Ratio of IL-8 in CSF Versus Serum Is Elevated in Patients with Unruptured Brain Aneurysm
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Sample Collection and Storage
2.3. IL-8 and MCP-1 Concentrations Evaluation
2.4. Statistical Analysis
3. Results
3.1. IL-8 Results
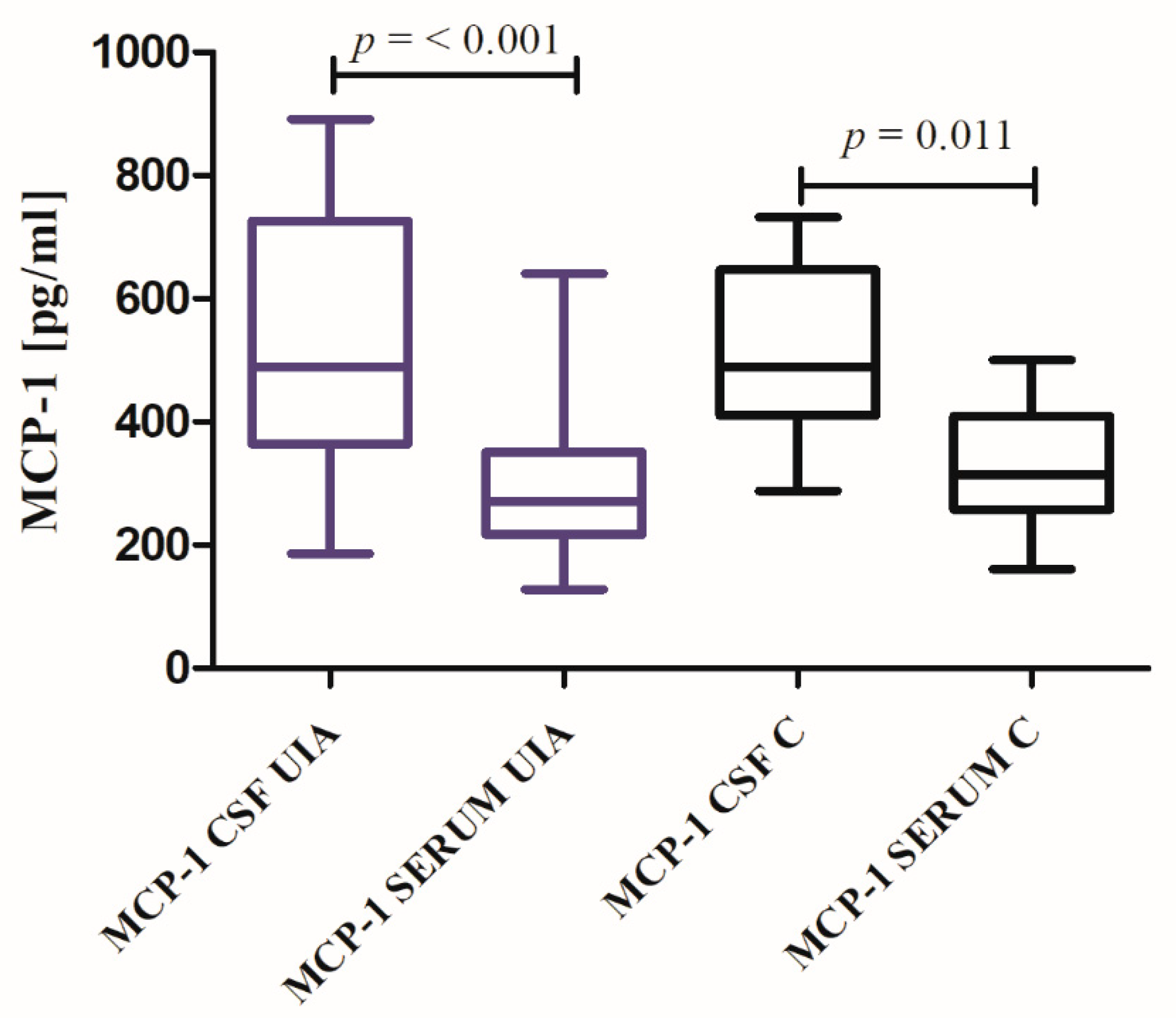
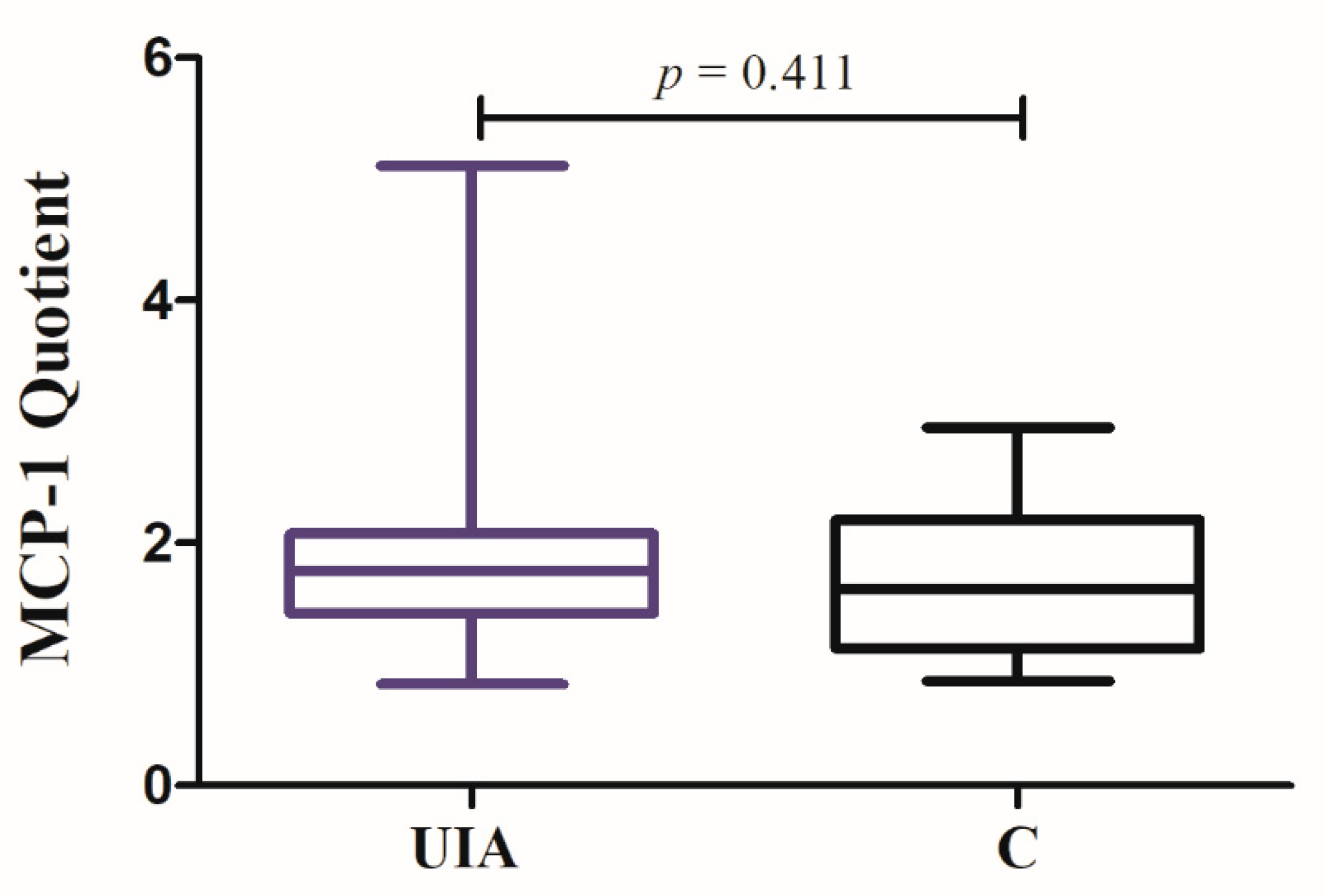
3.2. MCP-1 Results
3.3. Logistics Regression Analysis Results
3.4. Correlation Coefficient Results
3.5. Linear Regression Analysis Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wardlaw, J.M.; White, P.M. The detection and management of unruptured intracranial aneurysms. Brain 2000, 123, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Krex, D.; Schackert, H.K.; Schackert, G. Genesis of cerebral aneurysms—An update. Acta Neurochir. (Wien.) 2001, 143, 429–448; discussion 448–449. [Google Scholar] [PubMed]
- Francis, S.E.; Tu, J.; Qian, Y.; Avolio, A.P. A combination of genetic, molecular and haemodynamic risk factors contributes to the formation, enlargement and rupture of brain aneurysms. J. Clin. Neurosci. 2013, 20, 912–918. [Google Scholar] [CrossRef]
- Inci, S.; Spetzler, R.F. Intracranial aneurysms and arterial hypertension: A review and hypothesis. Surg. Neurol. 2000, 53, 530–540; discussion 540–542. [Google Scholar] [CrossRef]
- Hashimoto, T.; Meng, H.; Young, W.L. Intracranial aneurysms: Links among inflammation, hemodynamics and vascular remodeling. Neurol. Res. 2006, 28, 372–380. [Google Scholar] [CrossRef]
- Chalouhi, N.; Ali, M.S.; Starke, R.M.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. Cigarette smoke and inflammation: Role in cerebral aneurysm formation and rupture. Mediat. Inflamm. 2012, 2012, 271582. [Google Scholar] [CrossRef]
- Cebral, J.; Ollikainen, E.; Chung, B.J.; Mut, F.; Sippola, V.; Jahromi, B.R.; Tulamo, R.; Hernesniemi, J.; Niemelä, M.; Robertson, A.; et al. Flow Conditions in the Intracranial Aneurysm Lumen Are Associated with Inflammation and Degenerative Changes of the Aneurysm Wall. AJNR Am. J. Neuroradiol. 2017, 38, 119–126. [Google Scholar] [CrossRef]
