The Influence of Various Smoking Categories on The Risk of Gestational Hypertension and Pre-Eclampsia
Abstract
1. Introduction
2. Experimental Section
2.1. Method and Study Population
2.2. Studied Variables
2.3. Studied Pregnancy Complication
2.4. Statistical Analyses
3. Results
4. Discussion
Limitations and Benefits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; daSilva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Poniedziałek-Czajkowska, E.; Mierzyński, R.; Dłuski, D.; Leszczyńska-Gorzelak, B. Adipokines and Endothelium Dysfunction Markers in Pregnant Women with Gestational Hypertension. Int. J. Hypertens. 2019, 2019, 7541846. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, W.; Xiong, W.; Wang, F. Effects of blood pressure level management on maternal and perinatal outcomes in pregnant women with mild to moderate gestational hypertension. Ginekol. Pol. 2020, 91, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Smith, G.N.; Rodger, M.; White, R.R.; Walker, M.C.; Wen, S.W. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PloS ONE 2017, 12, e0175914. [Google Scholar] [CrossRef]
- Gusar, V.; Timofeeva, A.; Chagovets, V.; Kan, N.; Vasilchenko, O.; Prozorovskaya, K.; Ivanets, T.; Sukhikh, G. Preeclampsia: The Interplay Between Oxygen-Sensitive miRNAs and Erythropoietin. J. Clin. Med. 2020, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Rahman, R.A.; Murthi, P.; Singh, H.; Gurungsinghe, S.; Leaw, B.; Mockler, J.C.; Lim, R.; Wallace, E.M. Hydroxychloroquine Mitigates the Production of 8-Isoprostane and Improves Vascular Dysfunction: Implications for Treating Preeclampsia. Int. J. Mol. Sci. 2020, 21, 2504. [Google Scholar] [CrossRef]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. First Trimester Microelements and their Relationships with Pregnancy Outcomes and Complications. Nutrients 2020, 12, 1108. [Google Scholar] [CrossRef]
- Lewandowska, M.; Sajdak, S.; Lubiński, J. Serum Selenium Level in Early Healthy Pregnancy as a Risk Marker of Pregnancy Induced Hypertension. Nutrients 2019, 11, 1028. [Google Scholar] [CrossRef]
- Wei, J.; Liu, C.-X.; Gong, T.-T.; Wu, Q.-J.; Wu, L. Cigarette smoking during pregnancy and preeclampsia risk: A systematic review and meta-analysis of prospective studies. Oncotarget 2015, 6, 43667–43678. [Google Scholar] [CrossRef]
- Laule, C.F.; Wing, C.R.; Odean, E.J.; Wilcox, J.A.; Gilbert, J.S.; Regal, J.F. Effect of nicotine on placental ischemia-induced complement activation and hypertension in the rat. J. Immunotoxicol. 2017, 14, 235–240. [Google Scholar] [CrossRef] [PubMed]
- England, L.; Zhang, J. Smoking and risk of preeclampsia: A systematic review. Front. Biosci. J. Virtual Libr. 2007, 12, 2471–2483. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Althabe, F.; Belizán, J.M.; Kafury-Goeta, A.C. Cigarette smoking during pregnancy and risk of preeclampsia: A systematic review. Am. J. Obstet. Gynecol. 1999, 181, 1026–1035. [Google Scholar] [CrossRef]
- Gallus, S.; Lugo, A.; Liu, X.; Behrakis, P.; Boffi, R.; Bosetti, C.; Carreras, G.; Chatenoud, L.; Clancy, L.; Continente, X.; et al. Who smokes in Europe? Data from 12 European countries in the TackSHS survey (2017–2018). J. Epidemiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Probst, C.; Rehm, J.; Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e769–e776. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, L.; Shen, C.; Yao, H.; Zhu, Q.; Cheng, C.; Wang, R. Mothers’ prenatal tobacco smoke exposure is positively associated with the occurrence of developmental coordination disorder among children aged 3–6 years: A cross-sectional study in a rural area of Shanghai, China. Tob. Induc. Dis. 2020, 18, 25. [Google Scholar] [CrossRef]
- Trumble, B.C.; Finch, C.E. The exposome in human evolution: From dust to diesel. Q. Rev. Biol. 2019, 94, 333–394. [Google Scholar] [CrossRef] [PubMed]
