Disability of Dialysis Patients and the Condition of Blood Vessels
Abstract
1. Introduction
2. Material and Methods
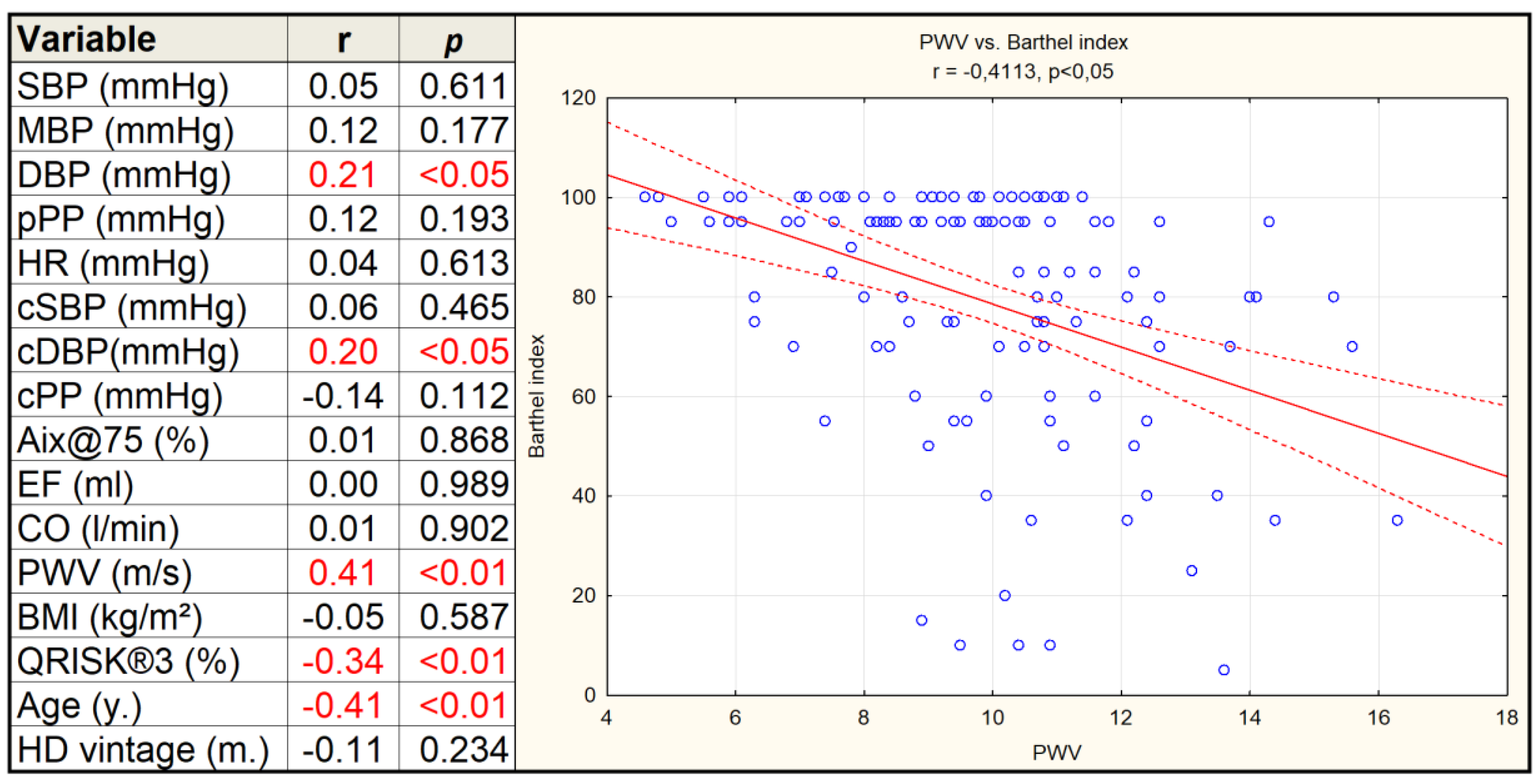
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018.