- Juvela, S.; Porras, M.; Poussa, K. Natural history of unruptured intracranial aneurysms: Probability of and risk factors for aneurysm rupture. J. Neurosurg. 2000, 93, 379–387. [Google Scholar] [CrossRef]
- Kotowski, M.; Naggara, O.; Darsaut, T.E.; Nolet, S.; Gevry, G.; Kouznetsov, E.; Raymond, J. Safety and occlusion rates of surgical treatment of unruptured intracranial aneurysms: A systematic review and meta-analysis of the literature from 1990 to 2011. J. Neurol. Neurosurg. Psychiatry 2013, 84, 42–48. [Google Scholar] [CrossRef]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J., 3rd; Meissner, I.; Brown, R.D., Jr.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [PubMed]
- Murayama, Y.; Takao, H.; Ishibashi, T.; Saguchi, T.; Ebara, M.; Yuki, I.; Arakawa, H.; Irie, K.; Urashima, M.; Molyneux, A.J. Risk Analysis of Unruptured Intracranial Aneurysms: Prospective 10-Year Cohort Study. Stroke 2016, 47, 365–371. [Google Scholar] [CrossRef]
- Campi, A.; Ramzi, N.; Molyneux, A.J.; Summers, P.E.; Kerr, R.S.; Sneade, M.; Yarnold, J.A.; Rischmiller, J.; Byrne, J.V. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 2007, 38, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.; Bogdan, G.; Radančević, D.; Pejanović-Škobić, N. Contrast-Induced Encephalopathy following Cerebral Angiography in a Hemodialysis Patient. Case Rep. Neurol. Med. 2020, 2020, 3985231. [Google Scholar] [PubMed]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef]
- Bryant, V.L.; Slade, C.A. Chemokines, their receptors and human disease: The good, the bad and the itchy. Immunol. Cell Biol. 2015, 93, 364–371. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Egashira, K.; Hashimoto, N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke 2009, 40, 942–951. [Google Scholar] [CrossRef]
- Nowicki, K.W.; Hosaka, K.; He, Y.; McFetridge, P.S.; Scott, E.W.; Hoh, B.L. Novel high-throughput in vitro model for identifying hemodynamic-induced inflammatory mediators of cerebral aneurysm formation. Hypertension 2014, 64, 1306–1313. [Google Scholar] [CrossRef]
- Aoki, T.; Nishimura, M. Targeting chronic inflammation in cerebral aneurysms: Focusing on NF-kappaB as a putative target of medical therapy. Expert Opin. Ther. Targets 2010, 14, 265–273. [Google Scholar] [CrossRef]
