centre: Lima, Peru, 2013; ISBN 9781845938291. [Google Scholar]
- Tonnang, H.E.Z.; Hervé, B.D.B.; Biber-Freudenberger, L.; Salifu, D.; Subramanian, S.; Ngowi, V.B.; Guimapi, R.Y.A.; Anani, B.; Kakmeni, F.M.M.; Affognon, H.; et al. Advances in crop insect modelling methods—Towards a whole system approach. Ecol. Modell. 2017, 354, 88–103. [Google Scholar] [CrossRef]
- Lampton, M. Damping-undamping strategies for the Levenberg-Marquardt nonlinear least-squares method. Comput. Phys. 1997, 11, 110–115. [Google Scholar] [CrossRef]
- Steiniger, S.; Hunter, A.J. Free and Open Source GIS Software for Building a Spatial Data Infrastructure. In Geospatial Free and Open Source Software in the 21st Century. In Proceedings of the First Open Source Geospatial Research Symposium, OGRS 2009, Nantes, France, 8–10 July 2009; Lecture Notes in Geoinformation and Cartography. Springer: Berlin/Heidelberg, Germany, 2012; pp. 247–261. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 20 August 2020).
- Ripley, B.D. The R Project in Statistical Computing. MSOR Connect. 2001, 1, 23–25. [Google Scholar] [CrossRef]
- Silva, V. Foundations of Eclipse RCP. In Practical Eclipse Rich Client Platform Projects; Apress: New York, NY, USA, 2009; pp. 1–19. [Google Scholar]
- Elzhov, T.; Mullen, K.; Spiess, A.; Bolker, B. Package “minpack. lm”. Available online: https://Cran.R-Project.Org./Web/Packages/Minpack.Lm/Minpack.Lm.Pdf (accessed on 15 June 2016).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Bivand, R.; Archer, E.; Baddeley, A.; Bearman, N.; Callahan, J.; Forrest, D.; Friendly, M.; Stokely, M. Package ‘maptools’ R Topics Documented. Available online: http://maptools.r-forge.r-project.org/ (accessed on 24 August 2020).
- Becker, R.A.; Wilks, A.R.; Brownrigg, R.; Minka, T.; Deckmyn, A. Maps: Draw Geographical Maps. R Package Version 3.3.0. 2018. Available online: http://cran.r-project.org/package=maps (accessed on 20 August 2020).
- Urbanek, S. Rserve: A Fast way to provide R functionality to ppplications. In Proceedings of the 3rd International Worshop on Distributed Statistical Computing, Vienna, Austria, 20–22 March 2003. [Google Scholar]
- Sharpe, P.J.H.; DeMichele, D.W. Reaction kinetics of poikilotherm development. J. Theor. Biol. 1977, 64, 649–670. [Google Scholar] [CrossRef]
- Yin, X.; Kropff, M.J.; McLaren, G.; Visperas, R.M. A nonlinear model for crop development as a function of temperature. Agric. For. Meteorol. 1995, 77, 1–16. [Google Scholar] [CrossRef]
- Grassini, P.; Pittelkow, C.M.; Cassman, K.G.; Yang, H.S.; Archontoulis, S.; Licht, M.; Lamkey, K.R.; Ciampitti, I.A.; Coulter, J.A.; Brouder, S.M.; et al. Robust spatial frameworks for leveraging research on sustainable crop intensification. Glob. Food Sec. 2017, 14, 18–22. [Google Scholar] [CrossRef]



Sample Availability: Samples are available from the authors. Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonnang, H.E.Z.; Guimapi, R.A.; Bruce, A.Y.; Makumbi, D.; Mudereri, B.T.; Balemi, T.; Craufurd, P. PPMaP: Reproducible and Extensible Open-Source Software for Plant Phenological Phase Duration Prediction and Mapping in Sub-Saharan Africa. Agriculture 2020, 10, 515. https://doi.org/10.3390/agriculture10110515
Tonnang HEZ, Guimapi RA, Bruce AY, Makumbi D, Mudereri BT, Balemi T, Craufurd P. PPMaP: Reproducible and Extensible Open-Source Software for Plant Phenological Phase Duration Prediction and Mapping in Sub-Saharan Africa. Agriculture. 2020; 10(11):515. https://doi.org/10.3390/agriculture10110515
Chicago/Turabian StyleTonnang, Henri E. Z., Ritter A. Guimapi, Anani Y. Bruce, Dan Makumbi, Bester T. Mudereri, Tesfaye Balemi, and Peter Craufurd. 2020. "PPMaP: Reproducible and Extensible Open-Source Software for Plant Phenological Phase Duration Prediction and Mapping in Sub-Saharan Africa" Agriculture 10, no. 11: 515. https://doi.org/10.3390/agriculture10110515
APA StyleTonnang, H. E. Z., Guimapi, R. A., Bruce, A. Y., Makumbi, D., Mudereri, B. T., Balemi, T., & Craufurd, P. (2020). PPMaP: Reproducible and Extensible Open-Source Software for Plant Phenological Phase Duration Prediction and Mapping in Sub-Saharan Africa. Agriculture, 10(11), 515. https://doi.org/10.3390/agriculture10110515




