Secondary Root Canal Treatment with Reciproc Blue and K-File: Radiographic and ESEM-EDX Analysis of Dentin and Root Canal Filling Remnants
Abstract
1. Introduction
2. Experimental Section
2.1. First Root Canal Treatment
2.2. Retreatment Procedure with Reciproc Blue
2.3. Retreatment Procedure with Manual K-File
2.4. Radiographic Evaluation of Root Canal Space Appearance
2.5. ESEM-EDX Sample Preparation
2.6. Statistical Analysis
3. Results
3.1. Radiographic Measurement of Root Canal Area
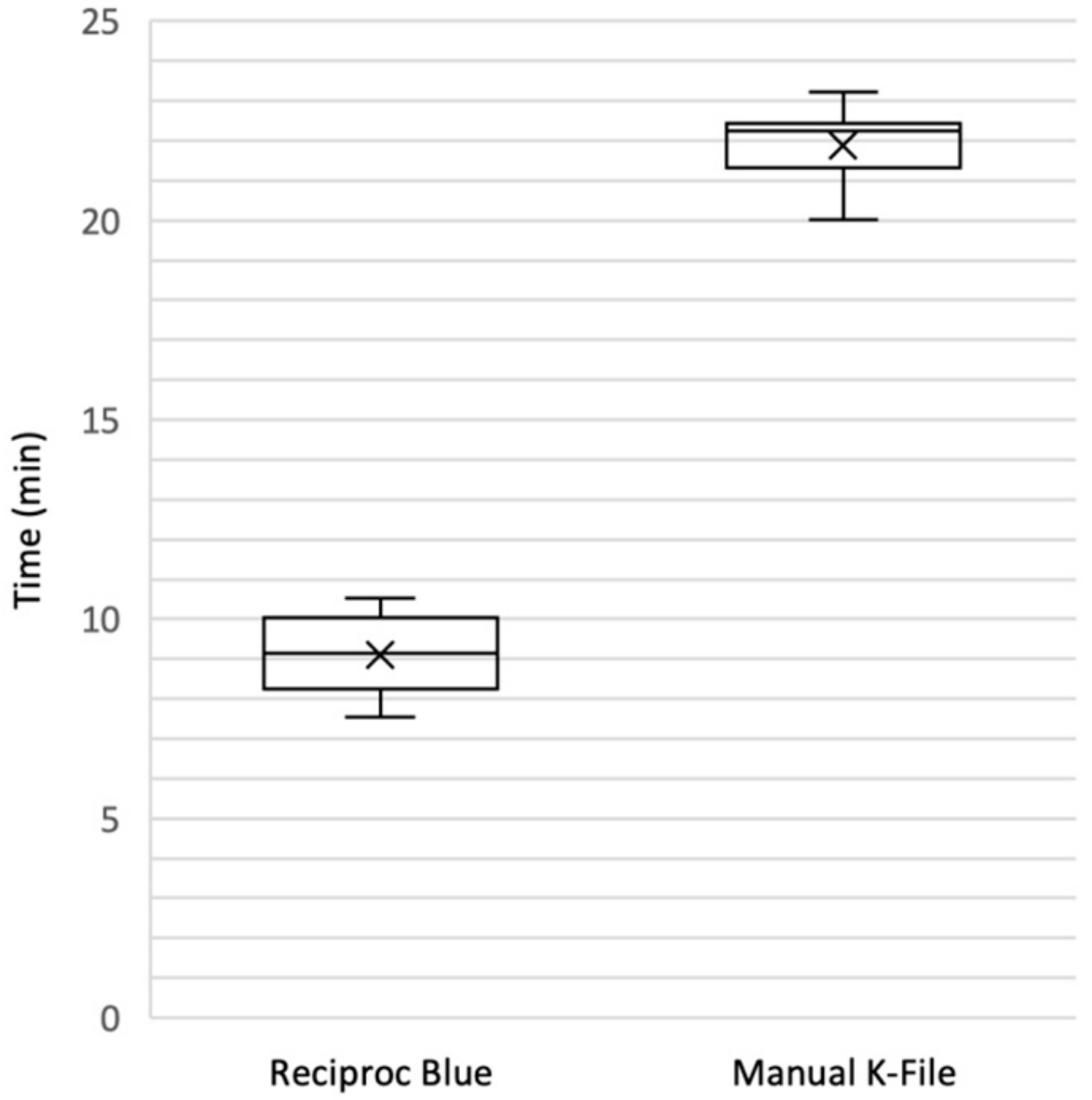
3.2. Working Time and Residual Radiopacity
3.3. Radiographic Evaluation
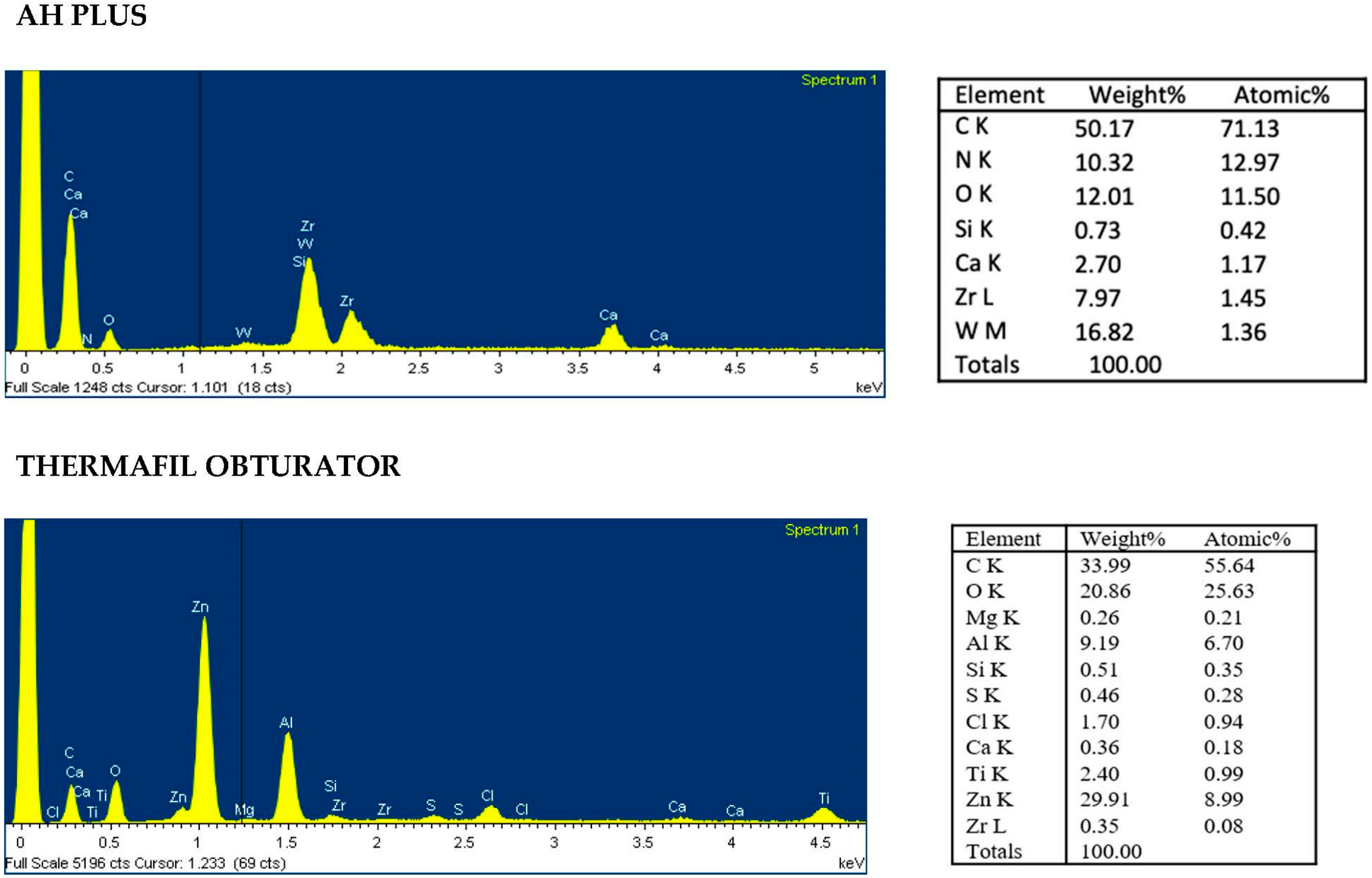
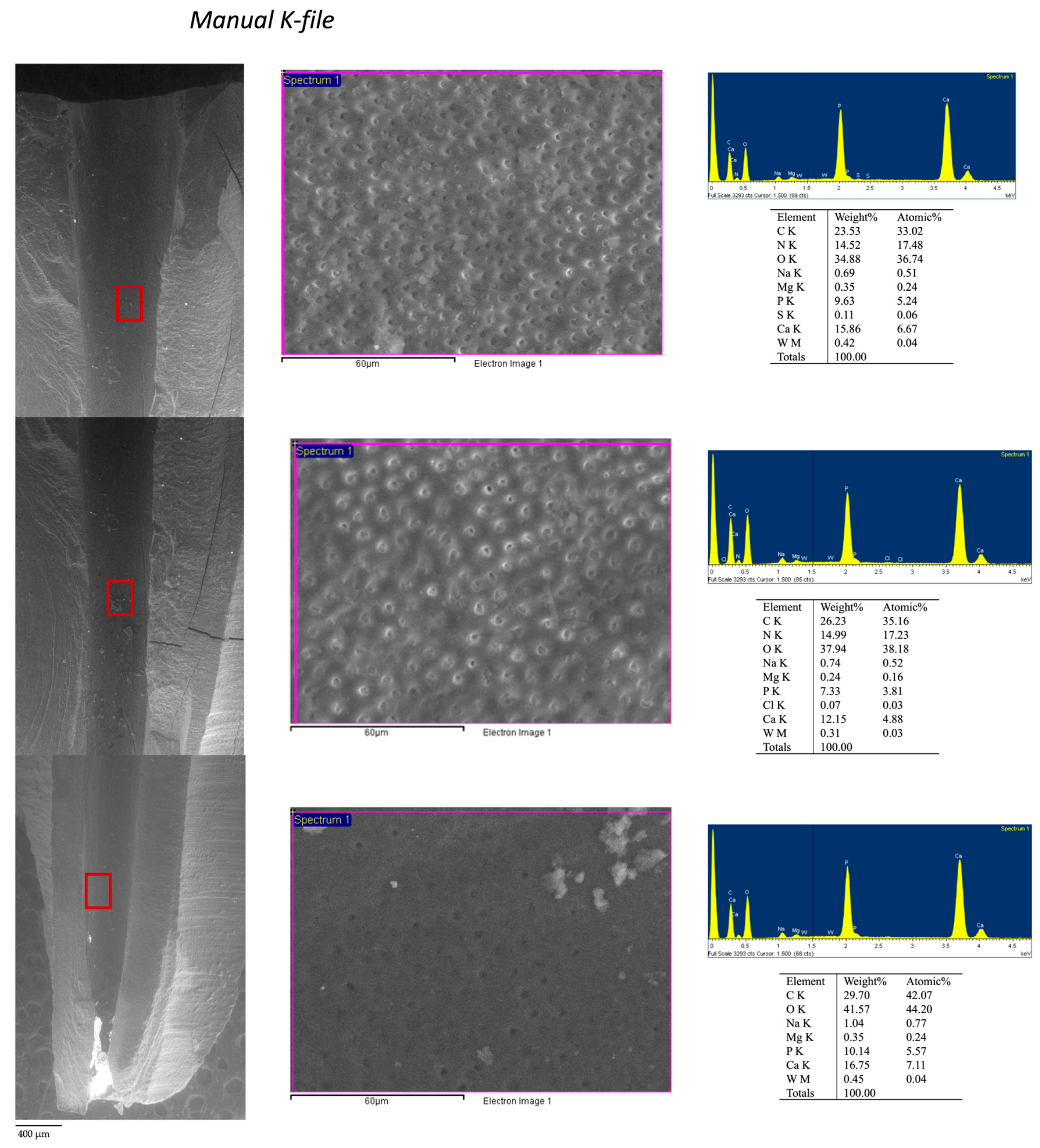
3.4. ESEM-EDX Analysis of Dentin Area
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gorni, F.; Gagliani, M. The outcome of endodontic retreatment: A 2-year follow-up. J. Endod. 2004, 30, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Rocas, I.N.; Alves, F.R.F.; Loghin, S.; Siqueira, J.F., Jr. Apically extruded sealers: Fate and influence on treatment outcome. J. Endod. 2016, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.R. On the causes of persistent apical periodontitis: A review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.-L.; Mann, V.; Gulabivala, K. Outcome of secondary root canal treatment: A systematic review of the literature. Int. Endod. J. 2008, 41, 1026–1046. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Corr, R.; Handysides, R.; Shabahang, S. Outcomes of nonsurgical retreatment and endodontic surgery: A systematic review. J. Endod. 2009, 35, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Bago, I.; Suk, M.; Katic, M.; Gabric, D.; Anic, I. Comparison of the effectiveness of various rotary and reciprocating systems with different surface treatments to remove gutta-percha and an epoxy resin-based sealer from straight root canals. Int. Endod. J. 2019, 52, 105–113. [Google Scholar] [CrossRef]
