Effects of Vermireactor Modifications on the Welfare of Earthworms Eisenia fetida (Sav.) and Properties of Vermicomposts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Material
2.1.1. Earthworms
2.1.2. Vegetable Waste—Sugar Beet Pulp
2.2. Experiment
2.2.1. Vermicomposting of Sugar Beet Pulp
- (1)
- Protective substrate (PS)—was constructed of 300 × 200 × 200 mm (length × width × height) containers. Vegetable waste in the form of sugar beet pulp (200 g of dry matter) was placed in plastic nets with the dimensions of 150 × 200 × 150 mm (length × width × height) and mesh size 3 × 4 mm. The nets with plant waste were placed in vermireactors, and the remaining capacity of the vermireactors was filled with a protective soil in the form of horticultural soil universal substrate for ornamental plants: composition: highmoor peat, lowmoor peat, pearlite, sand, microelements, mineral fertilizer NPK universal growing medium Kronen: pH in H2O 6.0–6.5; salinity NaCl 4.3 ± 0.8 (mg∙kg−1); N 1300 ± 80 (mg∙kg−1); P 210 ± 31 (mg∙kg−1); K 620 ± 82 (mg∙kg−1); Ca 3423 ± 97 (mg∙kg−1); Mg 470 ± 39 (mg∙dm−3), solid form, loose form, fraction of 0–20 mm.
- (2)
- No protective substrate (NPS)—was constructed of containers with dimensions of 150 × 200 × 200 mm (length × width × height). Vegetable waste in the form of sugar beet pulp was placed in the same amount (200 g of dry matter) in plastic nets with dimensions of 150 × 200 × 150 mm (length × width × height). The nets with waste were placed in vermireactors without the use of a protective substrate.
- −
- 5 PS vermireactors (200 g sugar beet pulp + 4.5 dm3 protective substrate + 20 mature E. fetida individuals);
- −
- 5 NPS vermireactors (200 g sugar beet pulp + 20 mature E. fetida individuals).
2.2.2. Analysis of the Protective Substrate Application Effectiveness
2.2.3. E. fetida Population Analysis
2.2.4. Physicochemical Analysis of Sugar Beet Pulp (SBP) Waste and Vermicomposts
2.3. Statistical Analysis
3. Results and Discussion
3.1. Changes in E. fetida Populations
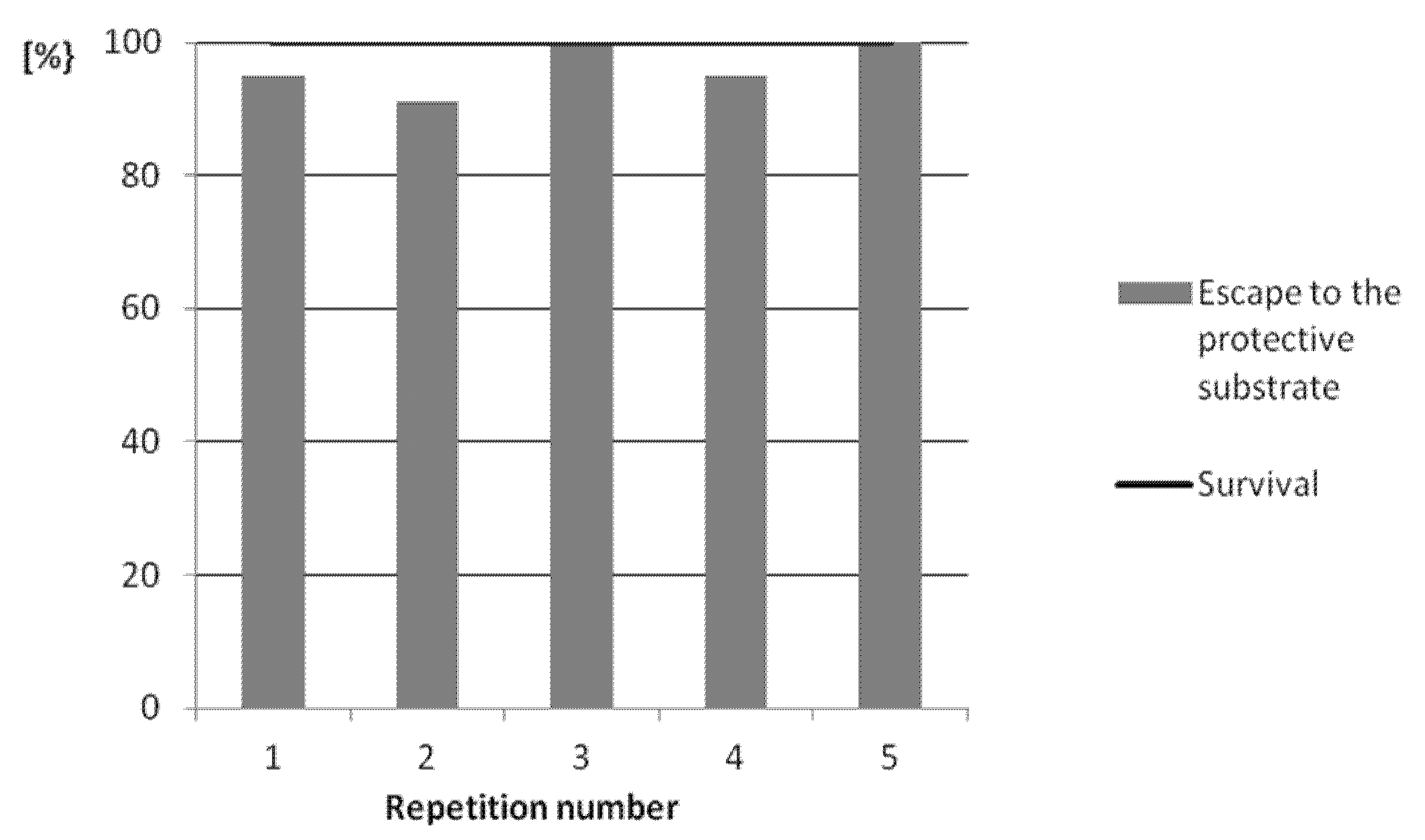
3.2. Analysis of the Protective Substrate Application Effectiveness
3.3. Physicochemical Properties of Waste and Vermicomposts
3.3.1. Macronutrients Content
3.3.2. Heavy Metals Contents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zorpas, A.A. Strategy development in the framework of waste management. Sci. Total Environ. 2020, 716. [Google Scholar] [CrossRef]
- Suthar, S. Potential utilization of guar gum industrial waste in vermicompost production. Bioresour. Technol. 2006, 97, 2474–2477. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Li, S.; Carson, M.A.; Chang, S.X.; Wu, Q.; Wang, L.; An, Z.; Sun, X. Spent mushroom substrate and cattle manure amendments enhance the transformation of garden waste into vermicomposts using the earthworm Eisenia fetida. J. Environ. Manag. 2019, 248, 109263. [Google Scholar] [CrossRef] [PubMed]
- Nogales, R.; Domínguez, J.; Mato, S. Vermicompostaje. In Compostaje; Moreno, J., Moral, R., Eds.; Ediciones Mundi Prensa: Madrid, Spain, 2008; pp. 187–208. [Google Scholar]