- Bizzarri, C.; Beccari, A.R.; Bertini, R.; Cavicchia, M.R.; Giorgini, S.; Allegretti, M. ELR + CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol. Ther. 2006, 112, 139–149. [Google Scholar] [CrossRef]
- Feniger-Barish, R.; Belkin, D.; Zaslaver, A.; Gal, S.; Dori, M.; Ran, M.; Ben-Baruch, A. GCP-2-induced internalization of IL-8 receptors: Hierarchical relationships between GCP-2 and other ELR(+)-CXC chemokines and mechanisms regulating CXCR2 internalization and recycling. Blood 2000, 95, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, S.L.; Strieter, R.M.; Chensue, S.W.; Basha, M.; Standiford, T.; Ham, J.; Remick, D.G. Tumor necrosis factor-alpha, interleukin-8 and chemotactic cytokines. Prog. Clin. Biol. Res. 1990, 349, 433–444. [Google Scholar] [PubMed]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Chyatte, D.; Bruno, G.; Desai, S.; Todor, D.R. Inflammation and intracranial aneurysms. Neurosurgery 1999, 45, 1137–1146; discussion 1146–1147. [Google Scholar] [CrossRef]
- Tulamo, R.; Frösen, J.; Hernesniemi, J.; Niemelä, M. Inflammatory changes in the aneurysm wall: A review. J. Neurointerv. Surg. 2018, 10, 58–67. [Google Scholar] [CrossRef]
- Meng, H.; Tutino, V.M.; Xiang, J.; Siddiqui, A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am. J. Neuroradiol. 2014, 35, 1254–1262. [Google Scholar] [CrossRef]
- Chalouhi, N.; Points, L.; Pierce, G.L.; Ballas, Z.; Jabbour, P.; Hasan, D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke 2013, 44, 2594–2597. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhao, M.G.; Liang, G.B.; Song, Z.Q.; Li, Z.Q. Expression of pro-inflammatory cytokines and the risk of intracranial aneurysm. Inflammation 2013, 36, 1195–1200. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Zhao, P.; Shen, J.; Li, X.; Wang, D.; Li, G.; Su, W. The Impact of Initial Systemic Inflammatory Response after Aneurysmal Subarachnoid Hemorrhage. Turk. Neurosurg. 2017, 27, 346–352. [Google Scholar]