- Borges, A.H.; Damiao, M.S.; Pereira, T.M.; Filho, G.S.; Miranda-Pedro, F.L.; Luiz de Oliveira da Rosa, W.; Piva, E.; Guedes, O.A. Influence of cervical preflaring on the incidence of root dentin defects. J. Endod. 2018, 44, 286–291. [Google Scholar] [CrossRef]
- Romeiro, K.; de Almeda, A.; Cassimiro, M.; Gominho, L.; Dantas, E.; Chagas, N.; Velozo, C.; Freire, L.; Albuquerque, D. Reciproc and Reciproc Blue in the removal of bioceramic and resin-base sealers in retreatment procedures. Clin. Oral Investig. 2020, 24, 1–12. [Google Scholar] [CrossRef]
- Iacono, F.; Pirani, C.; Arias, A.; de la Macorra, J.C.; Generali, L.; Gandolfi, M.G.; Prati, C. Impact of a modified motion on the fatigue life of NiTi reciprocating instruments: A Weibull analysis. Clin. Oral Investig. 2019, 23, 3095–3102. [Google Scholar] [CrossRef]
- De-Deus, G.; Belladonna, F.G.; Zuolo, A.S. Effectiveness of reciproc blue in removing canal filling material and regaining apical patency. Int. Endod. 2018, 52, 250–257. [Google Scholar] [CrossRef]
- Pirani, C.; Pelliccioni, G.A.; Marchionni, S.; Montebugnoli, L.; Piana, G.; Prati, C. Effectiveness of three different retreatment techniques in canals filled with compacted gutta-percha or Thermafil: A scanning electron microscope study. J. Endod. 2009, 35, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, L.; Moisiadis, P.; Van Meerbeek, B.; Quirynen, M.; Lambrechts, P. Microscopic observation of bacteria: Review highlighting the use of environmental SEM. Int. Endod. 2005, 38, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Zamparini, F.; Botticelli, D.; Ferri, M.; Yonezawa, D.; Piattelli, A.; Gandolfi, M.G. The use of ESEM-EDX as an innovative tool to analyse the mineral structure of peri-implant human bone. Materials 2020, 3, 1671. [Google Scholar] [CrossRef]
- Gurgel-Filho, E.D.; Andrade Feitosa, J.P.; Teixeira, F.B.; Monteiro de Paula, R.C.; Araújo Silva, J.B.; Souza-Filho, F.J. Chemical and X-ray analyses of five brands of dental gutta-percha cone. Int. Endod. J. 2003, 36, 302–307. [Google Scholar] [CrossRef]