- Huang, K.; Li, F.; Wei, Y.; Chen, X.; Fu, X. Changes of bacterial and fungal community compositions during vermicomposting of vegetable wastes by Eisenia foetida. Bioresour. Technol. 2013, 150, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.M.; Romero, E.; Nogales, R. Dynamics of microbial communities related to biochemical parameters during vermicomposting and maturation of agroindustrial lignocellulose wastes. Bioresour. Technol. 2013, 146, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Hanc, A.; Chadimova, Z. Nutrient recovery from apple pomace waste by vermicomposting technology. Bioresour. Technol. 2014, 168, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Swarnam, T.P.; Velmurugan, A.; Pandey, S.K.; Dam Roy, S. Enhancing nutrient recovery and compost maturity of coconut husk by vermicomposting technology. Bioresour. Technol. 2016, 207, 76–84. [Google Scholar] [CrossRef]
- Hanc, A.; Dreslova, M. Effect of composting and vermicomposting on properties of particle size fractions. Bioresour. Technol. 2016, 217, 186–189. [Google Scholar] [CrossRef]
- Sonia, V.; Felix, S.; Antony, C. Comparative study of growth and reproduction of earthworm Eudrilus eugeniae in different organic substrate. Int. J. Appl. Sci. 2016, 4, 2455–4499. [Google Scholar] [CrossRef] [Green Version]
- Taeporamaysamai, O.; Ratanatamskul, C. Co-composting of various organic substrates from municipal solid waste using an on-site prototype vermicomposting reactor. Int. Biodeterior. Biodegrad. 2016, 113, 357–366. [Google Scholar] [CrossRef]
- Pączka, G.; Mazur-Pączka, A.; Garczyńska, M.; Podolak, A.; Szura, R.; Butt, K.R.; Kostecka, J. Using of earthworms Eisenia fetida (Sav.) for vermicomposting expansive littoral plants biomass. Appl. Sci. 2019, 9, 3635. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Kalamdhad, A.S. Reduction of bioavailability and leachability of heavy metals during vermicomposting of water hyacinth. Environ. Sci. Pollut. Res. 2013, 20, 8974–8985. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Wu, W.; Hao, X.; Jiang, D.; Li, X.; Bai, L. Vermicomposting of sludge from animal wastewater treatment plant mixed with cow dung or swine manure using Eisenia fetida. Environ. Sci. Pollut. Res. 2016, 23, 7767–7775. [Google Scholar] [CrossRef]
- Molina, M.J.; Soriano, M.D.; Ingelmo, F.; Llinares, J. Stabilisation of sewage sludge and vinasse bio-wastes by vermicomposting with rabbit manure using Eisenia fetida. Bioresour. Technol. 2013, 137, 88–97. [Google Scholar] [CrossRef]
- Sharma, K.; Garg, V.K. Comparative analysis of vermicompost quality produced from rice straw and paper waste employing earthworm Eisenia fetida (Sav.). Bioresour. Technol. 2018, 250, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Garg, V.K. Recycling of lignocellulosic waste as vermicompost using earthworm Eisenia fetida. Environ. Sci. Pollut. Res. 2019, 26, 14024–14035. [Google Scholar] [CrossRef]