- Koper, O.M.; Kamińska, J.; Sawicki, K.; Reszeć, J.; Rutkowski, R.; Jadeszko, M.; Mariak, Z.; Dymicka-Piekarska, V.; Kemona, H. Cerebrospinal fluid and serum IL-8, CCL2, and ICAM-1 concentrations in astrocytic brain tumor patients. Ir. J. Med. Sci. 2018, 187, 767–775. [Google Scholar] [CrossRef]
- Koper, O.M.; Kamińska, J.; Grygorczuk, S.; Zajkowska, J.; Kemona, H. CXCL9 concentrations in cerebrospinal fluid and serum of patients with tick-borne encephalitis. Arch. Med. Sci. 2018, 14, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Koper-Lenkiewicz, O.M.; Kamińska, J.; Milewska, A.; Sawicki, K.; Jadeszko, M.; Mariak, Z.; Reszeć, J.; Dymicka-Piekarska, V.; Matowicka-Karna, J. Serum and cerebrospinal fluid Neudesin concentration and Neudesin Quotient as potential circulating biomarkers of a primary brain tumor. BMC Cancer 2019, 19, 319. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. External quality assessment in clinical neurochemistry: Survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin. Chem. 1995, 41, 256–263. [Google Scholar] [CrossRef]
- Reiber, H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin. Chim. Acta 2001, 310, 173–186. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007, 96, 644–647. [Google Scholar] [CrossRef]
- Bolboacă, S.D. Medical Diagnostic Tests: A Review of Test Anatomy, Phases, and Statistical Treatment of Data. Comput. Math. Methods Med. 2019, 2019, 1891569. [Google Scholar] [CrossRef]
- Wang, S.M.; Lei, H.Y.; Yu, C.K.; Wang, J.R.; Su, I.J.; Liu, C.C. Acute chemokine response in the blood and cerebrospinal fluid of children with enterovirus 71-associated brainstem encephalitis. J. Infect. Dis. 2008, 198, 1002–1006. [Google Scholar] [CrossRef]
- Tada, Y.; Yagi, K.; Kitazato, K.T.; Tamura, T.; Kinouchi, T.; Shimada, K.; Matsushita, N.; Nakajima, N.; Satomi, J.; Kageji, T.; et al. Reduction of endothelial tight junction proteins is related to cerebral aneurysm formation in rats. J. Hypertens. 2010, 28, 1883–1891. [Google Scholar] [CrossRef]
- Giron, S.E.; Bjurstrom, M.F.; Griffis, C.A.; Ferrante, F.M.; Wu, I.I.; Nicol, A.L.; Grogan, T.R.; Burkard, J.F.; Irwin, M.R.; Breen, E.C. Increased Central Nervous System Interleukin-8 in a Majority Postlaminectomy Syndrome Chronic Pain Population. Pain Med. 2018, 19, 1033–1043. [Google Scholar] [CrossRef]
