Background Glucocorticoid Therapy Has No Impact on Efficacy and Safety of Abatacept or Adalimumab in Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design and Analysis Population
2.2. Ethics Approval
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
3.1. Patient Population
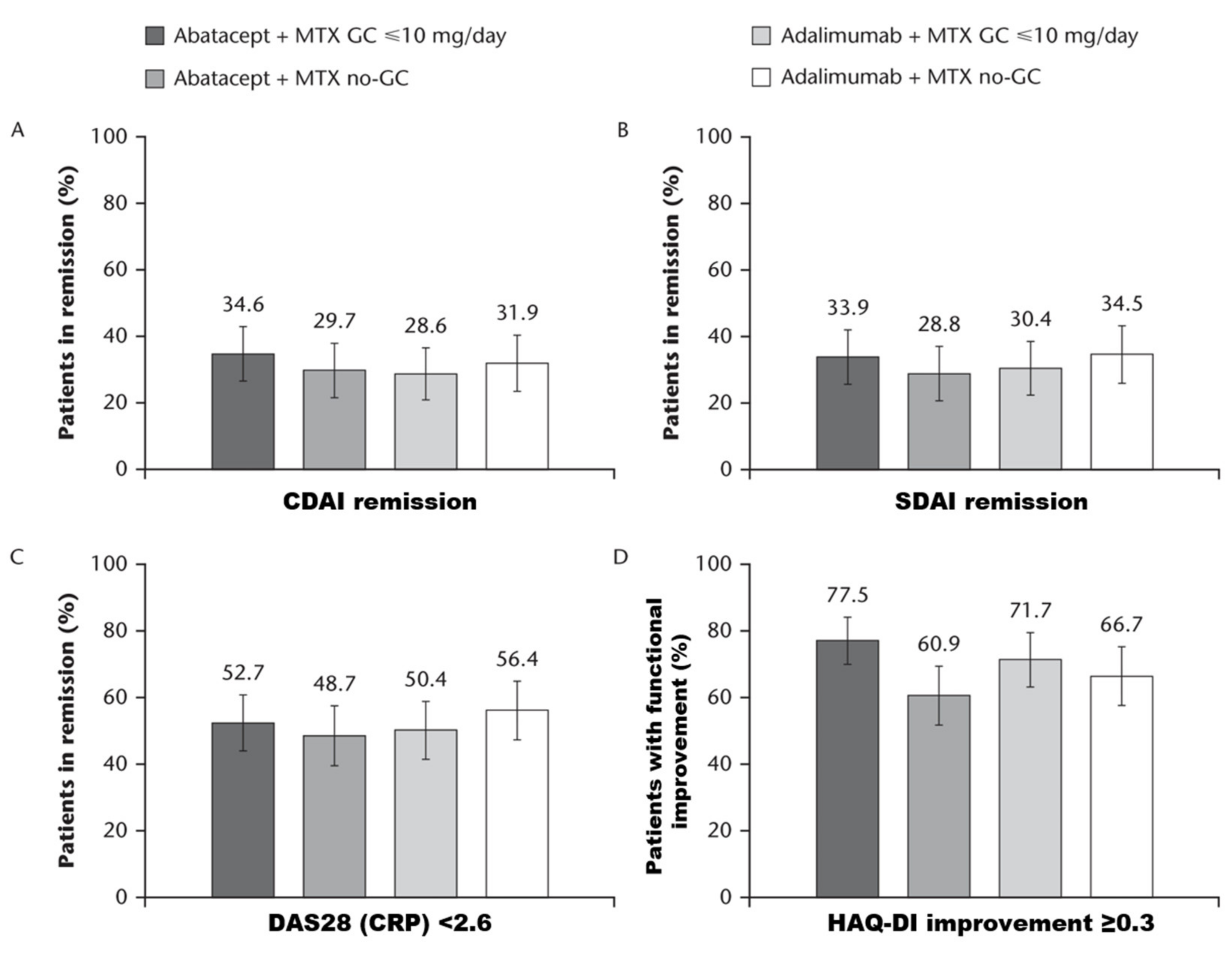
3.2. Clinical and Functional Evolution
3.3. Radiographic Evolution
3.4. Safety
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| SC Abatacept + MTX (n = 318) | SC Adalimumab + MTX (n = 328) | |||
|---|---|---|---|---|
| GC (n = 161) | No-GC (n = 156) | GC (n = 162) | No-GC (n = 164) | |
| DAS28 (CRP) | ||||
| Year 1, n | 142 | 132 | 138 | 128 |
| Adjusted mean change from baseline (95% CI) | −2.52 (−2.73, −2.31) | −2.37 (−2.58, −2.16) | −2.59 (−2.80, −2.38) | −2.50 (−2.71, −2.29) |
| Estimate of difference (95% CI) | −0.14 (−0.43, 0.14) | N/A | −0.09 (−0.38, 0.19) | N/A |
| Year 2, n | 131 | 119 | 125 | 117 |
| Adjusted mean change from baseline (95% CI) | −2.75 (−2.99, −2.52) | −2.55 (−2.79, −2.31) | −2.59 (−2.82, −2.35) | −2.62 (−2.86, −2.39) |
| Estimate of difference (95% CI) | −0.20 (−0.52, 0.12) | N/A | 0.04 (−0.28, 0.36) | N/A |
| HAQ-DI | ||||
| Year 1, n | 139 | 128 | 135 | 126 |
| Adjusted mean change from baseline (95% CI) | −0.72 (−0.83, −0.62) | −0.62 (−0.72, −0.51) | −0.75 (−0.85, −0.65) | −0.59 (−0.69, −0.49) |
| Estimate of difference (95% CI) | −0.11 (−0.25, 0.04) | N/A | −0.17 (−0.30, −0.03) | N/A |
| Year 2, n | 129 | 115 | 120 | 108 |
| Adjusted mean change from baseline (95% CI) | −0.74 (−0.85, −0.62) | −0.64 (−0.75, −0.53) | −0.73 (−0.84, −0.62) | −0.61 (−0.72, −0.50) |
| Estimate of difference (95% CI) | −0.10 (−0.25, 0.06) | N/A | −0.12 (−0.27, 0.03) | N/A |
| Change from Baseline ≤ SDC (2.2) | SC Abatacept + MTX (n = 318) | SC Adalimumab + MTX (n = 328) | ||
|---|---|---|---|---|
| GC (n = 161) | No-GC (n = 156) | GC (n = 162) | No-GC (n = 164) | |
| Year 1 | ||||
| Number of patients, n/N (%) | 123/146 (84.25) | 135/148 (91.22) | 125/147 (85.03) | 137/148 (92.57) |
| 95% CI | 78.34, 90.16 | 86.66, 95.78 | 79.27, 90.80 | 88.34, 96.79 |
| Estimate of difference (95% CI) | −7.0 (−15.1, 1.2) | N/A | −7.5 (−15.4, 0.3) | N/A |
| Year 2 | ||||
| Number of patients, n/N (%) | 105/130 (80.77) | 113/127 (88.98) | 107/134 (79.85) | 110/124 (88.71) |
| 95% CI | 73.99, 87.54 | 83.53, 94.42 | 73.06, 86.64 | 83.14, 94.28 |
| Estimate of difference (95% CI) | −8.2 (−17.7, 1.3) | N/A | −8.9 (−18.4, 0.7) | N/A |
References