- McAdams-Demarco, M.A.; Law, A.; Garonzik-Wang, J.M.; Gimenez, L.; Jaar, B.G.; Walston, J.D.; Segev, D.L. Activity of daily living disability and dialysis mortality: Better prediction using metrics of aging. J. Am. Geriatr. Soc. 2012, 60, 1981–1982. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Afifi, W.M.; Abo Elsaoud, A.M.; Elgawish, M.H.; Ghorab, A.M. Musculoskeletal manifestations in end-stage renal disease patients on hemodialysis and relation to parathyroid dysfunction. Saudi J. Kidney Dis.Transpl. 2019, 30, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Farragher, J.; Jassal, S.V. Rehabilitation of the geriatric dialysis patient. Semin. Dial. 2012, 25, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Diamond, J.; Vieco, A.; Chaudhuri, S.; Shinnar, E.; Cromer, S.; Perel, P.; Mensah, G.A.; Narula, J.; Johnson, C.O.; et al. Global Atlas of Cardiovascular Disease 2000–2016: The path to prevention and control. Glob. Heart 2018, 13, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Wade, D.T.; Collin, C. The Barthel ADL Index: A standard measure of physical disability? Int.Disabil. Stud. 1988, 10, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.; Brindle, P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: Prospective cohort study. BMJ 2017, 357, j2099. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Marino, C.; Di Napoli, A.; Agabiti, N.; Tazza, L.; Davoli, M.; Dialysis and Transplant Lazio Region Registry Scientific Committee. Functional impairment and risk of mortality in patients on chronic hemodialysis: Results of the Lazio Dialysis Registry. J. Nephrol. 2018, 31, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.A.; van Loon, I.N.; Morpey, M.I.; Verhaar, M.C.; Willems, H.C.; Emmelot-Vonk, M.H.; Bots, M.L.; Boereboom, F.T.J.; Hamaker, M.E. Geriatric assessment in elderly patients with end-stage kidney disease. Nephron 2019, 141, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Abdulan, I.M.; Onofriescu, M.; Stefaniu, R.; Mastaleru, A.; Mocanu, V.; Alexa, I.D.; Covic, A. The predictive value of malnutrition for functional and cognitive status in elderly hemodialysis patients. Int. Urol. Nephrol. 2019, 51, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, N.T.; Schiller, B.; Saxena, A.B.; Thomas, I.C.; Kurella Tamura, M. Prevalence and correlates of functional dependence among maintenance dialysis patients. Hemodial. Int. 2015, 19, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.L.; Jassal, S.V. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008, 73, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Ricci, N.A.; Pessoa, G.S.; Ferriolli, E.; Dias, R.C.; Perracini, M.R. Frailty and cardiovascular risk in community-dwelling elderly: A population-based study. Clin. Interv. Aging 2014, 9, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Stack, A.G.; Molony, D.A.; Rives, T.; Tyson, J.; Murthy, B.V. Association of physical activity with mortality in the US dialysis population. Am. J. Kidney Dis. 2005, 45, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M. Cause of death in patients with reduced kidney function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, R.; Tan, S.J.; Hawley, C.M.; Johnson, D.W.; Stanton, T.; Lee, K.; Mudge, D.W.; Campbell, S.; Elder, G.J.; Toussaint, N.D.; et al. Progression of arterial stiffness is associated with changes in bone mineral markers in advanced CKD. BMC Nephrol. 2017, 18, 281. [Google Scholar] [CrossRef] [PubMed]