- Negi, R.; Suthar, S. Degradation of paper mill wastewater sludge and cow dung by brown-rot fungi Oligoporus placenta and earthworm (Eisenia fetida) during vermicomposting. J. Clean. Prod. 2018, 201, 842–852. [Google Scholar] [CrossRef]
- Duncan, I.J.H.; Fraser, D. Understanding Animal Welfare. In Animal Welfare; Appleby, M.C., Hughes, B.O., Eds.; CAB International: Wallingford, UK, 1997; pp. 19–31. [Google Scholar]
- Fraser, D.; Weary, D.M.; Pajor, E.A.; Milligan, B.N. A scientific conception of animal welfare that reflects ethical concerns. Anim. Welf. 1997, 6, 187–205. [Google Scholar]
- Steffen, G.P.K.; Antoniolli, Z.I.; Steffen, R.B.; Jacques, R.J.S.; dos Santos, M.L. Earthworm extraction with onion solution. Appl. Soil Ecol. 2013, 69, 28–31. [Google Scholar] [CrossRef]
- MAFF. The Analysis of Agricultural Materials: A Manual of the Analytical Methods Used by the Agricultural Development and Advisory Service (ADAS); HMSO: London, UK, 1981. [Google Scholar]
- Sangwan, P.; Kaushik, C.P.; Garg, V.K. Vermiconversion of industrial sludge for recycling the nutrients. Bioresour. Technol. 2008, 99, 8699–8704. [Google Scholar] [CrossRef]
- Flack, F.M.; Hartenstein, R. Growth of the earthworm Eisenia fetida on microorganisms and cellulose. Soils Biol. Biochem. 1984, 16, 491–495. [Google Scholar] [CrossRef]
- Suthar, S. Nutrient changes and biodynamics of epigeic earthworm Perionyx excavatus (perrier) during recycling of some agriculture wastes. Bioresour. Technol. 2007, 98, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, S.A.; Prinsloo, M.W.; Reinecke, A.J. Resistance of Eisenia fetida (Oligochaeta) to cadmium after long-term exposure. Ecotoxicol. Environ. Saf. 1999, 42, 75–80. [Google Scholar] [CrossRef]
- Takacs, V.; Molnar, L.; Klimek, B.; Galuszka, A.; Morgan, A.J.; Plytycz, B. Exposure of Eisenia andrei (Oligochaeta; Lumbricidea) to cadmium polluted soil inhibits earthworm maturation and reproduction but not restoration of experimentally depleted coelomocytes or regeneration of amputated segments. Folia Biol. 2016, 64, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurgeon, D.J.; Hopkin, S.P. The development of genetically inherited resistance to zinc in laboratory-selected generations of the earthworm Eisenia fetida. Environ. Pollut. 2000, 109, 193–201. [Google Scholar] [CrossRef]
- Neuhauser, E.F.; Meyer, J.A.; Malecki, M.R.; Thomas, J.R. Dietary cobalt supplements and the growth and reproduction of the earthworm Eisenia fetida. Soil Biol. Biochem. 1984, 16, 521–523. [Google Scholar] [CrossRef]
- Van Gestel, C.A.M.; van Dis, W.A.; van Breemen, E.M.; Sparenburg, P.M.; Baerselman, R. Influence of cadmium, copper and pentachlorophenol on growth and sexual development of Eisenia andrei (Oligochaeta Annelida). Biol. Fertil. Soils 1991, 12, 117–121. [Google Scholar] [CrossRef]
- Klok, C.; De Roos, A.M. Population level consequences of toxicological influences on individual growth and reproduction in Lumbricus rubellus (Lumbricidae, Oligochaeta). Ecotoxicol. Environ. Saf. 1996, 33, 118–127. [Google Scholar] [CrossRef]