- Pirani, C.; Zamparini, F.; Peters, O.S.; Iacono, F.; Gatto, M.R.; Generali, L.; Gandolfi, M.G.; Prati, C. The fate of root canals obturated with thermafil: 10-years data of patients treated in a master’s program. Clin. Oral Investig. 2019, 23, 3367–3377. [Google Scholar] [CrossRef]
- Rodig, T.; Hausdorfer, T.; Konietschke, F.; Dullin, C.; Hahn, W.; Hulsmann, M. Efficacy of D-RaCe and ProTaper universal retreatment NiTi instrument s and hand files in removing gutta-percha from curved root canals—A micro-computed tomography study. Int. Endod. J. 2012, 45, 580–589. [Google Scholar] [CrossRef]
- Machado, A.G.; Guilherme, B.P.S.; Provenzano, J.C.; Marceliano-Alves, M.F.; Goncalves, L.S.; Siqueira, J.F.; Neves, M.A.S. Effect of preparation with the self-adjusting file, TRUShape and XP-endo shaper systems, and a supplementary step with XP-endo finisher R on filling materials removal during retreatment of mandibular molar canals. Int. Endod. J. 2019, 52, 709–715. [Google Scholar] [CrossRef]
- Yılmaz, F.; Koç, C.; Kamburoğlu, K.; Ocak, M.; Geneci, F.; Uzuner, M.B.; Çelik, H.H. Evaluation of 3 different retreatment techniques in maxillary molar teeth by using micro-computed tomography. J. Endod. 2018, 44, 480–484. [Google Scholar] [CrossRef]
- Azim, A.A.; Wang, H.H.; Tarrosh, M.; Azim, K.A.; Piasecki, L. Comparison between single-file rotary system: Part-1 efficiency, effectiveness, and adverse effects in endodontic retreatment. J. Endod. 2018, 44, 1720–1724. [Google Scholar] [CrossRef]
- Ballal, N.V.; Jain, I.; Tay, F.R. Evaluation of the smear layer removal and decalcification effect of QMix, maleic acid and EDTA on root dentin. J. Dent. 2016, 51, 62–68. [Google Scholar] [CrossRef]
- Taddei, P.; Prati, C.; Gandolfi, M.G. A poly (2-hydroxyethyl methacrylate)-based resin improves the dentin remineralizing ability of calcium silicates. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Foschi, F.; Nucci, C.; Montebugnoli, L.; Marchionni, S. Appearance of the root canal walls after preparation with NiTi rotary instruments: A comparative SEM investigation. Clin. Oral Investig. 2004, 8, 102–110. [Google Scholar] [CrossRef]