- Temmar, M.; Liabeuf, S.; Renard, C.; Czernichow, S.; Esper, N.E.; Shahapuni, I.; Presne, C.; Makdassi, R.; Andrejak, M.; Tribouilloy, C.; et al. Pulse wave velocity and vascular calcification at different stages of chronic kidney disease. J. Hypertens. 2010, 28, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Tripepi, G.; Agharazii, M.; Pannier, B.; D’Arrig o, G.; Mallamaci, F.; Zoccali, C.; London, G. Pulse wave velocity and prognosis in end-stage kidney disease. Hypertension 2018, 71, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Gillespie, I.A.; Eckardt, K.U.; Kronenberg, F.; Richards, S.; Drueke, T.B.; Stenvinkel, P.; Pisoni, R.L.; Robinson, B.M.; Marcelli, D.; et al. Development and validation of cardiovascular risk scores for haemodialysis patients. Int. J. Cardiol. 2016, 216, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Matschkal, J.; Mayer, C.C.; Sarafidis, P.A.; Lorenz, G.; Braunisch, M.C.; Guenthner, R.; Angermann, S.; Steubl, D.; Kemmner, S.; Bachmann, Q.; et al. Comparison of 24-h and office pulse wave velocity for prediction of mortality in hemodialysis patients. Am. J. Nephrol. 2019, 49, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; AgabitiRosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Boesby, L.; Elung-Jensen, T.; Strandgaard, S.; Kamper, A.L. Eplerenone attenuates pulse wave reflection in chronic kidney disease stage 3–4—A randomized controlled study. PLoS ONE 2013, 8, e64549. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Robertson, I.K.; Ball, M.J.; Geraghty, D.P.; Sharman, J.E.; Coombes, J.S. Effects of atorvastatin on arterial stiffness in chronic kidney disease: A randomised controlled trial. J. Atheroscler. Thromb. 2010, 17, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jassal, S.V.; Karaboyas, A.; Comment, L.A.; Bieber, B.A.; Morgenstern, H.; Sen, A.; Gillespie, B.W.; De Sequera, P.; Marshall, M.R.; Fukuhara, S.; et al. Functional dependence and mortality in the international dialysis outcomes and practice patterns study (DOPPS). Am. J. Kidney Dis. 2016, 67, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Hanafusa, N.; Yasunaga, H.; Nangaku, M. Association of dialysis with in-hospital disability progression and mortality in community-onset stroke. Nephrology 2019, 24, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Isoyama, N.; Qureshi, A.R.; Avesani, C.M.; Lindholm, B.; Barany, P.; Heimburger, O.; Cederholm, T.; Stenvinkel, P.; Carrero, J.J. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, R.; Roshanravan, B.; Shimoda, T.; Mamorita, N.; Yoneki, K.; Harada, M.; Watanabe, T.; Yoshida, A.; Takeuchi, Y.; Matsunaga, A. Physical activity dose for hemodialysis patients: Where to begin? Results from a prospective cohort study. J. Ren. Nutr. 2018, 28, 45–53. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, A.M.; Tawney, K.; Bacchetti, P.; Johansen, K.L. Decreased survival among sedentary patients undergoing dialysis: Results from the dialysis morbidity and mortality study wave 2. Am. J. Kidney Dis. 2003, 41, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Beusterien, K.M.; Nissenson, A.R.; Port, F.K.; Kelly, M.; Steinwald, B.; Ware, J.E., Jr. The effects of recombinant human erythropoietin on functional health and well-being in chronic dialysis patients. J. Am. Soc. Nephrol. 1996, 7, 763–773. [Google Scholar] [PubMed]
