Reactivating Ovarian Function through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women
Abstract
1. Introduction
2. Materials and Methods
2.1. General Exclusion Criteria
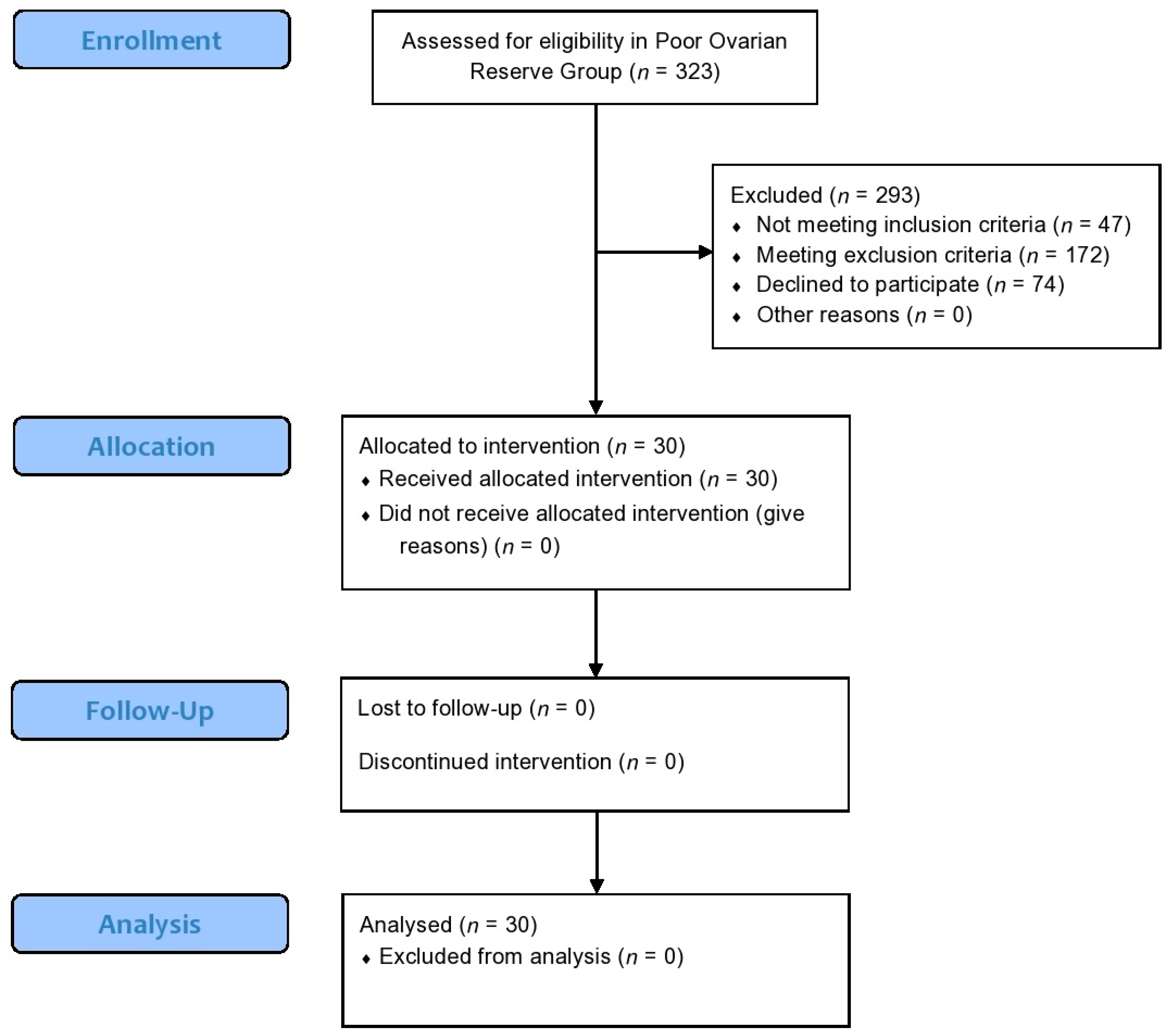
2.2. Participants in the Poor Ovarian Response Pilot Study
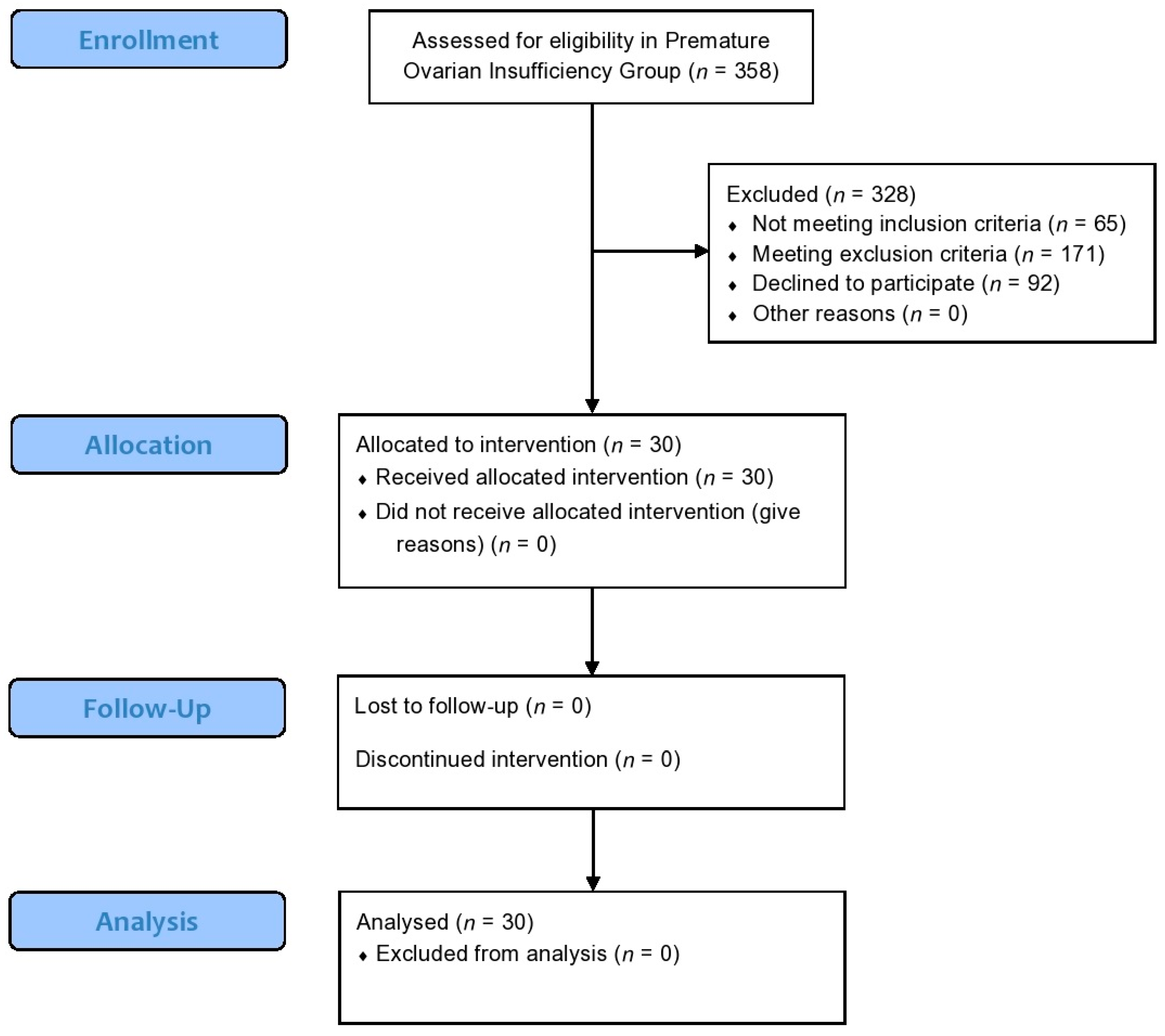
2.3. Participants in the Premature Ovarian Insufficiency Pilot Study
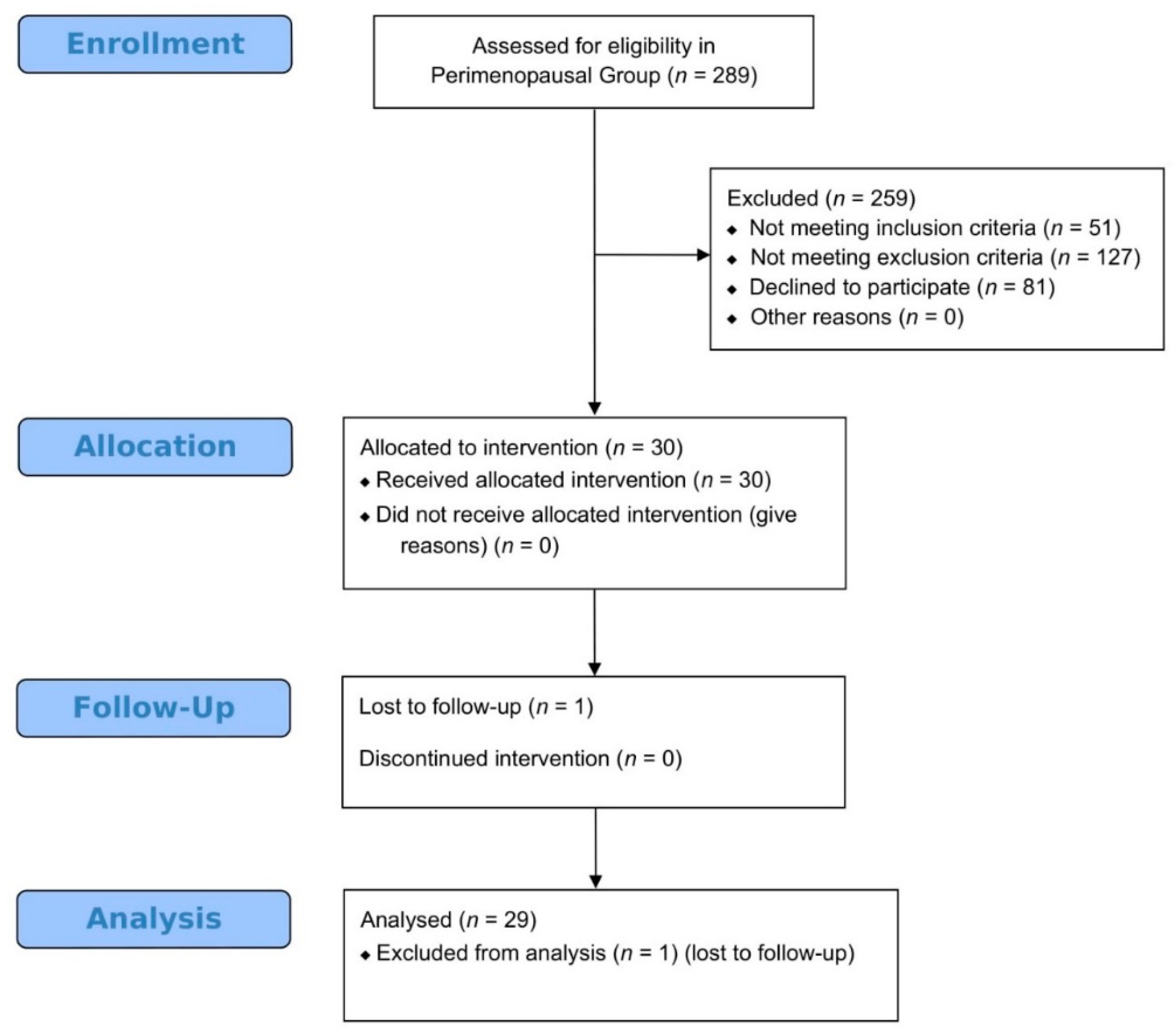
2.4. Participants in Perimenopause Pilot Study
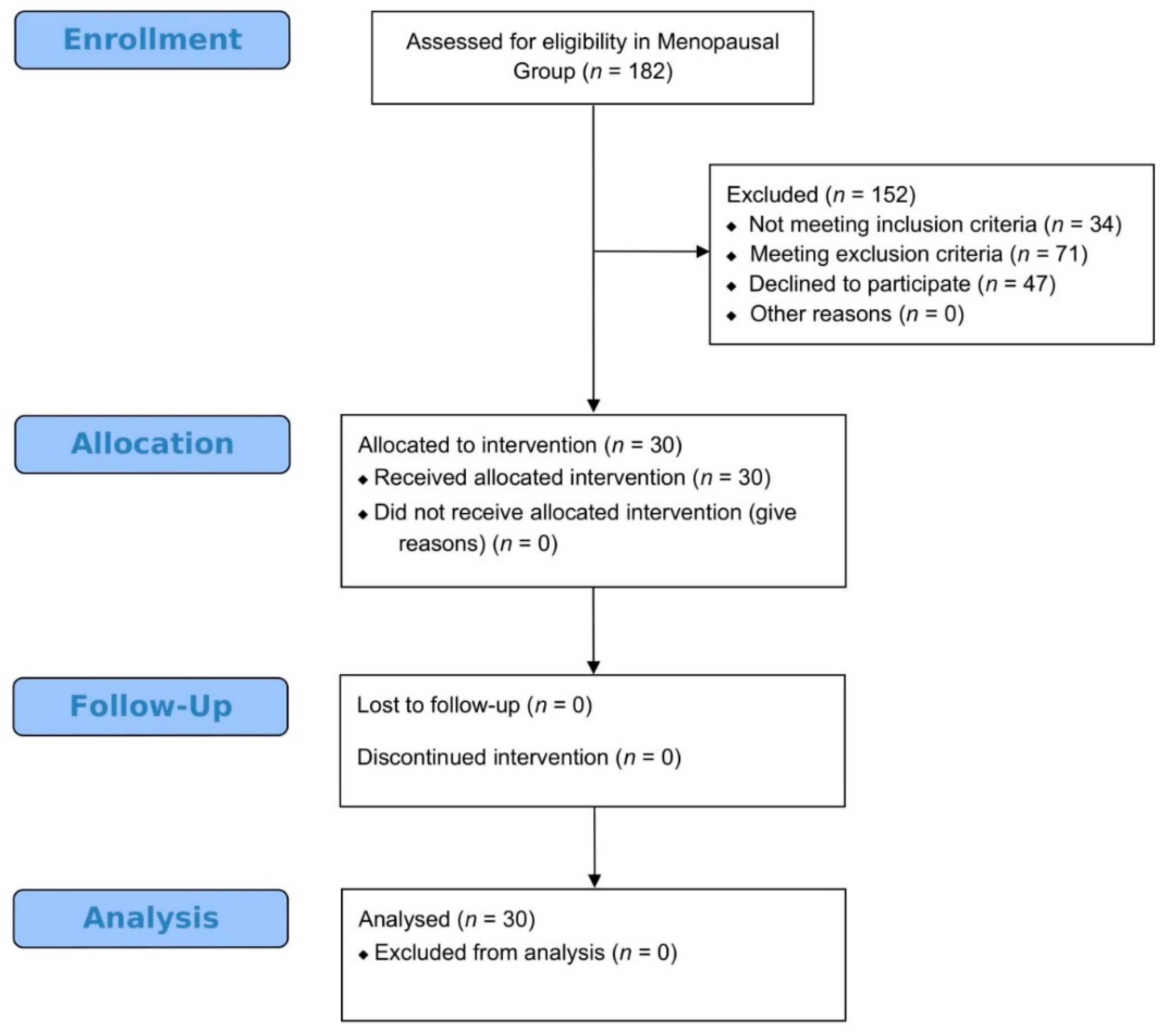
2.5. Participants in Menopause Pilot Study
2.6. Standard Examinations Prior to Platelet-Rich Plasma Treatment
2.7. Platelet-Rich Plasma Preparation Method
2.8. Platelet-Rich Plasma Intraovarian Infusion Method
2.9. Follow-Up Monitoring
2.10. Statistics
2.11. Study Approval
3. Results
3.1. Platelet-Rich Plasma Preparation and Infusion
3.2. Results Following Platelet-Rich Plasma Intraovarian Infusion in Women Presenting with Poor Ovarian Response
3.3. Results Following Platelet-Rich Plasma Intraovarian Infusion in Women Presenting with Premature Ovarian Insufficiency
3.4. Results Following Platelet-Rich Plasma Intraovarian Infusion Regarding Women in Perimenopause
3.5. Results Following Platelet-Rich Plasma Intraovarian Infusion Regarding Women in Menopause