- Ranney, L.M.; Kowitt, S.D.; Queen, T.L.; Jarman, K.L.; Goldstein, A.O. An Eye Tracking Study of Anti-Smoking Messages on Toxic Chemicals in Cigarettes. Int. J. Environ. Res. Public. Health 2019, 16, 4435. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E.; Boynton, M.H.; Noar, S.M.; Morgan, J.C.; Mendel, J.R.; Ribisl, K.M.; Stepanov, I.; Nylander-French, L.A.; Brewer, N.T. Effective Message Elements for Disclosures About Chemicals in Cigarette Smoke. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2018, 20, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.T.; Morgan, J.C.; Baig, S.A.; Mendel, J.R.; Boynton, M.H.; Pepper, J.K.; Byron, M.J.; Noar, S.M.; Agans, R.P.; Ribisl, K.M. Public understanding of cigarette smoke constituents: Three US surveys. Tob. Control 2016, 26, 592–599. [Google Scholar] [CrossRef]
- Chang, J.J.; Strauss, J.F.; Deshazo, J.P.; Rigby, F.B.; Chelmow, D.P.; Macones, G.A. Reassessing the impact of smoking on preeclampsia/eclampsia: Are there age and racial differences? PloS ONE 2014, 9, e106446. [Google Scholar] [CrossRef]
- Bakker, R.; Steegers, E.A.; Mackenbach, J.P.; Hofman, A.; Jaddoe, V.W. Maternal smoking and blood pressure in different trimesters of pregnancy: The Generation R study. J. Hypertens. 2010, 28, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Rauchfuss, M.; Fischer, T.; Bogner, G.; Maier, B. Influence of so far neglected psychosomatic factors, BMI and smoking on pregnancy-induced hypertension (PIH). Pregnancy Hypertens. 2012, 2, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-C.; Julien, P.; Wei, S.-Q.; Audibert, F.; Smith, G.N.; Fraser, W.D. MIROS study group Plasma cotinine indicates an increased risk of preeclampsia in previous and passive smokers. Am. J. Obstet. Gynecol. 2014, 210, 232.e1–232.e5. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, O.A.; Grjibovski, A.M.; Krettek, A.; Nieboer, E.; Odland, J.Ø. First-trimester smoking cessation in pregnancy did not increase the risk of preeclampsia/eclampsia: A Murmansk County Birth Registry study. PLoS ONE 2017, 12, e0179354. [Google Scholar] [CrossRef]
- Radoń-Pokracka, M.; Adrianowicz, B.; Płonka, M.; Danił, P.; Nowak, M.; Huras, H. Evaluation of Pregnancy Outcomes at Advanced Maternal Age. Open Access Maced. J. Med. Sci. 2019, 7, 1951–1956. [Google Scholar] [CrossRef]
- LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group; Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.-A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; et al. Association of Gestational Weight Gain With Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702–1715. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Dvorakova, L.; Krofta, L. Evaluation of Vascular Endothelial Function in Young and Middle-Aged Women with Respect to a History of Pregnancy, Pregnancy-Related Complications, Classical Cardiovascular Risk Factors, and Epigenetics. Int. J. Mol. Sci. 2020, 21, 430. [Google Scholar] [CrossRef]
- Nemmar, A.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H.; Adeghate, E. Nose-Only Water-Pipe Smoke Exposure in Mice Elicits Renal Histopathological Alterations, Inflammation, Oxidative Stress, DNA Damage, and Apoptosis. Front. Physiol. 2020, 11, 46. [Google Scholar] [CrossRef]
- Tong, X.; Chaudhry, Z.; Lee, C.-C.; Bone, R.N.; Kanojia, S.; Maddatu, J.; Sohn, P.; Weaver, S.A.; Robertson, M.A.; Petrache, I.; et al. Cigarette smoke exposure impairs β-cell function through activation of oxidative stress and ceramide accumulation. Mol. Metab. 2020, 37, 100975. [Google Scholar] [CrossRef]
- Lewandowska, M.; Sajdak, S.; Lubiński, J. Can Serum Iron Concentrations in Early Healthy Pregnancy Be Risk Marker of Pregnancy-Induced Hypertension? Nutrients 2019, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Sajdak, S.; Marciniak, W.; Lubiński, J. First Trimester Serum Copper or Zinc Levels, and Risk of Pregnancy-Induced Hypertension. Nutrients 2019, 11, 2479. [Google Scholar] [CrossRef]