- Moore, G.E.; Parsons, D.B.; Stray-Gundersen, J.; Painter, P.L.; Brinker, K.R.; Mitchell, J.H. Uremic myopathy limits aerobic capacity in hemodialysis patients. Am. J. Kidney Dis. 1993, 22, 277–287. [Google Scholar] [CrossRef]
- Chou, F.F.; Lee, C.H.; Chen, J.B. General weakness as an indication for parathyroid surgery in patients with secondary hyperparathyroidism. Arch. Surg. 1999, 134, 1108–1111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- United States Renal Data System. The USRDS dialysis morbidity and mortality study: Wave 2. United States renal data system. Am. J. Kidney Dis. 1997, 30, S67–S85. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Wuerth, D.; Finkelstein, S.H.; Finkelstein, F.O. The identification and treatment of depression in patients maintained on dialysis. Semin. Dial. 2005, 18, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Malluche, H.H.; Mawad, H.W.; Monier-Faugere, M.C. Renal osteodystrophy in the first decade of the new millennium: Analysis of 630 bone biopsies in black and white patients. J. Bone Miner. Res. 2011, 26, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Moyses, R.M.; Neves, K.R.; Canziani, M.E.; Draibe, S.A.; Jorgetti, V.; Carvalho, A.B. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008, 73, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.C.; Sung, J.M.; Chang, Y.T.; Hwang, J.S.; Wang, J.D. Estimation of physical functional disabilities and long-term care needs for patients under maintenance hemodialysis. Med. Care 2014, 52, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kurella Tamura, M.; Covinsky, K.E.; Chertow, G.M.; Yaffe, K.; Landefeld, C.S.; McCulloch, C.E. Functional status of elderly adults before and after initiation of dialysis. N. Engl. J. Med. 2009, 361, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Nonoyama, M.L.; Brooks, D.; Ponikvar, A.; Jassal, S.V.; Kontos, P.; Devins, G.M.; Spanjevic, L.; Heck, C.; Laprade, J.; Naglie, G. Exercise program to enhance physical performance and quality of life of older hemodialysis patients: A feasibility study. Int. Urol. Nephrol. 2010, 42, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Golebiowski, T.; Kusztal, M.; Weyde, W.; Dziubek, W.; Wozniewski, M.; Madziarska, K.; Krajewska, M.; Letachowicz, K.; Strempska, B.; Klinger, M. A program of physical rehabilitation during hemodialysis sessions improves the fitness of dialysis patients. Kidney Blood Press. Res. 2012, 35, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Wiebe, N.; Padwal, R.S.; Gyenes, G.; Headley, S.A.E.; Radhakrishnan, J.; Graham, M. The effect of exercise on blood pressure in chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2019, 14, e0211032. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.E.; La Gerche, A.; Coombes, J.S. Exercise and cardiovascular risk in patients with hypertension. Am. J. Hypertens. 