- Liu, X.; Kumar, A. Differential signaling mechanism for HIV-1 Nef-mediated production of IL-6 and IL-8 in human astrocytes. Sci. Rep. 2015, 5, 9867. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Scotter, E.L.; Jansson, D.; Kho, D.T.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; Faull, R.L.; Curtis, M.A.; Graham, S.E.; et al. An anti-inflammatory role for C/EBPδ in human brain pericytes. Sci. Rep. 2015, 5, 12132. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S. Plasma endothelin concentrations after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2000, 92, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Seifert, V.; Löffler, B.M.; Zimmermann, M.; Roux, S.; Stolke, D. Endothelin concentrations in patients with aneurysmal subarachnoid hemorrhage. Correlation with cerebral vasospasm, delayed ischemic neurological deficits, and volume of hematoma. J. Neurosurg. 1995, 82, 55–62. [Google Scholar] [CrossRef]
- Koper, O.M.; Kamińska, J.; Kemona, H.; Dymicka-Piekarska, V. Application of the Bead-Based Technique in Neurodegeneration: A Literature Review. Neurodegener. Dis. 2015, 15, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.; Gwank, H.S.; Kim, S. Retrospective Analysis of Cerebrospinal Fluid Profiles in 228 Patients with Leptomeningal Carcinomatosis: Differences According to the Sampling Site, Symptoms and Sytemic Factors. J. Korean Neurosurg. Soc. 2016, 59, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Kalamatianos, T.; Markianos, M.; Margetis, K.; Bourlogiannis, F.; Stranjalis, G. Higher Orexin A levels in lumbar compared to ventricular CSF: A study in idiopathic normal pressure hydrocephalus. Peptides 2014, 51, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Brander, S.; Thaler, C.; Lewczuk, P.; Lelental, N.; Buchfelder, M.; Kleindienst, A. Neuroprotein Dynamics in the Cerebrospinal Fluid: Intraindividual Concomitant Ventricular and Lumbar Measurements. Eur. Neurol. 2013, 70, 189–194. [Google Scholar] [CrossRef]




| UIA Group | Control Group | p-Value | |
|---|---|---|---|