- Violich, D.R.; Chandler, N.P. The smear layer in endodontics—A review. Int. Endod. J. 2010, 43, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Saleh, I.M.; Ruyter, I.E.; Haapasalo, M.P.; Orstavik, D. Adhesion of endodontic sealers: scanning electron microscopy and energy dispersive spectroscopy. J. Endod. 2003, 29, 595–601. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Taddei, P.; Pondrelli, A.; Zamparini, F.; Prati, C.; Spagnuolo, G. Demineralization, collagen modification and remineralization degree of human dentin after EDTA and citric acid treatments. Materials 2018, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N.; Luo, X.J.; Li, G.H.; Bortoluzzi, E.A.; Mao, J.; Chen, J.H.; Gutmann, J.L.; Pashley, D.H.; Tay, F.R. Effects of different sonic activation protocols on debridement efficacy in teeth with single-rooted canals. J. Dent. 2014, 42, 1001–1009. [Google Scholar] [CrossRef]
- Leon-Mancilla, B.H.; Araiza-Tellez, M.A.; Flores-Flores, J.O.; Pina-Barba, M.C. Physico-chemical characterization of collagen scaffold for tissue engineering. J. Appl. Res. Technol. 2016, 14, 77–85. [Google Scholar] [CrossRef]
- Hülsmann, M.; Bluhm, V. Efficacy, cleaning ability and safety of different rotary NiTi instruments in root canal retreatment. Int. Endod. J. 2004, 37, 468–476. [Google Scholar] [CrossRef]
- Saleh, I.M.; Ruyter, I.E.; Haapasalo, M.P.; Orstavik, D. The effect of dentin pretreatment on the adhesion of root canal sealers. Int. Endod. J. 2002, 35, 859–866. [Google Scholar] [CrossRef]
- Venturi, M.; Prati, C.; Capelli, G.; Falconi, M.; Breschi, L. A preliminary analysis of the morphology of lateral canals after root canal filling using a tooth-clearing technique. Int. Endod. 2003, 36, 54–63. [Google Scholar] [CrossRef]