- Singh, J.A.; Saag, K.G.; Bridges, S.L.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; Van Vollenhoven, R.F.; De Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Strehl, C.; Bijlsma, J.W.J.; De Wit, M.; Boers, M.; Caeyers, N.; Cutolo, M.; Dasgupta, B.; Dixon, W.G.; Geenen, R.; Huizinga, T.W.J.; et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: Viewpoints from an EULAR task force. Ann. Rheum. Dis. 2016, 75, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Palmowski, Y.; Buttgereit, T.; Dejaco, C.; Bijlsma, J.W.; Matteson, E.L.; Voshaar, M.; Boers, M.; Buttgereit, F. “Official View” on Glucocorticoids in Rheumatoid Arthritis: A Systematic Review of International Guidelines and Consensus Statements. Arthritis Rheum. 2017, 69, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, T.; O’Dell, J.R. It is the Best of Treatments, it is the Worst of Treatments: The Continuing Love‐Hate Relationship with Glucocorticoids in Rheumatoid Arthritis. Arthritis Rheum. 2017, 69, 1131–1133. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Bijlsma, J.W. Glucocorticoids in rheumatoid arthritis: The picture is shaping up. Ann. Rheum. Dis. 2017, 76, 1785–1787. [Google Scholar] [CrossRef] [PubMed]
- Chatzidionysiou, K.; Emamikia, S.; Nam, J.; Ramiro, S.; Smolen, J.; Van Der Heijde, D.; Dougados, M.; Bijlsma, J.; Burmester, G.; Scholte, M.; et al. Efficacy of glucocorticoids, conventional and targeted synthetic disease-modifying antirheumatic drugs: A systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Weinblatt, M.E.; Schiff, M.; Valente, R.; Van Der Heijde, D.; Citera, G.; Zhao, C.; Maldonado, M.; Fleischmann, R. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: Findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2012, 65, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.; Weinblatt, M.E.; Valente, R.; Van Der Heijde, D.; Citera, G.; Elegbe, A.; Maldonado, M.; Fleischmann, R. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: Two-year efficacy and safety findings from AMPLE trial. Ann. Rheum. Dis. 2013, 73, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Charles-Schoeman, C.; Van Der Heijde, D.; Burmester, G.R.; Nash, P.; Zerbini, C.A.; Connell, C.A.; Fan, H.; Kwok, K.; Bananis, E.; Fleischmann, R. Effect of Glucocorticoids on the Clinical and Radiographic Efficacy of Tofacitinib in Patients with Rheumatoid Arthritis: A Posthoc Analysis of Data from 6 Phase III Studies. J. Rheumatol. 2017, 45, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Wollenhaupt, J.; Cohen, S.; Smolen, J.S.; Dahl, P.; Iikuni, N.; Shi, H.; Tatulych, S.; Takiya, L. SAT0247 Impact of glucocorticoids on efficacy and safety of tofacitinib with and without methotrexate and adalimumab with methotrexate for rheumatoid arthritis: Results from a phase 3b/4 randomised trial. Ann. Rheum. Dis. 2018, 77, 985–986. [Google Scholar] [CrossRef]