- Ma, W. Sublethal toxic effects of copper on growth, reproduction and litter breakdown in the earthworm Lumbricus rubellus, with observations on the influence of temperature and soil pH. Environ. Pollut. Ser. A 1984, 33, 207–219. [Google Scholar] [CrossRef]
- Owojori, O.J.; Reinecke, A.J.; Rozanov, A.B. The combined stress effects of salinity and copper on the earthworm Eisenia fetida. Appl. Soil Ecol. 2009, 41, 277–285. [Google Scholar] [CrossRef]
- Fischer, E.; Molnar, L. Growth and reproduction of Eisenia fetida (Oligochaeta, Lumbricidae) in various metal chlorides containing semi-natural soil. Soil Biol. Biochem. 1997, 29, 667–670. [Google Scholar] [CrossRef]
- Huang, K.; Fusheng, L.; Yongfen, W.; Xiaoyong, F.; Xuemin, C. Effects of earthworms on physicochemical properties and microbial profiles during vermicomposting of fresh fruit and vegetable wastes. Bioresour. Technol. 2014, 170, 45–52. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Vig, A.P. Effect on Growth of Earthworm and Chemical Parameters during Vermicomposting of Pressmud Sludge Mixed with Cattle Dung Mixture. Procedia Environ. Sci. 2016, 35, 425–434. [Google Scholar] [CrossRef]
- Ndegwa, P.M.; Thompson, S.A. Integrating composting and vermicomposting in the treatment and bioconversion of biosolids. Bioresour. Technol. 2001, 76, 107–112. [Google Scholar] [CrossRef]
- Pramanik, P.; Ghosh, G.K.; Ghosal, P.K.; Banik, P. Changes in Organic-C, N, P and K and enzyme activities in vermicomposts of biodegradable organic wastes under liming and microbial inoculants. Bioresour. Technol. 2007, 98, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Kaviraj, S.S.; Sharma, S. Municipal solid waste management through vermicomposting employing exotic and local species of earthworms. Bioresour. Technol. 2003, 90, 169–173. [Google Scholar] [CrossRef]
- Suthar, S. Bioremediation of aerobically treated distillery sludge mixed with cow dung by using an epigeic earthworm Eisenia fetida. Environmentalist 2008, 28, 76–84. [Google Scholar] [CrossRef]
- Viel, M.; Sayag, D.; Peyre, A.; Andre, L. Optimization of In-vessel Co-composting through heat recovery. Biol. Wastes 1987, 20, 167–185. [Google Scholar] [CrossRef]
- Plaza, C.; Nogales, R.; Senesi, N.; Benitez, E.; Polo, A. Organic matter humification by vermicomposting of cattle manure alone and mixed with two-phase olive pomace. Bioresour. Technol. 2007, 9, 5085–5089. [Google Scholar] [CrossRef]
- Deka, H.; Deka, S.; Baruah, C.K.; Das, J.; Hoque, S.; Sarma, H.; Sarma, N.S. Vermicomposting potential of Perionyx excavates for recycling of waste biomass of java citronella—An aromatic oil yielding plant. Bioresour. Technol. 2011, 102, 11212–11217. [Google Scholar] [CrossRef]
- Ghosh, M.; Chattopadhyay, G.N.; Baral, K. Transformation of phosphorus during vermicomposting. Bioresour. Technol. 1999, 69, 149–154. [Google Scholar] [CrossRef]
- Prakash, M.; Karmegam, N. Dynamics of nutrients and microflora during vermicomposting of mango leaf litter (Mangifera indica) using Perionyx ceylanensis. Int. J. Glob. Environ. Issues 2010, 10, 339–353. [Google Scholar] [CrossRef]