2015, 28, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, N.D.; Polkinghorne, K.R.; Kerr, P.G. Impact of intradialytic exercise on arterial compliance and B-type natriuretic peptide levels in hemodialysis patients. Hemodial. Int. 2008, 12, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Green, S.; Fiatarone Singh, M.A.; Barnard, R.; Bonder, C.S.; Cheema, B.S. Effect of intradialytic resistance training on pulse wave velocity and associated cardiovascular disease biomarkers in end stage renal disease. Nephrology 2018, 23, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Debowska, M.; Poleszczuk, J.; Dabrowski, W.; Wojcik-Zaluska, A.; Zaluska, W.; Waniewski, J. Impact of hemodialysis on cardiovascular system assessed by pulse wave analysis. PLoS ONE 2018, 13, e0206446. [Google Scholar] [CrossRef] [PubMed]
| Independent (n = 67) | Dependent (n = 62) | ||||
|---|---|---|---|---|---|
| Blood pressure and HR | Mean | ± | Mean | ± | p-value |
| SBP (mmHg) | 142.8 | 26.3 | 139.7 | 29.9 | 0.530 |
| MBP (mmHg) | 112.5 | 20.0 | 106.2 | 20.8 | 0.083 |
| DBP (mmHg) | 86.9 | 17.0 | 77.8 | 15.7 | <0.05 |
| pPP (mmHg) | 55.9 | 17.5 | 61.9 | 20.9 | 0.078 |
| cSBP (mmHg) | 127.8 | 22.6 | 123.8 | 24.8 | 0.341 |
| cDBP (mmHg) | 89.0 | 17.5 | 79.6 | 16.0 | <0.05 |
| cPP (mmHg) | 38.7 | 12.7 | 44.2 | 15.2 | <0.05 |
| HR (1/min.) | 73.5 | 14.3 | 72.1 | 11.6 | 0.541 |
| Hemodynamic | |||||
| EF (mL) | 72.9 | 14.9 | 73.3 | 14.4 | 0.898 |
| CO (L/min.) | 5.3 | 1.1 | 5.2 | 1.1 | 0.869 |
| TVR (s × mmHg/mL) | 1.3 | 0.3 | 1.3 | 0.4 | 0.251 |
| Cardiac index (L/min. × 1/m2) | 2.9 | 0.7 | 2.9 | 0.8 | 0.677 |
| Vascular stiffness | |||||
| PWV (m/s) | 8.8 | 2.0 | 10.9 | 2.2 | <0.05 |
| Aix@75 (%) | 21.6 | 14.2 | 22.9 | 12.9 | 0.602 |
| Augmentation pressure (mmHg) | 10.0 | 8.4 | 12.6 | 9.6 | 0.102 |
| Reflex (%) | 60.6 | 10.8 | 63.0 | 9.9 | 0.197 |
| Sex, Age, and body size | |||||
| Female. No. (%) * | 28 (41.8) | 26 (41.9) | 0.992 | ||
| Age (y.) | 57.9 | 14.7 | 71.6 | 12.5 | <0.05 |
| BMI (m2/kg2) | 26.4 | 5.3 | 26.5 | 5.2 | 0.862 |
| Weight (kg) | 75.4 | 19.2 | 73.1 | 16.0 | 0.458 |
| Cardiovascular | |||||
| QRISK®3 (%) | 22.6 | 13.0 | 32.9 | 11.8 | <0.05 |
| History of CV events: No (%) * | 30 (45) | 55 (89) | <0.05 | ||
| Antihypertensive agents | |||||
| ꞵ-blockers No (%) * | 50 (74.6) | 42 (67.7) | 0.723 | ||
| CCB No (%) * | 46 (68.7) | 26 (41.9) | 0.102 | ||
| ACEI/ABR No (%) * | 7 (10.4) | 5 (8.0) | 0.671 | ||
| Diuretics No (%) * | 14 (20.9) | 15 (24.2) | 0.722 | ||
| α-blockers No (%) * | 20 (29.9) | 20 (32.3) | 0.830 | ||
| Cause of Renal Failure | All Patients (n = 129) | Independent (n = 67) | Dependent (n = 62) | p-Value * |
|---|---|---|---|---|
| Ischemic nephropathy/hypertension No (%) | 45 (34.9) | 13 (19.4) | 32 (51.6) | <0.01 |
| Diabetic nephropathy No (%) | 34 (26.4) | 16 (23.9) | 18 (29.0) | 0.613 |
| Glomerulonephritis No (%) | 24 (18.6) | 19 (28.4) | 5 (8.1) | <0.05 |
| ADPKD No (%) | 8 (6.2) | 6 (9.0) | 2 (3.2) | 0.205 |
| Pyelonephritis (reflux, stones) No (%) | 9 (6.98) | 7 (10.4) | 2 (3.2) | 0.133 |
| Urologic cancer (kidney, prostate) No (%) | 6 (4.65) | 4 (6.0) | 2 (3.2) | 0.480 |
| Unknown No (%) | 2 (1.55) | 1 (1.5) | 1 (1.6) | 0.956 |
| Myeloma No (%) | 1 (0.78) | 1 (1.5) | 0 (0) | 0.337 |