- Mamootil, K.; Messer, H.H. Penetration of dentinal tubules by endodontic sealer cements in extracted teeth and in vivo. Int. Endod. J. 2007, 40, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Pirani, F.; Zamparini, M.R.; Gatto, M.G.; Gandolfi, A. 20-year historical prospective cohort study of root canal treatments. A multilevel analysis. Int. Endod. J. 2018, 1, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Grischke, J.; Müller-Heine, A.; Hülsmann, M. The effect of four different irrigation systems in the removal of a root canal sealer. Clin. Oral Investig. 2014, 18, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- De Souza, P.F.; Goncalves, L.C.O.; Marques, A.A.F.; Junior, E.C.S.; Garcia, L.D.F.R.; de Carvalho, F.M.A. Root canal retreatment using reciprocating and continuous rotary nickel-titanium instruments. Eur. J. Dent. 2015, 9, 234–239. [Google Scholar] [CrossRef]
- Marques da Silva, B.; Baratto-Filho, F.; Leonardi, D.P.; Henrique Borges, A.; Volpato, L.; Branco Barletta, F. Effectiveness of ProTaper, D-RaCe, and Mtwo retreatment files with and without supplementary instruments in the removal of root canal filling material. Int. Endod. J. 2012, 45, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Doganay Yildiz, E.; Arslan, H. The effect of blue thermal treatment on endodontic instruments and apical debris extrusion during retreatment procedures. Int. Endod. J. 2019, 52, 1629–1634. [Google Scholar] [CrossRef]
- Alharmoodi, R.; Al-Salehi, S. Assessment of the quality of endodontic re-tratment and changes in periapical status on a postgraduate endodontic clinic. J. Dent. 2020, 92, 103–261. [Google Scholar] [CrossRef]
- Ng, Y.L.; Mann, V.; Gulabivala, K. Tooth survival following non-surgical root canal treatment: A systematic review of the literature. Int. Endod. J. 2010, 43, 171–189. [Google Scholar] [CrossRef]


| Root Canal Area | Reciproc Blue (n = 12) | Manual K-File (n = 12) |
|---|---|---|
| Pre-operative area (mm2) | 7.01 ± 1.96 NS | 6.93 ± 1.72 |
| Treatment area (mm2) | 9.68 ± 2.23 NS | 8.42 ± 1.61 |
| Re-treatment area (mm2) | 10.49 ± 1.50 NS | 10.95 ± 2.61 |
| Reciproc Blue (n = 12) | 9.10 ± 1.02 p < 0.0001 |
| Manual K-File (n = 12) | 21.54 ± 4.46 |