- Suthar, S.; Pandey, B.; Gusain, R.; Gaur, R.Z.; Kumar, K. Nutrient changes and biodynamics of Eisenia fetida during vermicomposting of water lettuce (Pistia sp.) biomass: A noxious weed of aquatic system. Environ. Sci. Pollut. Res. 2017, 24, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S.; Sharma, P. Vermicomposting of toxic weed-Lantana camara biomass: Chemical and microbial properties changes and assessment of toxicity of end product using seed bioassay. Ecotoxicol. Environ. Saf. 2013, 95, 179–187. [Google Scholar] [CrossRef]
- Puyuelo, B.; Arizmendiarrieta, J.S.; Irigoyen, I.; Plana, R. Quality assessment of composts officially registered as organic fertilisers in Spain. Span. J. Agric. Res. 2019, 17, 1101. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Bhattacharyya, P.; Ghosh, B.C.; Banik, P. Effect of vermicomposting on calcium, sulphur and some heavy metal content of different biodegradable organic wastes under liming and microbial inoculation. J. Environ. Sci. Health B 2012, 47, 205–211. [Google Scholar] [CrossRef]
- Liu, X.; Hu, C.; Zhang, S. Effects of earthworm activity on fertility and heavy metal bioavailability in sewage sludge. Environ. Int. 2005, 31, 874–879. [Google Scholar] [CrossRef]
- Heavy Metals and Organic Compounds from Wastes Used as Organic Fertilizers. Annex 2 Compost Quality Definition—Legislation and Standards. Available online: http://ec.europa.eu/environment/waste/compost/pdf/hm_annex2.pdf (accessed on 7 August 2020).
- Deolalikar, A.V.; Mitra, A.; Bhattacharyee, S.; Chakraborty, S. Effect of vermicomposting process on metal content of paper mill solid waste. J. Environ. Sci. Eng. 2005, 47, 81–84. [Google Scholar] [PubMed]
- Hait, S.; Tare, V. Transformation and availability of nutrients and heavy metalsduring integrated composting–vermicomposting of sewage sludge. Ecotoxicol. Environ. Saf. 2012, 79, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, A.; Thangaraj, R.; Maheswaran, R. Decomposition of poultry litter through vermicomposting using earthworm Drawida sulcata and its effect on plant growth. Int. J. Environ. Sci. Technol. 2019, 16, 7241–7254. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Vig, A.P. Vermiremediation of dyeing sludge from textile mill with the help of exotic earthworm Eisenia fetida Savigny. Environ. Sci. Pollut. Res. 2013, 20, 5975–5982. [Google Scholar] [CrossRef]
- Gupta, R.; Garg, V.K. Stabilization of primary sewage sludge during vermicomposting. J. Hazard. Mater. 2008, 153, 1023–1030. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.; Wu, J.; Tian, G. Bioaccumulation of heavy metals in the earthworm Eisenia fetida in relation to bioavailable metal concentrations in pig manure. Bioresour. Technol. 2010, 101, 3430–3436. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Assessment of bioavailability and leachability of heavy metals during rotary drum composting of green waste (Water hyacinth). Ecol. Eng. 2013, 52, 59–69. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Sharma, A. Manganese in cell metabolism of higher plants. Bot. Rev. 1991, 57, 117–149. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.S.; Eaton, J.W. Environmental contamination through residual trace metal dispersal from a derelict lead-zinc mine. J. Environ. Qual. 1980, 9, 175–179. [Google Scholar] [CrossRef]