| Disorders Leading to Disability | All Patients (n = 129) | Independent (n = 67) | Dependent (n = 62) | p-Value * |
|---|---|---|---|---|
| Heart failure/coronary heart disease/acquired valvular heart disease | 27 (20.9) | 3 (4.5) | 24 (38.7) | <0.01 |
| Lower limb ischemia, diabetic foot and amputation | 17 (13.2) | 4 (6.0) | 13 (21.0) | <0.05 |
| Central nervous system disorder | 14 (10.9) | 3 (4.5) | 11 (17.7) | <0.05 |
| Sarcopenia/weakness associated with dialysis | 28 (21.7) | 26 (38.8) | 2 (3.2) | <0.01 |
| Join and skeletal system manifestations | 18 (14.0) | 9 (13.4) | 9 (14.5) | 0.878 |
| Vision and hearing loss | 4 (3.1) | 2 (3.0) | 2 (3.2) | 0.939 |
| Respiratory tract disorders | 3 (2.3) | 2 (3.0) | 1 (1.6) | 0.614 |
| Fully independent | 18 (14.0) | 18 (26.9) | 0 (0) | <0.01 |
| Variable | Estimate | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Age (year.) | 0.075 | 1.08 | 1.04 | 1.11 | <0.05 |
| PWV (m/s) | 0.499 | 1.65 | 1.33 | 2.04 | <0.05 |
| Aix@75 (%) | 0.007 | 1.01 | 0.98 | 1.03 | 0.60 |
| BMI (kg/m2) | 0.006 | 1.01 | 0.94 | 1.08 | 0.86 |
| QRISK®3 (%) | 0.066 | 1.07 | 1.04 | 1.10 | <0.05 |
| HD (m) | 0.004 | 1.00 | 1.00 | 1.01 | 0.15 |
| SBP (mmHg) | −0.004 | 1.00 | 0.98 | 1.01 | 0.53 |
| MBP (mmHg) | −0.015 | 0.98 | 0.97 | 1.00 | 0.09 |
| DBP (mmHg) | −0.034 | 0.97 | 0.94 | 0.99 | <0.05 |
| pPP (mmHg) | 0.017 | 1.02 | 1.00 | 1.04 | 0.08 |
| HR (1/min) | −0.008 | 0.99 | 0.97 | 1.02 | 0.54 |
| cSBP (mmHg) | −0.007 | 0.99 | 0.98 | 1.01 | 0.34 |
| cDBP (mmHg) | −0.034 | 0.97 | 0.95 | 0.99 | <0.05 |
| cpPP (mmHg) | 0.029 | 1.03 | 1.00 | 1.06 | <0.05 |
| EF (mL) | 0.002 | 1.00 | 0.98 | 1.03 | 0.90 |
| CO (mL/min.) | −0.003 | 1.00 | 0.97 | 1.03 | 0.87 |
| History of CV (1) | 2.271 | 9.69 | 3.85 | 24.37 | <0.05 |
| Variable | Estimate | adj.OR * | 95% CI | p-Value | |
|---|---|---|---|---|---|
| History of CV (1) | 1.576 | 4.83 | 1.74 | 13.41 | <0.05 |
| PWV (m/s) | 0.370 | 1.45 | 1.15 | 1.82 | <0.05 |
| cDBP (mmHg) | −0.031 | 0.97 | 0.94 | 0.99 | <0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołębiowski, T.; Kusztal, M.; Konieczny, A.; Letachowicz, K.; Gawryś, A.; Skolimowska, B.; Ostrowska, B.; Zmonarski, S.; Janczak, D.; Krajewska, M. Disability of Dialysis Patients and the Condition of Blood Vessels. J. Clin. Med. 2020, 9, 1806. https://doi.org/10.3390/jcm9061806
Gołębiowski T, Kusztal M, Konieczny A, Letachowicz K, Gawryś A, Skolimowska B, Ostrowska B, Zmonarski S, Janczak D, Krajewska M. Disability of Dialysis Patients and the Condition of Blood Vessels. Journal of Clinical Medicine. 2020; 9(6):1806. https://doi.org/10.3390/jcm9061806
Chicago/Turabian StyleGołębiowski, Tomasz, Mariusz Kusztal, Andrzej Konieczny, Krzysztof Letachowicz, Ada Gawryś, Beata Skolimowska, Bożena Ostrowska, Sławomir Zmonarski, Dariusz Janczak, and Magdalena Krajewska. 2020. "Disability of Dialysis Patients and the Condition of Blood Vessels" Journal of Clinical Medicine 9, no. 6: 1806. https://doi.org/10.3390/jcm9061806
APA StyleGołębiowski, T., Kusztal, M., Konieczny, A., Letachowicz, K., Gawryś, A., Skolimowska, B., Ostrowska, B., Zmonarski, S., Janczak, D., & Krajewska, M. (2020). Disability of Dialysis Patients and the Condition of Blood Vessels. Journal of Clinical Medicine, 9(6), 1806. https://doi.org/10.3390/jcm9061806