- Fleischmann, R.; Wollenhaupt, J.; Cohen, S.; Wang, L.; Fan, H.; Bandi, V.; Andrews, J.; Takiya, L.; Bananis, E.; Weinblatt, M.E. Effect of Discontinuation or Initiation of Methotrexate or Glucocorticoids on Tofacitinib Efficacy in Patients with Rheumatoid Arthritis: A Post Hoc Analysis. Rheumatol. Ther. 2018, 5, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Safy-Khan, M.; Jacobs, J.W.G.; De Hair, M.J.H.; Welsing, P.M.J.; Edwardes, M.D.; Teitsma, X.M.; Luder, Y.; Devenport, J.; Van Laar, J.M.; Pethoe-Schramm, A.; et al. Effect on efficacy and safety trial outcomes of also enrolling patients on ongoing glucocorticoid therapy in rheumatoid arthritis clinical trials of tocilizumab or adalimumab or methotrexate monotherapy. Ann. Rheum. Dis. 2020, 79, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Bruynesteyn, K.; Boers, M.; Kostense, P.; Van Der Linden, S.; Van Der Heijde, D. Deciding on progression of joint damage in paired films of individual patients: Smallest detectable difference or change. Ann. Rheum. Dis. 2004, 64, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Sebba, A.; Gu, J.; Lowenstein, M.B.; Calvo, A.; Gomez-Reino, J.J.; Siri, D.A.; Tomšič, M.; Alecock, E.; Woodworth, T.; et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION study. Ann. Rheum. Dis. 2009, 69, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Emery, P.; Van Vollenhoven, R.; Dikranian, A.; Alten, R.; Pavelka, K.; Klearman, M.; Musselman, D.; Agarwal, S.; Green, J.; et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): A randomised, double-blind, controlled phase 4 trial. Lancet 2013, 381, 1541–1550. [Google Scholar] [CrossRef]
- Dougados, M.; Kissel, K.; Sheeran, T.; Tak, P.P.; Conaghan, P.G.; Mola, E.M.; Schett, G.; Amital, H.; Navarro-Sarabia, F.; Hou, A.; et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann. Rheum. Dis. 2012, 72, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Rigby, W.F.; Van Vollenhoven, R.F.; Kay, J.; Rubbert-Roth, A.; Blanco, R.; Kadva, A.; Dimonaco, S. Tocilizumab combination therapy or monotherapy or methotrexate monotherapy in methotrexate-naive patients with early rheumatoid arthritis: 2-year clinical and radiographic results from the randomised, placebo-controlled FUNCTION trial. Ann. Rheum. Dis. 2017, 76, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
| SC Abatacept + MTX (n = 318) | SC Adalimumab + MTX (n = 328) | |||
|---|---|---|---|---|
| GC (n = 161) | No-GC (n = 156) | GC (n = 162) | No-GC (n = 164) | |