- Blaylock, M.J.; Salt, D.E.; Dushenkov, S.; Zakarova, O.; Gussman, C.; Kapulnik, Y.; Ensley, B.D.; Raskin, I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 1997, 31, 860–865. [Google Scholar] [CrossRef]
- Gong, X.Q.; Li, S.Y.; Chang, S.X.; Wu, Q.; Cai, L.L.; Sun, X.Y. Alkyl polyglycoside and earthworm (Eisenia fetida) enhance biodegradation of green waste and its use for growing vegetables. Ecotoxicol. Environ. Saf. 2019, 167, 459–466. [Google Scholar] [CrossRef]
- Soobhany, N.; Mohee, R.; Garg, V.K. Comparative assessment of heavy metals content during the composting and vermicomposting of Municipal Solid Waste employing Eudrilus eugeniae. Waste Manag. 2015, 39, 130–145. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Y.; Sun, J.; She, J.; Yin, M.; Fang, F.; Liu, J. Geochemical transfer of cadmium in river sediments near a lead-zinc smelter. Ecotoxicol. Environ. Saf. 2020, 196, 110529. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.; Zia-ur-Rehman, M.; Zahir, Z.A.; Rinklebe, J.; Tack, F.M.G.; Ok, Y.S. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]



| 10 | 20 | 30 | 40 | 50 | 60 | Mean ± sd | |
|---|---|---|---|---|---|---|---|
| PS | 0.89 ± 0.04 a | 1.63 ± 0.05 a | 2.14 ± 0.09 a | 2.54 ± 0.07 a | 3.14 ± 0.15 a | 2.85 ± 0.08 a | 2.19 ± 0.83 a |
| NPS | 0.72 ± 0.05 a | 1.35 ± 0.06 a | 1.62 ± 0.04 a | 1.69 ± 0.06 b | 2.00 ± 0.07 b | 2.13 ± 0.04 b | 1.59 ± 0.51 b |
| % difference | 19.1 | 17.2 | 24.3 | 33.5 | 36.3 | 25.3 | 27.4 |
| Day of Experiment | E. fetida | Protective Substrate (PS) | Waste SBP | Percentage | |
|---|---|---|---|---|---|
| PS | SBP | ||||
| 10 | Adult | 5.6 ± 1.14 a | 14.2 ± 1.30 b | 28.6 | 71.4 |
| Immature | 0 | 0 | 0 | 0 | |
| Cocoons | 13.0 ± 1.22 a | 4.8 ± 0.84 b | 73.0 | 27.0 | |
| 20 | Adult | 5.4 ± 1.52 a | 14.4 ± 1.52 b | 27.3 | 72.7 |
| Immature | 6.4 ± 2.07 a | 12.0 ± 2.55 b | 34.8 | 65.2 | |
| Cocoons | 23.8 ± 3.96 a | 8.4 ± 2.88 b | 73.9 | 26.1 | |
| 30 | Adult | 6.0 ± 1.00 a | 13.8 ± 1.30 b | 30.3 | 69.7 |
| Immature | 6.0 ± 1.00 a | 21.8 ± 1.64 b | 21.6 | 78.4 | |
| Cocoons | 32.6 ± 2.70 a | 9.8 ± 3.56 b | 76.9 | 23.1 | |
| 40 | Adult | 5.4 ± 2.07 a | 14.2 ± 1.92 b | 27.6 | 72.4 |
| Immature | 13.4 ± 2.88 a | 56.2 ± 3.27 b | 19.3 | 80.7 | |
| Cocoons | 40.0 ± 3.81 a | 9.8 ± 3.42 b | 80.3 | 19.7 | |
| 50 | Adult | 5.8 ± 0.84 a | 13.8 ± 0.84 b | 29.3 | 70.7 |
| Immature | 15.6 ± 3.71 a | 73.8 ± 4.09 b | 17.4 | 82.6 | |
| Cocoons | 46.6 ± 2.97 a | 14.8 ± 3.70 b | 75.9 | 24.1 | |
| 60 | Adult | 4.8 ± 1.30 a | 14.8 ± 0.84 b | 24.5 | 75.5 |
| Immature | 17.2 ± 4.02 a | 70.4 ± 5.22 b | 19.6 | 80.4 | |
| Cocoons | 44.6 ± 4.09 a | 11.2 ± 3.77 b | 79.9 | 20.1 | |