| Reciproc Blue (n = 12) | 2.59 ± 1.94 NS |
| Manual K-File (n = 12) | 3.18 ± 2.61 |
| Type of Dentin Areas | Definition | ESEM | EDX |
|---|---|---|---|
| Area 1a | Clean, smooth, regular and sound instrumented dentine. No grooves and pits observed. | 100×: Absence of high electron dense areas. 3000×: Dentinal tubules well visible with no high electron dense remnants and no smear layer. Absence of debris and remnants | Presence of Ca, P and N elements with apatite Ca/P ratio and dentin Ca/N ratio. No/limited presence of sealer and gutta-percha tracer elements (Zr, Zn, Si, W, Ti on dentin surface). |
| Area 1b | Instrumented dentine with signs of deprotenized dentin. No smear layer | 100×: Presence of low high electron dense debris. 3000×: Dentinal tubules partially covered with smear layer | Presence of Ca and P. Absence of N. Absence (or low presence) of tracer elements of sealer and gutta-percha (Zr, Zn Si, W). |
| Area 2 | Smooth dentin surface covered by a collagen-free deprotenized smear layer | 100×: few high electron dense debris. 3000×: Dentinal tubules covered by a thin smear layer with free from any remnants and electron dense particles | Presence of Ca and P. Absence of N. No/rare presence of sealer and gutta-percha tracer elements (Zr, Zn, Si, W). |
| Area 3 | Contaminated smear layer containing debris from filling materials. Absence of collagen | 100×: widespread electron dense deposits and presence of remnants. 3000×: Many remnants and materials spread or immersed into thick smear layer with only sporadic dentinal tubules noticeable | Moderate presence of Ca and P elements Absence of N Moderate presence of sealer and more gutta-percha tracer elements (Zr, Zn, Si, W). |
| Area 4 | Heavy contaminated dentin surface for presence of remnants and debris of filling material. Irregular surface | 100×: uniform and compact high electron dense deposits. 3000×: All surface covered by spread remnants Dentinal tubules completely masks by uniform and electron dense layer. Debris and remnants visible of dentine. | Reduced presence of P and Ca and N. High presence of sealer and gutta-percha tracer elements (Zr, Zn, Si, W). |