| Age (mean, range) (years) | 56 (30–70) | 57 (25–78) | 0.235 |
| Gender: Female/Male | 20/5 | 14/6 | 0.670 |
| LABORATORY PARAMETERS | |||
| WBC (×103/µL) RBC (×106/µL) HGB (g/dL) HCT (%) PLT (×103/µL) MPV (fL) P-LCR(%) PT (s) INR APTT (s) Fibrinogen (mg/dL) | 6.94 (5.31–8.11) 4.52 (4.20–4.80) 13.40 (12.60–14.40) 40.20 (37.60–42.40) 231 (187–246) 11.0 (10.3–11.6) 33.7 (27.1–38.1) 12.2 (11.9–12.5) 0.91 (0.88–0.93) 28.8 (27.3–30.1) 339 (306–391) | 6.17 (4.71–7.52) 4.51 (4.12–4.90) 13.45 (12.70–14.90) 40.80 (38.10–43.60) 254 (196–289) 10.8 (10.3–11.1) 30.5 (27.3–33.9) 12.7 (12.5–12.9) 0.99 (0.94–0.99) 26.6 (25.9–29.0) 277 (206–307) | 0.195 0.985 0.869 0.742 0.432 0.400 0.371 0.006 0.001 0.034 0.003 |
| Na+ (mmol/L) | 138 (137–140) | 140 (136–140) | 0.941 |
| K+ (mmol/L) | 4.3 (4.0–4.6) | 4.5 (4.2–4.7) | 0.281 |
| Glucose (mg/dL) | 92 (87–102) | 89 (86–103) | 0.584 |
| Urea (mg/dL) | 29 (24–37) | 30 (20–49) | 0.812 |
| Creatinine (mg/dL) | 0.73 (0.66–0.85) | 0.78 (0.69–0.83) | 0.534 |
| CLINICAL CHARACTERISTICS OF UIA GROUP | |||
| Ethnicity: | All patients belong to Caucasian ethnicity | ||
| Prior SAH: | No | - | - |
| Number of aneurysm: Single/Multiple | 15/10 | - | - |
| Polycyclic aneurysms: n (%) | 10 (40%) | - | - |
| Hypertension: n (%) | 16 (64%) | 10 (50%) | 0.521 |
| Smoking: n (%) | 10 (40%) | 2 (10%) | 0.055 |
| Obesity: n (%) | 6 (24%) | 8 (40%) | 0.408 |
| No. | Aneurysm Location | Side: L/R | Aneurysm Size # (mm) | Number of Aneurysms | Polycyclic Aneurysm Yes/No |
|---|---|---|---|---|---|
| 1. | MCA | R | 5.0 | 4 | Yes |
| 2. | MCA | R | 6.0 | 1 | No |
| 3. | MCA | L | 5.6 | 2 | No |
| 4. | MCA, ICA | R | 5.6 | 6 | No |
| 5. | MCA | R | 7.0 | 1 | No |
| 6. | MCA | R | 5.0 | 1 | No |
| 7. | MCA | L | 9.5 | 1 | Yes |
| 8. | ICA | L | 3.2 | 2 | Yes |
| 9. | AcomA | R | 4.3 | 3 | Yes |
| 10. | MCA | L | 4.7 | 1 | No |
| 11. | MCA | L | 3.0 | 1 | No |
| 12. | MCA | L | 8.0 | 1 | Yes |
| 13. | MCA | L | 4.5 | 2 | No |
| 14. | ICA | L | 4.0 | 1 | No |
| 15. | ICA | L | 4.2 | 1 | Yes |
| 16. | MCA | L | 6.0 | 1 | Yes |
| 17. | MCA | L | 3.4 | 1 | No |
| 18. | MCA | R | 15.0 | 4 | Yes |
| 19. | MCA | L | 5.0 | 1 | No |