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lew, R. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 55, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, A.; Ardiles, G.; Neuspiller, F.; Remohí, J.; Simón, C.; Bonilla-Musoles, F. Evaluation of the ovarian reserve in young low responders with normal basal levels of follicle-stimulating hormone using three-dimensional ultrasonography. Fertil. Steril. 1998, 70, 671–675. [Google Scholar] [CrossRef]
- Rudnicka, E.; Kruszewska, J.; Klicka, K.; Kowalczyk, J.; Grymowicz, M.; Skórska, J.; Pięta, W.; Smolarczyk, R. Premature ovarian insufficiency–aetiopathology, epidemiology, and diagnostic evaluation. Przeglad Menopauzalny Menopause Rev. 2018, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Ubaldi, F.M.; Cimadomo, D.; Vaiarelli, A.; Fabozzi, G.; Venturella, R.; Maggiulli, R.; Mazzilli, R.; Ferrero, S.; Palagiano, A.; Rienzi, L. Advanced maternal age in IVF: Still a challenge? The present and the future of its treatment. Front. Endocrinol. 2019, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Chabbert-Buffet, N.; Darai, E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder—A plea for universal definitions. J. Assist. Reprod. Genet. 2015, 32, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Pastore, L.M.; Christianson, M.S.; Stelling, J.; Kearns, W.G.; Segars, J.H. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J. Assist. Reprod. Genet. 2018, 35, 17–23. [Google Scholar] [CrossRef]
- Ferraretti, A.P.; La Marca, A.; Fauser, B.C.J.M.; Tarlatzis, B.; Nargund, G.; Gianaroli, L.; ESHRE working group on Poor Ovarian Response Definition. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: The Bologna criteria. Hum. Reprod. Oxf. Engl. 2011, 26, 1616–1624. [Google Scholar] [CrossRef]
- Humaidan, P.; Alviggi, C.; Fischer, R.; Esteves, S.C. The novel POSEIDON stratification of ‘Low prognosis patients in Assisted Reproductive Technology’and its proposed marker of successful outcome. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Poseidon Group (Patient-Oriented Strategies Encompassing IndividualizeD Oocyte Number); Alviggi, C.; Andersen, C.Y.; Buehler, K.; Conforti, A.; De Placido, G.; Esteves, S.C.; Fischer, R.; Galliano, D.; Polyzos, N.P.; et al. A new more detailed stratification of low responders to ovarian stimulation: From a poor ovarian response to a low prognosis concept. Fertil. Steril. 2016, 105, 1452–1453. [Google Scholar] [CrossRef]
- Overview|Menopause: Diagnosis and management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng23 (accessed on 11 December 2019).