| Parameter | Units | PS | NPS | |
|---|---|---|---|---|
| C | mg kg−1(d.m.) | Initial | 206321.5 ± 3689.2 a | |
| Final | 98189.8 ± 2699.7 b | 99391.7 ± 2811.4 b | ||
| % of change | −52.4 | −51.8 | ||
| N | Initial | 3851.4 ± 33.4 a | ||
| Final | 4964.2 ± 49.5 b | 4610.1 ± 93.4 b | ||
| % of change | 28.9 | 19.7 | ||
| P | Initial | 114.9 ± 13.2 a | ||
| Final | 289.4 ± 28.1 b | 263.7 ± 44.1 b | ||
| % of change | 151.9 | 129.5 | ||
| K | Initial | 2778.3 ± 86.6 a | ||
| Final | 3509.2 ± 71.1 b | 3489.7 ± 95.8 b | ||
| % of change | 26.3 | 25.6 | ||
| Ca | Initial | 1486.3 ± 35.1 a | ||
| Final | 1911.8 ± 29.5 b | 1883.1 ± 95.8 b | ||
| % of change | 28.6 | 26.7 | ||
| Mg | Initial | 163.9 ± 12.2 a | ||
| Final | 266.8 ± 29.4 b | 261.1 ± 85.6 b | ||
| % of change | 62.8 | 59.3 | ||
| C/N ratio | - | Initial | 53.57 ± 9.78 a | |
| Final | 19.78 ± 1.16 b | 21.56 ± 1.09 b | ||
| % of change | −63.1 | −59.8 | ||
| pH in H2O | - | Initial | 7.07 ± 0.09 a | |
| Final | 6.29 ± 0.17 b | 5.94 ± 0.89 b | ||
| % of change | −12.4 | −19.0 | ||
| Electrical conductivity | mS·cm−1 | Initial | 1.74 ± 0.05 a | |
| Final | 2.36 ± 0.07 b | 2.17 ± 0.03 b | ||
| % of change | 35.6 | 24.7 | ||
| Properties | Units | Characterized Substrates | PS | NPS |
|---|---|---|---|---|
| Cu | mg kg−1(d.m.) | Initial | 22.3 ± 4.1 a | |
| Final | 45.9 ± 6.4 b | 47.1 ± 11.3 b | ||
| % of change | 105.8 | 111.2 | ||
| Mn | Initial | 456.3 ± 27.1 a | ||
| Final | 388.1 ± 19.7 b | 362.5 ± 21.1 b | ||
| % of change | −15.0 | −20.6 | ||
| Zn | Initial | 19.5 ± 2.7 a | ||
| Final | 51.7 ± 9.1 b | 56.2 ± 9.9 b | ||
| % of change | 165.1 | 188.2 | ||
| Cd | Initial | 0.7 ± 0.0 a | ||
| Final | 0.9 ± 0.1 b | 0.9 ± 0.1 b | ||
| % of change | 28.6 | 28.6 | ||
| Pb | Initial | 1.1 ± 0.1a | ||
| Final | 0.6 ± 0.1 b | 0.5 ± 0.1 b | ||
| % of change | −45.5 | −54.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pączka, G.; Mazur-Pączka, A.; Garczyńska, M.; Kostecka, J.; Butt, K.R. Effects of Vermireactor Modifications on the Welfare of Earthworms Eisenia fetida (Sav.) and Properties of Vermicomposts. Agriculture 2020, 10, 481. https://doi.org/10.3390/agriculture10100481
Pączka G, Mazur-Pączka A, Garczyńska M, Kostecka J, Butt KR. Effects of Vermireactor Modifications on the Welfare of Earthworms Eisenia fetida (Sav.) and Properties of Vermicomposts. Agriculture. 2020; 10(10):481. https://doi.org/10.3390/agriculture10100481
Chicago/Turabian StylePączka, Grzegorz, Anna Mazur-Pączka, Mariola Garczyńska, Joanna Kostecka, and Kevin R. Butt. 2020. "Effects of Vermireactor Modifications on the Welfare of Earthworms Eisenia fetida (Sav.) and Properties of Vermicomposts" Agriculture 10, no. 10: 481. https://doi.org/10.3390/agriculture10100481
APA StylePączka, G., Mazur-Pączka, A., Garczyńska, M., Kostecka, J., & Butt, K. R. (2020). Effects of Vermireactor Modifications on the Welfare of Earthworms Eisenia fetida (Sav.) and Properties of Vermicomposts. Agriculture, 10(10), 481. https://doi.org/10.3390/agriculture10100481