| 20. | ICA | R | 8.0 | 1 | Yes |
| 21. | MCA | R | 5.6 | 1 | No |
| 22. | MCA | R | 5.4 | 2 | No |
| 23. | MCA | L | 7.0 | 3 | No |
| 24. | MCA | R | 4.8 | 1 | Yes |
| 25. | MCA | R | 7.0 | 2 | No |
| Cut-Off | Youden Index | AUC ± SE | Se [%] | Sp [%] | PPV [%] | NPV [%] | ACC [%] | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| IL-8 Quotient | 2.28 | 0.42 | 0.720 ± 0.092 | 72 | 70 | 86 | 50 | 71 | 0.017 |
| Variable | OR | 95%CI | p-Value |
|---|---|---|---|
| Univariate logistic regression analysis | |||
| IL-8 Quotient | 1.84 | 1.001–3.308 | 0.050 |
| Univariate linear regression analysis | ||||
| No | Variable | β | eβ (95%CI) | p-Value |
| 1 | CSF IL-8 (pg/mL) | 0.013 | 1.013 (1.002–1.024) | 0.020 |
| 2 | BMI (kg/m2) | 0.034 | 1.035 (1.004–1.067) | 0.029 |
| Multivariate linear regression analysis | ||||
| No | Variable | β | eβ (95%CI) | p-Value |
| 1 | CSF IL-8 (pg/mL) | 0.014 | 1.014 (1.003–1.025) | 0.014 |
| 2 | BMI (kg/m2) | 0.031 | 1.031 (1.004–1.059) | 0.026 |
| Aneurysm Size (mm) | p-Value | ||
|---|---|---|---|
| <5.4 | ≥5.4 | ||
| CSF IL-8 (pg/mL) | 28.2 (22.9–32.5) | 39.9 (30.1–44.3) | 0.036 |
| Serum IL-8 (pg/mL) | 9.6 (8.7–12.1) | 11.1 (10.2–16.4) | 0.123 |
| IL-8 Quotient | 2.5 (2.3–3.4) | 3.4 (1.9–5.0) | 0.503 |
| CSF MCP-1 (pg/mL) | 425.3 (364.1–606.9) | 603.6 (378.0–740.0) | 0.538 |
| Serum MCP-1 (pg/mL) | 238.6 (209.1–359.9) | 294.0 (217.6–340.8) | 0.852 |
| MCP-1 Quotient | 1.9 (1.4–2.0) | 1.7 (1.5–2.1) | 0.726 |
| NUMBER OF ANEURYSM | |||
| Single | Multiple | ||
| CSF IL-8 (pg/mL) | 30.9 (23.4–36.9) | 31.5 26.2–41.9) | 0.461 |
| Serum IL-8 (pg/mL) | 9.6 (8.8–11.3) | 12.6 (10.2–58.1) | 0.062 |
| IL-8 Quotient | 3.0 (2.3–3.7) | 2.3 (0.7–4.4) | 0.397 |
| CSF MCP-1 (pg/mL) | 380.0 (362.2–540.6) | 650.2 (537.4–746.0) | 0.036 |
| Serum MCP-1 (pg/mL) | 235.0 (216.6–278.0) | 345.6 (294.0–367.4) | 0.055 |
| MCP-1 Quotient | 1.7 (1.3–1.9) | 1.9 (1.7–3.1) | 0.367 |
| POLYCYCLIC ANEURYSMS | |||
| No | Yes | ||
| CSF IL-8 (pg/mL) | 31.7 (24.2–41.6) | 30.7 (28.1–36.9) | 0.807 |
| Serum IL-8 (pg/mL) | 10.2(9.3–16.1) | 11.1 (9.6–13.0) | 0.892 |
| IL-8 Quotient | 2.6 (2.0–3.6) | 3.3 1.7–5.0) | 0.367 |