- Vollenhoven, B.; Hunt, S. Ovarian ageing and the impact on female fertility. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Holloway, D. Menopause Symptom Management in the United Kingdom. Nurs. Clin. 2018, 53, 263–277. [Google Scholar] [CrossRef]
- Lamarche, C.; Levy, R.; Felloni, B.; Denis-Belicard, E.; Huss, M.; Maubon, I.; Aknin, I.; Seffert, P. Assisted reproductive techniques in women aged 38 years or more. Gynecol. Obstet. Fertil. 2007, 35, 420–429. [Google Scholar] [CrossRef]
- Remohi, J.; Vidal, A.; Pellicer, A. Oocyte donation in low responders to conventional ovarian stimulation for in vitro fertilization. Fertil. Steril. 1993, 59, 1208–1215. [Google Scholar] [CrossRef]
- Sauer, M.V.; Paulson, R.J.; Lobo, R.A. Pregnancy after age 50: Application of oocyte donation to women after natural menopause. Lancet 1993, 341, 321–323. [Google Scholar] [CrossRef]
- Silber, S.J.; Lenahan, K.M.; Levine, D.J.; Pineda, J.A.; Gorman, K.S.; Friez, M.J.; Crawford, E.C.; Gosden, R.G. Ovarian transplantation between monozygotic twins discordant for premature ovarian failure. N. Engl. J. Med. 2005, 353, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.H.; Abbas, S.H.; Al-Obaidi, M.T.; Abdulraheem, Y. The Efficacy of Platelets Rich Plasma (PRP) for Ovarian Rejuvenation. Indian J. Public Health Res. Dev. 2019, 10, 1211–1217. [Google Scholar] [CrossRef]
- Bos-Mikich, A.; de Oliveira, R.; Frantz, N. Platelet-rich plasma therapy and reproductive medicine. J. Assist. Reprod. Genet. 2018, 35, 753–756. [Google Scholar] [CrossRef]
- Qureshi, A.H.; Chaoji, V.; Maiguel, D.; Faridi, M.H.; Barth, C.J.; Salem, S.M.; Singhal, M.; Stoub, D.; Krastins, B.; Ogihara, M.; et al. Proteomic and Phospho-Proteomic Profile of Human Platelets in Basal, Resting State: Insights into Integrin Signaling. PLoS ONE 2009, 4, e7627. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A review of platelet-rich plasma: History, biology, mechanism of action, and classification. Skin Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Krüger, J.P.; Freymann, U.; Vetterlein, S.; Neumann, K.; Endres, M.; Kaps, C. Bioactive factors in platelet-rich plasma obtained by apheresis. Transfus. Med. Hemother. 2013, 40, 432–440. [Google Scholar] [CrossRef]
- Bastami, F.; Vares, P.; Khojasteh, A. Healing Effects of Platelet-Rich Plasma on Peripheral Nerve Injuries. J. Craniofac. Surg. 2017, 28, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- Borrione, P.; Gianfrancesco, A.D.; Pereira, M.T.; Pigozzi, F. Platelet-rich plasma in muscle healing. Am. J. Phys. Med. Rehabil. 2010, 89, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Hersant, B.; Sid-Ahmed, M.; Braud, L.; Jourdan, M.; Baba-Amer, Y.; Meningaud, J.-P.; Rodriguez, A.-M. Platelet-Rich Plasma Improves the Wound Healing Potential of Mesenchymal Stem Cells through Paracrine and Metabolism Alterations. Stem Cells Int. 2019, 2019, 1234263. [Google Scholar] [CrossRef] [PubMed]
- L Alio, J.; Arnalich-Montiel, F.; E Rodriguez, A. The role of “eye platelet rich plasma”(E-PRP) for wound healing in ophthalmology. Curr. Pharm. Biotechnol. 2012, 13, 1257–1265. [Google Scholar] [CrossRef]
- Li, X.-H.; Zhou, X.; Zeng, S.; Ye, F.; Yun, J.-L.; Huang, T.-G.; Li, H.; Li, Y.-M. Effects of intramyocardial injection of platelet-rich plasma on the healing process after myocardial infarction. Coron. Artery Dis. 2008, 19, 363–370. [Google Scholar] [CrossRef]
- Chang, Y.; Li, J.; Chen, Y.; Wei, L.; Yang, X.; Shi, Y.; Liang, X. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int. J. Clin. Exp. Med. 2015, 8, 1286. [Google Scholar]
- Zadehmodarres, S.; Salehpour, S.; Saharkhiz, N.; Nazari, L. Treatment of thin endometrium with autologous platelet-rich plasma: A pilot study. JBRA Assist. Reprod. 2017, 21, 54. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Simopoulou, M.; Nitsos, N.; Lazaros, L.; Rapani, A.; Pantou, A.; Koutsilieris, M.; Nikas, Y.; Pantos, K. Successful Implantation and Live Birth Following Autologous Platelet-rich Plasma Treatment for a Patient with Recurrent Implantation Failure and Chronic Endometritis. In Vivo 2019, 33, 515–521. [Google Scholar] [CrossRef]
- Pantos, K.; Nitsos, N.; Kokkali, G.; Vaxevanoglou, T.; Markomichali, C.; Pantou, A.; Grammatis, M.; Lazaros, L.; Sfakianoudis, K. Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment. In Proceedings of the Abstracts, ESHRE 32nd Annual Meeting, Helsinki, Finland, 3–6 July 2016; pp. 3–6. [Google Scholar]
- Sills, E.S.; Rickers, N.S.; Li, X.; Palermo, G.D. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol. Endocrinol. 2018, 34, 756–760. [Google Scholar] [CrossRef]
- Farimani, M.; Heshmati, S.; Poorolajal, J.; Bahmanzadeh, M. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol. Biol. Rep. 2019, 46, 1611–1616. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Simopoulou, M.; Nitsos, N.; Rapani, A.; Pantou, A.; Vaxevanoglou, T.; Kokkali, G.; Koutsilieris, M.; Pantos, K. A Case Series on Platelet-Rich Plasma Revolutionary Management of Poor Responder Patients. Gynecol. Obstet. Investig. 2018, 1–8. [Google Scholar] [CrossRef]
- Pantos, K.; Simopoulou, M.; Pantou, A.; Rapani, A.; Tsioulou, P.; Nitsos, N.; Syrkos, S.; Pappas, A.; Koutsilieris, M.; Sfakianoudis, K. A Case Series on Natural Conceptions Resulting in Ongoing Pregnancies in Menopausal and Prematurely Menopausal Women Following Platelet-Rich Plasma Treatment. Cell Transplant. 2019, 28, 1333–1340. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Simopoulou, M.; Nitsos, N.; Rapani, A.; Pappas, A.; Pantou, A.; Chronopoulou, M.; Deligeoroglou, E.; Koutsilieris, M.; Pantos, K. Autologous Platelet-Rich Plasma Treatment Enables Pregnancy for a Woman in Premature Menopause. J. Clin. Med. 2019, 8, 1. [Google Scholar] [CrossRef]
- Urman, B.; Boza, A.; Balaban, B. Platelet-rich plasma another add-on treatment getting out of hand? How can clinicians preserve the best interest of their patients? Hum. Reprod. Oxf. Engl. 2019, 34, 2099–2103. [Google Scholar] [CrossRef]
- Guideline on the Management of Premature Ovarian Insufficiency. Available online: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Management-of-premature-ovarian-insufficiency (accessed on 11 December 2019).
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 11 December 2019).