| Age, years | 50.5 (12.7) | 52.4 (12.5) | 49.2 (12.1) | 52.9 (13.0) |
| Female sex, n (%) | 136 (84.5) | 122 (78.2) | 129 (79.6) | 139 (84.8) |
| Disease duration, years | 2.0 (1.4) | 1.8 (1.4) | 2.0 (1.4) | 1.5 (1.3) |
| Physical function (HAQ-DI) | 1.6 (0.7) | 1.4 (0.6) | 1.5 (0.7) | 1.4 (0.7) |
| Number of patients, na | 146 | 148 | 147 | 148 |
| mTSS | 23.0 (36.6) | 15.6 (27.1) | 23.1 (62.7) | 14.8 (22.4) |
| ESS | 11.9 (19.1) | 8.7 (15.9) | 12.1 (17.1) | 8.9 (13.2) |
| NSS | 11.1 (18.5) | 6.9 (12.4) | 11.0 (17.6) | 6.0 (10.6) |
| CRP, mg/dL | 2.0 (2.6) | 1.2 (1.3) | 1.5 (1.6) | 1.5 (3.7) |
| DAS28 (CRP) | 5.7 (1.1) | 5.3 (1.2) | 5.6 (1.1) | 5.5 (1.1) |
| MTX dose, mg/week | 18.1 (7.9) | 16.9 (4.0) | 17.2 (3.8) | 17.5 (7.9) |
| GC dose, mg/day | 6.6 (2.6) | N/A | 6.6 (2.3) | N/A |
| Anti-CCP2 positive, n (%) | 70 (43.5) | 73 (46.8) | 62 (38.3) | 91 (55.5) |
| RF positive, n (%) | 124 (77.0) | 115 (73.7) | 133 (82.1) | 120 (73.2) |
| SC Abatacept + MTX (n = 318) | SC Adalimumab + MTX (n = 328) | |||
|---|---|---|---|---|
| GC (n = 161) | No-GC (n = 156) | GC (n = 162) | No-GC (n = 164) | |
| Year 1 | ||||
| Total score | ||||
| n | 146 | 148 | 147 | 148 |
| Baseline mean (SD) | 23.02 (36.64) | 15.61 (27.11) | 23.07 (32.65) | 14.83 (22.42) |
| Mean change from baseline (SD) | 0.88 (2.90) | 0.24 (2.28) | 1.43 (9.06) | 0.03 (2.09) |
| Difference from no-GC (95% CI) | 0.67 (0.07, 1.27) | N/A | 1.08 (−0.42, 2.58) | N/A |
| Erosion score | ||||
| n | 146 | 148 | 147 | 148 |
| Baseline mean (SD) | 11.93 (19.09) | 8.67 (15.93) | 12.05 (17.11) | 8.85 (13.23) |
| Mean change from baseline (SD) | 0.27 (1.87) | 0.14 (1.76) | 0.57 (5.12) | −0.09 (1.66) |
| Difference from no-GC (95% CI) | 0.17 (−0.24, 0.59) | N/A | 0.59 (−0.28, 1.47) | N/A |
| Joint space narrowing score | ||||
| n | 146 | 148 | 147 | 148 |
| Baseline mean (SD) | 11.10 (18.54) | 6.94 (12.35) | 11.02 (17.60) | 5.98 (10.58) |
| Mean change from baseline (SD) | 0.62 (2.14) | 0.09 (0.95) | 0.86 (4.17) | 0.12 (0.94) |
| Difference from no-GC (95% CI) | 0.49 (0.11, 0.88) | N/A | 0.53 (−0.16, 1.22) | N/A |
| Year 2 | ||||
| Total score | ||||
| n | 130 | 127 | 134 | 124 |
| Baseline mean (SD) | 21.56 (33.49) | 15.42 (28.25) | 22.82 (31.08) | 14.00 (21.37) |
| Mean change from baseline (SD) | 1.25 (4.82) | 0.52 (3.26) | 1.88 (11.51) | 0.22 (3.47) |
| Difference from no-GC (95% CI) | 0.75 (−0.27, 1.77) | N/A | 1.17 (−0.95, 3.29) | N/A |
| Erosion score | ||||
| n | 130 | 127 | 134 | 124 |
| Baseline mean (SD) | 11.30 (17.03) | 8.81 (16.77) | 12.09 (17.22) | 8.43 (12.14) |