| CSF MCP-1 (pg/mL) | 427.6 (362.2–712.0) | 513.6 (366.2–740.0) | 0.765 |
| Serum MCP-1 (pg/mL) | 271.6 (230.6–352.4) | 249.7 (196.7–312.4) | 0.567 |
| MCP-1 Quotient | 1.8 (1.4–3.0) | 1.7 (1.5–2.7) | 0.807 |
| Cut-Off (pg/mL) | Youden Index | AUC ± SE | Se (%) | Sp (%) | PPV (%) | NPV (%) | ACC (%) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| CSF IL-8 | 36.9 | 0.615 | 0.763 ± 0.102 | 62 | 100 | 100 | 71 | 80 | 0.010 |
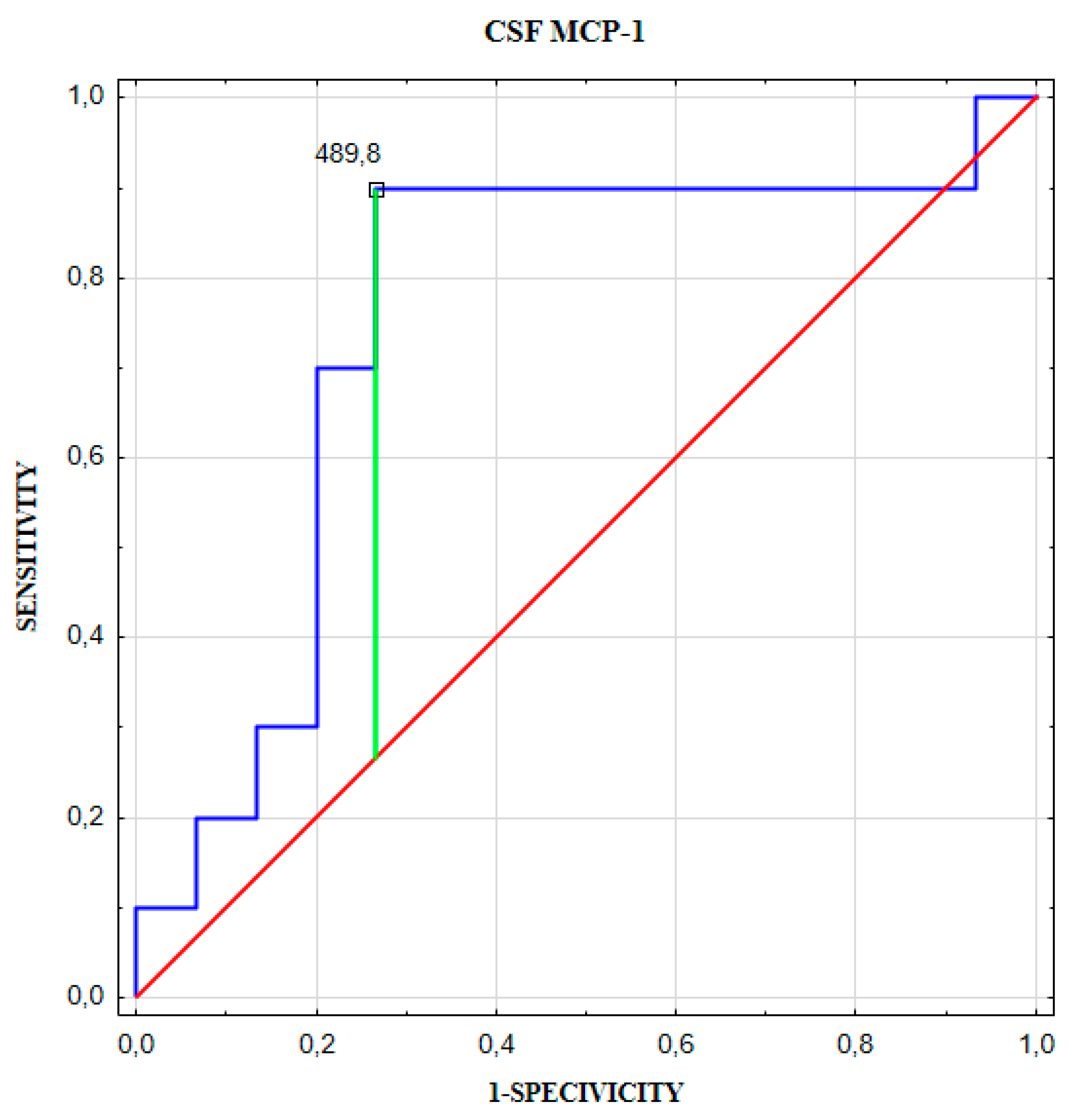
| Cut-off (pg/mL) | Youden index | AUC ± SE | Se (%) | Sp (%) | PPV (%) | NPV (%) | ACC (%) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| CSF MCP-1 | 489.8 | 0.615 | 0.753 ± 0.108 | 90 | 73 | 69 | 92 | 80 | 0.019 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska, J.; Lyson, T.; Chrzanowski, R.; Sawicki, K.; Milewska, A.J.; Tylicka, M.; Zińczuk, J.; Matowicka-Karna, J.; Dymicka-Piekarska, V.; Mariak, Z.; et al. Ratio of IL-8 in CSF Versus Serum Is Elevated in Patients with Unruptured Brain Aneurysm. J. Clin. Med. 2020, 9, 1761. https://doi.org/10.3390/jcm9061761
Kamińska J, Lyson T, Chrzanowski R, Sawicki K, Milewska AJ, Tylicka M, Zińczuk J, Matowicka-Karna J, Dymicka-Piekarska V, Mariak Z, et al. Ratio of IL-8 in CSF Versus Serum Is Elevated in Patients with Unruptured Brain Aneurysm. Journal of Clinical Medicine. 2020; 9(6):1761. https://doi.org/10.3390/jcm9061761
Chicago/Turabian StyleKamińska, Joanna, Tomasz Lyson, Robert Chrzanowski, Karol Sawicki, Anna J. Milewska, Marzena Tylicka, Justyna Zińczuk, Joanna Matowicka-Karna, Violetta Dymicka-Piekarska, Zenon Mariak, and et al. 2020. "Ratio of IL-8 in CSF Versus Serum Is Elevated in Patients with Unruptured Brain Aneurysm" Journal of Clinical Medicine 9, no. 6: 1761. https://doi.org/10.3390/jcm9061761
APA StyleKamińska, J., Lyson, T., Chrzanowski, R., Sawicki, K., Milewska, A. J., Tylicka, M., Zińczuk, J., Matowicka-Karna, J., Dymicka-Piekarska, V., Mariak, Z., & Koper-Lenkiewicz, O. M. (2020). Ratio of IL-8 in CSF Versus Serum Is Elevated in Patients with Unruptured Brain Aneurysm. Journal of Clinical Medicine, 9(6), 1761. https://doi.org/10.3390/jcm9061761