- World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Bates, G.W. Is hope on the horizon for premature ovarian insufficiency? Fertil. Steril. 2018, 109, 800–801. [Google Scholar] [CrossRef]
- Ye, H.; Zheng, T.; Li, W.; Li, X.; Fu, X.; Huang, Y.; Hu, C.; Li, J.; Huang, J.; Liu, Z.; et al. Ovarian Stem Cell Nests in Reproduction and Ovarian Aging. Cell. Physiol. Biochem. 2017, 43, 1917–1925. [Google Scholar] [CrossRef]
- Badawy, A.; Wageah, A.; El Gharib, M.; Osman, E.E. Prediction and Diagnosis of Poor Ovarian Response: The Dilemma. J. Reprod. Infertil. 2011, 12, 241–248. [Google Scholar]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian Aging: Mechanisms and Clinical Consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Fenton, A.J. Premature ovarian insufficiency: Pathogenesis and management. J. Life Health 2015, 6, 147–153. [Google Scholar] [CrossRef]
- Şükür, Y.E.; Kıvançlı, İ.B.; Özmen, B. Ovarian aging and premature ovarian failure. J. Turk. Ger. Gynecol. Assoc. 2014, 15, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Younis, J.S.; Haddad, S.; Matilsky, M.; Radin, O.; Ben-Ami, M. Undetectable basal ovarian stromal blood flow in infertile women is related to low ovarian reserve. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2007, 23, 284–289. [Google Scholar] [CrossRef]
- Abedini, A.; Zamberlam, G.; Lapointe, E.; Tourigny, C.; Boyer, A.; Paquet, M.; Hayashi, K.; Honda, H.; Kikuchi, A.; Price, C.; et al. WNT5a is required for normal ovarian follicle development and antagonizes gonadotropin responsiveness in granulosa cells by suppressing canonical WNT signaling. FASEB J. 2016, 30, 1534–1547. [Google Scholar] [CrossRef]
- Devesa, J.; Caicedo, D. The Role of Growth Hormone on Ovarian Functioning and Ovarian Angiogenesis. Front. Endocrinol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Devesa, M.; Martinez, F.; Coroleu, B.; Tur, R.; Gonzalez, C.; Rodriguez, I.; Barri, P.N. Poor prognosis for ovarian response to stimulation: Results of a randomised trial comparing the flare-up GnRH agonist protocol vs. the antagonist protocol. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2010, 26, 509–515. [Google Scholar] [CrossRef]
- Liu, J.; Deutsch, U.; Jeong, J.; Lobe, C.G. Constitutive notch signaling in adult transgenic mice inhibits bFGF-induced angiogenesis and blocks ovarian follicle development. Genes. N. Y. N 2000 2014, 52, 809–816. [Google Scholar] [CrossRef]
- Price, C.A. Mechanisms of fibroblast growth factor signaling in the ovarian follicle. J. Endocrinol. 2016, 228, R31–R43. [Google Scholar] [CrossRef]
- Skinner, M.K. Regulation of primordial follicle assembly and development. Hum. Reprod. Update 2005, 11, 461–471. [Google Scholar] [CrossRef]
- Herraiz, S.; Romeu, M.; Buigues, A.; Martínez, S.; Díaz-García, C.; Gómez-Seguí, I.; Martínez, J.; Pellicer, N.; Pellicer, A. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil. Steril. 2018, 110, 496–505.e1. [Google Scholar] [CrossRef]
- Ozcan, P.; Takmaz, T.; Tok, O.E.; Islek, S.; Yigit, E.N.; Ficicioglu, C. The protective effect of platelet-rich plasma administrated on ovarian function in female rats with Cy-induced ovarian damage. J. Assist. Reprod. Genet. 2020, 37, 865–873. [Google Scholar] [CrossRef]
- Sills, E.S.; Wood, S.H. Autologous activated platelet-rich plasma injection into adult human ovary tissue: Molecular mechanism, analysis, and discussion of reproductive response. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Peterson, J.E.; Zurakowski, D.; Italiano, J.E.; Michel, L.V.; Fox, L.; Klement, G.L.; Folkman, J. Normal ranges of angiogenesis regulatory proteins in human platelets. Am. J. Hematol. 2010, 85, 487–493. [Google Scholar] [CrossRef]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef]
- Yang, S.; Chen, X.; Zhen, X.; Wang, H.; Ma, C.; Li, R.; Liu, P.; Qiao, J. The Prognosis of IVF in Poor Responders Depending on the Bologna Criteria: A Large Sample Retrospective Study from China. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Goldfarb, J.M. Cumulative pregnancy rates in women with poor ovarian response. Fertil. Steril. 2018, 109, 1004–1005. [Google Scholar] [CrossRef]
- Melo, P.; Navarro, C.; Jones, C.; Coward, K.; Coleman, L. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: A non-randomized interventional study. J. Assist. Reprod. Genet. 2020, 1–9. [Google Scholar] [CrossRef]
- Ford, E.; Beckett, E.L.; Roman, S.; McLaughlin, E.A.; Sutherland, J. Advances in human primordial follicle activation and premature ovarian insufficiency. Reprod. Camb. Engl. 2019. [Google Scholar] [CrossRef]