| Mean change from baseline (SD) | 0.49 (2.56) | 0.32 (2.58) | 0.75 (6.65) | −0.01 (2.26) |
| Difference from no-GC (95% CI) | 0.21 (−0.42, 0.84) | N/A | 0.63 (−0.61, 1.87) | N/A |
| Joint space narrowing score | ||||
| n | 130 | 127 | 134 | 124 |
| Baseline mean (SD) | 10.26 (17.31) | 6.61 (12.53) | 10.74 (15.80) | 5.58 (10.52) |
| Mean change from baseline (SD) | 0.75 (2.87) | 0.20 (1.05) | 1.13 (5.05) | 0.23 (1.51) |
| Difference from no-GC (95% CI) | 0.53 (−0.01, 1.07) | N/A | 0.60 (−0.32, 1.53) | N/A |
| System Organ Class, n (%) | SC Abatacept + MTX (n = 318) | p Value | SC Adalimumab + MTX (n = 328) | p Value | ||
| GC (n = 161) | No-GC (n = 156) | GC (n = 162) | No-GC (n = 164) | |||
| Deaths | 0 | 1 (0.6) | 0.492 | 1 (0.6) | 0 | 0.497 |
| SAEs | 22 (13.7) | 22 (14.1) | 0.999 | 25 (15.4) | 29 (17.7) | 0.656 |
| Discontinued due to SAEs | 2 (1.2) | 3 (1.9) | 0.681 | 8 (4.9) | 8 (4.9) | 1.000 |
| Infectious SAEs | 6 (3.7) | 6 (3.8) | 1.000 | 11 (6.8) | 8 (4.9) | 0.488 |
| Major cardiovascular events | 8 (5.0) | 10 (6.4) | 0.633 | 8 (4.9) | 5 (3.0) | 0.412 |
| Malignancies | 3 (1.9) | 4 (2.6) | 0.720 | 2 (1.2) | 5 (3.0) | 0.448 |
| Autoimmune events a | 5 (3.1) | 7 (4.5) | 0.569 | 3 (1.9) | 2 (1.2) | 0.684 |
| Local injection-site reactions | 7 (4.3) | 6 (3.8) | 1.000 | 11 (6.8) | 23 (14.0) | 0.045 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degboé, Y.; Schiff, M.; Weinblatt, M.; Fleischmann, R.; Ahmad, H.A.; Constantin, A. Background Glucocorticoid Therapy Has No Impact on Efficacy and Safety of Abatacept or Adalimumab in Patients with Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 2017. https://doi.org/10.3390/jcm9062017
Degboé Y, Schiff M, Weinblatt M, Fleischmann R, Ahmad HA, Constantin A. Background Glucocorticoid Therapy Has No Impact on Efficacy and Safety of Abatacept or Adalimumab in Patients with Rheumatoid Arthritis. Journal of Clinical Medicine. 2020; 9(6):2017. https://doi.org/10.3390/jcm9062017
Chicago/Turabian StyleDegboé, Yannick, Michael Schiff, Michael Weinblatt, Roy Fleischmann, Harris A. Ahmad, and Arnaud Constantin. 2020. "Background Glucocorticoid Therapy Has No Impact on Efficacy and Safety of Abatacept or Adalimumab in Patients with Rheumatoid Arthritis" Journal of Clinical Medicine 9, no. 6: 2017. https://doi.org/10.3390/jcm9062017
APA StyleDegboé, Y., Schiff, M., Weinblatt, M., Fleischmann, R., Ahmad, H. A., & Constantin, A. (2020). Background Glucocorticoid Therapy Has No Impact on Efficacy and Safety of Abatacept or Adalimumab in Patients with Rheumatoid Arthritis. Journal of Clinical Medicine, 9(6), 2017. https://doi.org/10.3390/jcm9062017