| Manual | Coronal | Medium | Apical |
|---|---|---|---|
| Area 1 | 69.0 ± 26.1 | 60.0 ± 7.1 | 65.5 ± 4.9 |
| Area 2 | 18.0 ± 7.7 | 24.5 ± 0.7 | 24.1 ± 1.4 |
| Area 3 | 6.9 ± 5.9 | 12.0 ± 4.3 | 5.5 ± 3.5 |
| Area 4 | 5.5 ± 4.8 | 4.1 ± 2.4 | 8.5 ± 0.7 |
| Total | 100.0 | 100.0 | 100.0 |
| Reciproc Blue | Coronal | Medium | Apical |
|---|---|---|---|
| Area 1 | 69.0 ± 4.2 | 81.0 ± 8.4 | 72.5 ± 10.5 |
| Area 2 | 19.0 ± 7.1 | 14.0 ±5.2 | 16.5 ± 12.4 |
| Area 3 | 4.5 ± 0.7 | 2.5 ± 0.6 | 6.0 ± 0.3 |
| Area 4 | 6.5 ± 0.7 | 2.5 ± 0.5 | 5.0 ± 0.2 |
| Total | 100.0 | 100.0 | 100.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prati, C.; Zamparini, F.; Spinelli, A.; Pelliccioni, G.A.; Pirani, C.; Gandolfi, M.G. Secondary Root Canal Treatment with Reciproc Blue and K-File: Radiographic and ESEM-EDX Analysis of Dentin and Root Canal Filling Remnants. J. Clin. Med. 2020, 9, 1902. https://doi.org/10.3390/jcm9061902
Prati C, Zamparini F, Spinelli A, Pelliccioni GA, Pirani C, Gandolfi MG. Secondary Root Canal Treatment with Reciproc Blue and K-File: Radiographic and ESEM-EDX Analysis of Dentin and Root Canal Filling Remnants. Journal of Clinical Medicine. 2020; 9(6):1902. https://doi.org/10.3390/jcm9061902
Chicago/Turabian StylePrati, Carlo, Fausto Zamparini, Andrea Spinelli, Gian Andrea Pelliccioni, Chiara Pirani, and Maria Giovanna Gandolfi. 2020. "Secondary Root Canal Treatment with Reciproc Blue and K-File: Radiographic and ESEM-EDX Analysis of Dentin and Root Canal Filling Remnants" Journal of Clinical Medicine 9, no. 6: 1902. https://doi.org/10.3390/jcm9061902
APA StylePrati, C., Zamparini, F., Spinelli, A., Pelliccioni, G. A., Pirani, C., & Gandolfi, M. G. (2020). Secondary Root Canal Treatment with Reciproc Blue and K-File: Radiographic and ESEM-EDX Analysis of Dentin and Root Canal Filling Remnants. Journal of Clinical Medicine, 9(6), 1902. https://doi.org/10.3390/jcm9061902