- Kawamura, K.; Kawamura, N.; Hsueh, A.J.W. Activation of dormant follicles: A new treatment for premature ovarian failure? Curr. Opin. Obstet. Gynecol. 2016, 28, 217–222. [Google Scholar] [CrossRef]
- Jeppesen, J.V.; Anderson, R.A.; Kelsey, T.W.; Christiansen, S.L.; Kristensen, S.G.; Jayaprakasan, K.; Raine-Fenning, N.; Campbell, B.K.; Yding Andersen, C. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol. Hum. Reprod. 2013, 19, 519–527. [Google Scholar] [CrossRef]
- Diaz-Garcia, C.; Domingo, J.; Garcia-Velasco, J.A.; Herraiz, S.; Mirabet, V.; Iniesta, I.; Cobo, A.; Remohí, J.; Pellicer, A. Oocyte vitrification versus ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: A prospective cohort study. Fertil. Steril. 2018, 109, 478–485.e2. [Google Scholar] [CrossRef]
- Torrealday, S.; Kodaman, P.; Pal, L. Premature Ovarian Insufficiency-an update on recent advances in understanding and management. F1000Research 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Chae-Kim, J.J.; Gavrilova-Jordan, L. Premature Ovarian Insufficiency: Procreative Management and Preventive Strategies. Biomedicines 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.H.; Jadeja, Y.D.; Bhadarka, H.K.; Patel, M.N.; Patel, N.H.; Sodagar, N.R. Insight into Different Aspects of Surrogacy Practices. J. Hum. Reprod. Sci. 2018, 11, 212–218. [Google Scholar] [CrossRef]
- Klein, J.U.; Sauer, M.V. Ethics in egg donation: Past, present, and future. Semin. Reprod. Med. 2010, 28, 322–328. [Google Scholar] [CrossRef]
- Santoro, N. Perimenopause: From Research to Practice. J. Womens Health 2016, 25, 332–339. [Google Scholar] [CrossRef]
- Delamater, L.; Santoro, N. Management of the Perimenopause. Clin. Obstet. Gynecol. 2018, 61, 419–432. [Google Scholar] [CrossRef]
- Pangas, S.A.; Rajkovic, A. Chapter 21-Follicular Development: Mouse, Sheep, and Human Models. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T.M., Zeleznik, A.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 947–995. ISBN 978-0-12-397175-3. [Google Scholar]
- Richardson, S.J.; Nelson, J.F. Follicular Depletion during the Menopausal Transition. Ann. N. Y. Acad. Sci. 1990, 592, 13–20. [Google Scholar] [CrossRef]
- Benetti-Pinto, C.L.; de Almeida, D.M.B.; Makuch, M.Y. Quality of life in women with premature ovarian failure. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2011, 27, 645–649. [Google Scholar] [CrossRef]
- Garel, M.; Blondel, B.; Karpel, L.; Blanchet, V.; Breart, G.; Frydman, R.; Olivennes, F. Women’s views on Friendly IVF: A qualitative preliminary study. J. Psychosom. Obstet. Gynaecol. 2009, 30, 101–104. [Google Scholar] [CrossRef]
- Jylhävä, J.; Pedersen, N.L.; Hägg, S. Biological Age Predictors. EBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef]
- Wang, K.; Li, Z.; Li, J.; Liao, W.; Qin, Y.; Zhang, N.; Huo, X.; Mao, N.; Zhu, H. Optimization of the Platelet-Rich Plasma Concentration for Mesenchymal Stem Cell Applications. Tissue Eng. Part A 2019, 25, 333–351. [Google Scholar] [CrossRef]
- Nurden, A.T.; Freson, K.; Seligsohn, U. Inherited platelet disorders. Haemoph. Off. J. World Fed. Hemoph. 2012, 18 (Suppl. 4), 154–160. [Google Scholar] [CrossRef]
- Marques, L.F.; Stessuk, T.; Camargo, I.C.C.; Sabeh Junior, N.; dos Santos, L.; Ribeiro-Paes, J.T. Platelet-rich plasma (PRP): Methodological aspects and clinical applications. Platelets 2015, 26, 101–113. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The biology of platelet-rich plasma. J. Oral Maxillofac. Surg. 2001, 59, 1119–1120. [Google Scholar] [CrossRef]
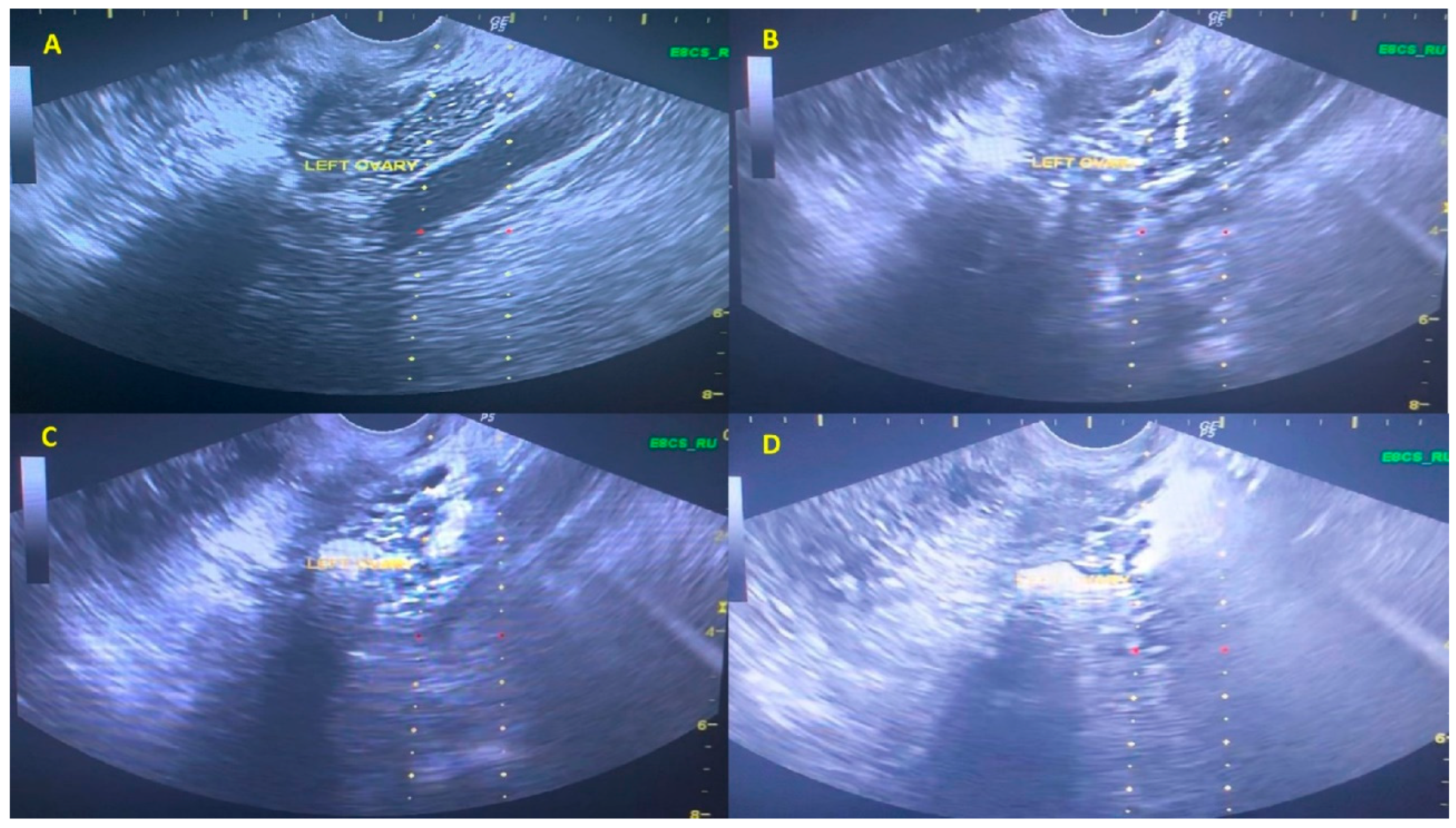
| Pilot Study | Screening for Eligibility | Standard Examination Prior to PRP Treatment | PRP Intraovarian Infusion | Follow-Up Monitoring |
|---|---|---|---|---|
| POR (n = 30) | Bologna Criteria | - Assessment of AFC, serum FSH, LH, AMH and E2 - HSG and semen analysis | Performed at least 2 months following the last ICSI-ET cycle | - AFC, serum FSH, LH, AMH and E2 assessment for three consecutive months following PRP treatment - In the third month following PRP participants were subjected to an ICSI-ET cycle |
| POI (n = 30) | - Age <40 years - Amenorrhea for at least four months, and elevated FSH >25 IU/L | -Assessment of AFC, serum FSH, LH, AMH and E2 -HSG and semen analysis | Performed immediately following standard investigation or at least six months following HR discontinuation | - AFC, serum FSH, LH, AMH and E2 assessment for three consecutive months following PRP treatment. - Participants were invited to conceive naturally |
| Perimenopause (n = 30) | -Age ≥40 years -Menstrual cycle irregularities | |||
| Menopause (n = 30) | - Age 45–55 years - Amenorrhea for at least 12 months without HR supplementation, and FSH >30 IU/L |
| Parameters | Poor Ovarian Response Group (n = 30) | |||
| General Participants’ Characteristics | ||||
| Patient age (years) | 38.40 ± 2.01 | |||
| Partner age (years) | 40.9 ± 2.52 | |||
| Years of infertility | 5.83 ± 1.02 | |||
| Body Mass Index (kg/m2) | 23.12 ± 2.52 | |||
| Ovarian Reserve Markers | Prior ICSI Cycle | 1st Menstrual Cycle | 2nd Menstrual Cycle | Post ICSI Cycle |
| FSH (IU/mL) | 10.71 ± 1.62 | 9.05 ± 1.76 a(**) | 8.87 ± 1.68 a(**) | 8.95 ± 1.40 a(**) |
| LH (IU/mL) | 9.02 ± 0.80 | 7.10 ± 1.03 a(**) | 6.50 ± 0.98 a(**) | 6.08 ± 0.92 a(**) |
| AMH (ng/mL) | 0.66 ± 0.20 | 0.85 ± 0.26 a(*) | 1.17 ± 0.28 a(**),b(**) | 1.14 ± 0.26 a(**),b(**) |
| AFC | 2.63 ± 0.93 | 3.80 ± 1.06 a(*) | 5.33 ± 1.32 a(**),b(**) | 5.20 ± 1.35 a(**),b(**) |
| ICSI Cycle’s Performance | Prior ICSI Cycle | 1st Menstrual Cycle | 2nd Menstrual Cycle | Post ICSI Cycle |
| Dur. of stimulation (Days) | 10.57 ± 0.90 | NA | NA | 9.40 ± 1.10 a(**) |
| Gonadotropin Dose (IU) | 4234.30 ± 261.58 | NA | NA | 4316.70 ± 217.93 |
| E2 (pg/mL) (hCG Trigger) | 710.33 ± 226.36 | NA | NA | 1522.90 ± 472.02 a(**) |
| Retrieved Oocytes | 1.20 ± 0.76 | NA | NA | 3.37 ± 1.54 a(**) |
| MII Oocytes Obtained | 1.00 ± 0.79 | NA | NA | 2.97 ± 1.38 a(**) |
| 2PN Embryos Obtained | 0.73 ± 0.52 | NA | NA | 2.43 ± 1.38 a(**) |
| Cleavage Stage Embryos | 0.60 ± 0.56 | NA | NA | 1.93 ± 1.26 (**) |
| Total Number of Cleavage Stage Embryos | 18 | NA | NA | 58 |
| Good quality (Grade 1 & 2) | 8/18 (44.4%) | NA | NA | 28/58 (48.2%) |
| Cancellation Rate | 19/30 (63.3%) | NA | NA | 9/30 (30%) a(*) |
| General Participants’ Characteristics | PRP Success (n = 18) | PRP Failure (n = 12) | p Value | |||||||||
| Participant age (years) | 35.11 ± 1.57 | 35.92 ± 1.93 | NS | |||||||||
| Partner age (years) | 37.94 ± 1.21 | 37.88 ± 1.70 | NS | |||||||||
| Duration of amenorrhea (months) | 10.06 ± 2.62 | 10.17 ± 4.76 | NS | |||||||||
| Time to menstrual cycle recovery (days) | 42.06 ± 6.43 | NA | NA | |||||||||
| Outcome Measures Following PRP | Prior to Treatment | Follow-Up Month 1 | Follow-Up Month 2 | Follow-Up Month 3 | ||||||||
| PRP Success | PRP Failure | p Value | PRP Success | PRP Failure | p Value | PRP Success | PRP Failure | p Value | PRP Success | PRP Failure | p Value | |
| Menstrual cycle duration (days) | NA | NA | NA | 34.22 ± 4.10 | NA | NA | 34.06 ± 4.33 | NA | NA | 33.44 ± 3.57 | NA | NA |
| Menstruation duration (days) | NA | NA | NA | 4.22 ± 1.31 | NA | NA | 4.56 ± 1.20 | NA | NA | 4.89 ± 1.18 | NA | NA |
| FSH (IU/mL) | 40.61 ± 6.05 | 63.65 ± 6.41 | <0.001 | 35.87 ± 4.77 | 54.07 ± 8.98 | <0.001 | 27.18 ± 5.82 | 52.47 ± 8.09 | <0.001 | 20.67 ± 3.58 | 59.40 ± 9.47 | <0.001 |
| LH (IU/mL) | 25.14 ± 3.10 | 24.33 ± 3.04 | NS | 21.91 ± 2.00 | 22.23 ± 2.38 | NS | 20.11 ± 1.45 | 17.51 ± 2.53 | NS | 19.31 ± 1.93 | 23.50 ± 4.37 | 0.001 |
| AMH (ng/mL) | 0.18 ± 0.04 | 0.15 ± 0.04 | NS | 0.53 ± 0.10 | 0.21 ± 0.06 | <0.001 | 0.65 ± 0.08 | 0.27 ± 0.09 | <0.001 | 0.75 ± 0.06 | 0.30 ± 0.05 | <0.001 |
| E2 (pg/mL) | 17.13 ± 2.22 | 17.38 ± 2.61 | NS | 26.75 ± 4.56 | 23.43 ± 2.86 | NS | 38.92 ± 9.46 | 25.14 ± 1.99 | <0.001 | 48.08 ± 6.28 | 20.86 ± 7.11 | <0.001 |
| AFC | 0 | 0 | NS | 1.56 ± 0.51 | 0 | <0.001 | 2.06 ± 0.73 | 0 | <0.001 | 2.33 ± 0.49 | 0 | <0.001 |
| Spontaneous Pregnancies Recorded Following PRP | PRP Success | PRP Failure | p Value | |||||||||
| Number of Pregnancies achieved | 3 | 0 | NS | |||||||||
| Number of Live Births | 3 | 0 | NS | |||||||||
| General Participants’ Characteristics | PRP Success (n = 24) | PRP Failure (n = 5) | p Value | |||||||||
| Participant age (years) | 43.25 ± 1.42 | 44 ± 2.55 | NS | |||||||||
| Partner age (years) | 44.92 ± 3.23 | 49 ± 2.00 | <0.001 | |||||||||
| Menstrual cycle irregularities (months) | 16 ± 2.43 | 17 ± 2.35 | NS | |||||||||
| Outcome Measures Following PRP | Prior to Treatment | Follow-Up Month 1 | Follow-Up Month 2 | Follow-Up Month 3 | ||||||||
| PRP Success | PRP Failure | p Value | PRP Success | PRP Failure | p Value | PRP Success | PRP Failure | p Value | PRP Success | PRP Failure | p Value | |
| Differences in menstrual cycle duration between two consecutive cycle (days) | 10.08 ± 2.10 | 12.00 ± 1.58 | NS | NA | NA | NA | 0.79 ± 0.66 | 12.40 ± 3.91 | <0.001 | 1.29 ± 1.27 | 10.20 ± 2.68 | <0.001 |
| Menstrual cycle duration (days) | 41.13 ± 1.85 | 47 ± 10.42 | NS | 37.08 ± 5.32 | 40.80 ± 8.61 | NS | 36.88 ± 5.62 | 36.80 ± 9.36 | NS | 36.75 ± 4.93 | 38.60 ± 4.88 | NS |
| Menstruation duration (days) | 3.17 ± 1.17 | 3.60 ± 1.52 | NS | 3.92 ± 1.72 | 4.40 ± 1.52 | NS | 5.33 ± 1.69 | 4.60 ± 1.95 | NS | 4.92 ± 1.38 | 5.00 ± 1.41 | NS |
| FSH (IU/mL) | 18.51 ± 2.62 | 18.32 ± 1.78 | NS | 17.03 ± 3.57 | 17.82 ± 1.41 | NS | 15.10 ± 3.65 | 18.24 ± 1.89 | NS | 15.28 ± 4.03 | 18.14 ± 1.82 | NS |
| LH (IU/mL) | 16.28 ± 2.64 | 16.62 ± 1.97 | NS | 14.72 ± 3.43 | 15.90 ± 1.84 | NS | 13.20 ± 3.53 | 16.22 ± 1.80 | NS | 13.18 ± 3.52 | 16.30 ± 1.36 | NS |
| AMH (ng/mL) | 0.96 ± 0.28 | 0.86 ± 0.36 | NS | 1.42 ± 0.16 | 0.80 ± 0.37 | <0.001 | 1.41 ± 0.17 | 0.82 ± 0.38 | <0.001 | 1.41 ± 0.23 | 0.66 ± 0.36 | <0.001 |
| E2 (pg/mL) | 29.67 ± 3.82 | 27.80 ± 6.87 | NS | 39.50 ± 2.06 | 28.70 ± 5.97 | <0.001 | 39.29 ± 5.82 | 31.34 ± 7.14 | 0.026 | 40.83 ± 4.30 | 30.24 ± 9.86 | <0.001 |
| AFC | 1.54 ± 0.51 | 1.00 ± 0.71 | NS | 2.79 ± 0.78 | 1.20 ± 0.45 | <0.001 | 3.38 ± 0.92 | 1.40 ± 0.89 | <0.001 | 4.25 ± 0.68 | 1.20 ± 1.10 | <0.001 |
| Spontaneous Pregnancies Recorded Following PRP | PRP Success | PRP Failure | p Value | |||||||||
| Number of Pregnancies achieved | 4 | 0 | NS | |||||||||
| Number of Live Births | 3 | 0 | NS | |||||||||
| General Participants’ Characteristics | PRP Success (n = 13) | PRP Failure (n = 12) | p Value | |||||||||
| Participant age (years) | 48.85 ± 1.57 | <0.05 | ||||||||||
| Partner age (years) | 49.15 ± 3.91 | NS | ||||||||||
| Duration of amenorrhea (months) | 15.69 ± 1.75 | NS | ||||||||||
| Time to menstrual cycle recovery (days) | 40.92 ± 7.57 | NA | ||||||||||
| Outcome Measures Following PRP | Prior to Treatment | Follow-Up Month 1 | Follow-Up Month 2 | Follow-Up Month 3 | ||||||||
| PRP Success | PRP Failure | p Value | PRP Success | PRP Failure | p Value | PRP Success | PRP Failure | p Value | PRP Success | PRP Failure | p Value | |
| Menstrual cycle duration (days) | NA | NA | NA | 35.62 ± 3.62 | NA | NA | 34.38 ± 4.52 | NA | NA | 32.77 ± 4.19 | NA | NA |
| Menstruation duration (days) | NA | NA | NA | 4.69 ± 0.95 | NA | NA | 4.69 ± 1.18 | NA | NA | 5.31 ± 1.65 | NA | NA |
| FSH (IU/mL) | 80.27 ± 5.03 | 81.15 ± 6.19 | NS | 43.63 ± 6.10 | 68.54 ± 7.26 | <0.001 | 38.42 ± 2.50 | 65.40 ± 6.50 | <0.001 | 30.55 ± 2.50 | 66.98 ± 8.12 | <0.001 |
| LH (IU/mL) | 30.82 ± 3.78 | 30.18 ± 5.04 | NS | 22.62 ± 3.79 | 25.41 ± 4.23 | NS | 18.80 ± 3.80 | 24.65 ± 3.10 | 0.001 | 17.04 ± 3.08 | 26.60 ± 2.84 | <0.001 |
| AMH (ng/mL) | 0.13 ± 0.03 | 0.11 ± 0.05 | NS | 0.32 ± 0.08 | 0.18 ± 0.08 | <0.001 | 0.40 ± 0.13 | 0.24 ± 0.06 | <0.001 | 0.61 ± 0.19 | 0.19 ± 0.04 | <0.001 |
| E2 (pg/mL) | 14.01 ± 2.59 | 13.26 ± 2.30 | NS | 22.19 ± 5.63 | 16.31 ± 2.10 | 0.002 | 32.95 ± 2.73 | 15.29 ± 1.78 | <0.001 | 41.53 ± 8.61 | 13.63 ± 2.30 | <0.001 |
| AFC | 0 | 0 | NS | 1.31 ± 0.48 | 0 | <0.001 | 1.77 ± 0.60 | 0 | <0.001 | 2.38 ± 0.65 | 0 | <0.001 |
| Spontaneous Pregnancies Recorded Following PRP | PRP Success | PRP Failure | p Value | |||||||||
| Number of Pregnancies achieved | 1 | 0 | NS | |||||||||
| Number of Live Births | 1 | 0 | NS | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfakianoudis, K.; Simopoulou, M.; Grigoriadis, S.; Pantou, A.; Tsioulou, P.; Maziotis, E.; Rapani, A.; Giannelou, P.; Nitsos, N.; Kokkali, G.; et al. Reactivating Ovarian Function through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women. J. Clin. Med. 2020, 9, 1809. https://doi.org/10.3390/jcm9061809
Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, Rapani A, Giannelou P, Nitsos N, Kokkali G, et al. Reactivating Ovarian Function through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women. Journal of Clinical Medicine. 2020; 9(6):1809. https://doi.org/10.3390/jcm9061809
Chicago/Turabian StyleSfakianoudis, Konstantinos, Mara Simopoulou, Sokratis Grigoriadis, Agni Pantou, Petroula Tsioulou, Evangelos Maziotis, Anna Rapani, Polina Giannelou, Nikolaos Nitsos, Georgia Kokkali, and et al. 2020. "Reactivating Ovarian Function through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women" Journal of Clinical Medicine 9, no. 6: 1809. https://doi.org/10.3390/jcm9061809
APA StyleSfakianoudis, K., Simopoulou, M., Grigoriadis, S., Pantou, A., Tsioulou, P., Maziotis, E., Rapani, A., Giannelou, P., Nitsos, N., Kokkali, G., Koutsilieris, M., & Pantos, K. (2020). Reactivating Ovarian Function through Autologous Platelet-Rich Plasma Intraovarian Infusion: Pilot Data on Premature Ovarian Insufficiency, Perimenopausal, Menopausal, and Poor Responder Women. Journal of Clinical Medicine, 9(6), 1809. https://doi.org/10.3390/jcm9061809