- Mazurek, D.; Łoźna, K.; Bronkowska, M. The concentration of selected elements in the placenta according to selected sociodemographic factors and their effect on birth mass and birth length of newborns. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2020, 58, 126425. [Google Scholar] [CrossRef]
- Laine, J.E.; Ray, P.; Bodnar, W.; Cable, P.H.; Boggess, K.; Offenbacher, S.; Fry, R.C. Placental Cadmium Levels Are Associated with Increased Preeclampsia Risk. PloS ONE 2015, 10, e0139341. [Google Scholar] [CrossRef] [PubMed]
- Chełchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Lewandowski, L.; Reśko-Zachara, M.; Maciejewski, T.M. Influence of Active Exposure to Tobacco Smoke on Nitric Oxide Status of Pregnant Women. Int. J. Environ. Res. Public. Health 2018, 15, 2719. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.; Nizamov, F.; Woodruff, B.A.; Ishmakova, R.; Komilov, J.; Wegmüller, R.; Wirth, J.P.; Arifdjanova, D.; Guo, S.; Rohner, F. Risk Factors for Anemia and Micronutrient Deficiencies among Women of Reproductive Age-The Impact of the Wheat Flour Fortification Program in Uzbekistan. Nutrients 2020, 12, 714. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Ng, M.J.; Chern, B.; Yeo, G.S.; Tan, K.H. Angiogenic factors during pregnancy in Asian women with elevated blood pressure in early pregnancy and the risk of preeclampsia: A longitudinal cohort study. BMJ Open 2019, 9, e032237. [Google Scholar] [CrossRef]
| Normotensives (n = 775) | Cases (n = 137) * | ||
|---|---|---|---|
| Maternal Characteristics | Mean (SD); Median or n (%) | Mean (SD); Median or n (%) | p ** |
| Controls (n = 775) | GH cases (n = 113) | ||
| Maternal age (years) | 33.5 (4.8); 35.0 | 35.0 (4.3); 36.0 | 0.005 |
| Primiparous women | 318 (41.0%) | 53 (46.9%) | 0.237 |
| Prior PIH | 4 (0.5%) | 12 (10.6%) | <0.00001 |
| Infertility treatment | 29 (3.7%) | 8 (7.1%) | 0.097 |
| Pre-pregnancy BMI (kg/m2) | 23.3 (4.1); 22.5 | 26.7 (5.3); 25.5 | < 0.001 |
| GWG/week (kg/week) *** | 0.35 (0.14); 0.34 | 0.38 (0.21); 0.38 | 0.057 |
| Smokers | 133 (17.2%) | 31 (27.4%) | 0.009 |
| Smokers in the first trimester | 37 (4.8%) | 17 (15.0%) | 0.00002 |
| Fetal sex—son | 405 (52.3%) | 55 (48.7%) | 0.476 |
| Gestational age at delivery (week) | 38.9 (1.6); 39.0 | 38.3 (2.2); 39.0 | 0.016 |
| Newborn birthweight (g) | 3416.5 (511.7); 3449.0 | 3174.1 (734.3); 3200.0 | 0.001 |
| Gestational diabetes mellitus | 121 (15.6%) | 22 (19.5%) | 0.298 |
| Controls (n = 775) | PE cases (n = 24) | ||
| Maternal age (years) | 33.5 (4.8); 35.0 | 34.1 (5.0); 35.0 | 0.434 |
| Primiparous women | 318 (41.0%) | 12 (50.0%) | 0.380 |
| Prior PIH | 4 (0.5%) | 3 (12.5%) | <0.00001 |
| Infertility treatment | 29 (3.7%) | 3 (12.5%) | 0.031 |
| Pre-pregnancy BMI (kg/m2) | 23.3 (4.1); 22.5 | 26.5 (6.2); 25.0 | 0.008 |
| GWG/week (kg/week) *** | 0.35 (0.14); 0.34 | 0.42 (0.21); 0.38 | 0.155 |
| Smokers | 133 (17.2%) | 4 (16.7%) | 0.950 |
| Smokers in the first trimester | 37 (4.8%) | 3 (12.5%) | 0.084 |
| Fetal sex—son | 405 (52.3%) | 13 (54.2%) | 0.854 |
| Gestational age at delivery (week) | 38.9 (1.6); 39.0 | 35.1 (3.7); 36.0 | < 0.001 |
| Newborn birthweight (g) | 3416.5 (511.7); 3449.0 | 2294.2 (927.5); 2445.0 | < 0.001 |
| Gestational diabetes mellitus | 121 (15.6%) | 3 (12.5%) | 0.678 |
| PE beginning: ≤31st week | - | 7 (29.2%) | - |
| PE beginning 32–33rd week | - | 4 (16.7%) | - |
| PE beginning ≥34th week | - | 13 (54.2%) | - |
| Odds Ratios of Two Forms of GH and PE for Smoking Categories | |||
|---|---|---|---|
| Cases/Controls | OR (95% CI:); p | AOR * (95% CI:); p | |
| Gestational hypertension (GH) risk | |||
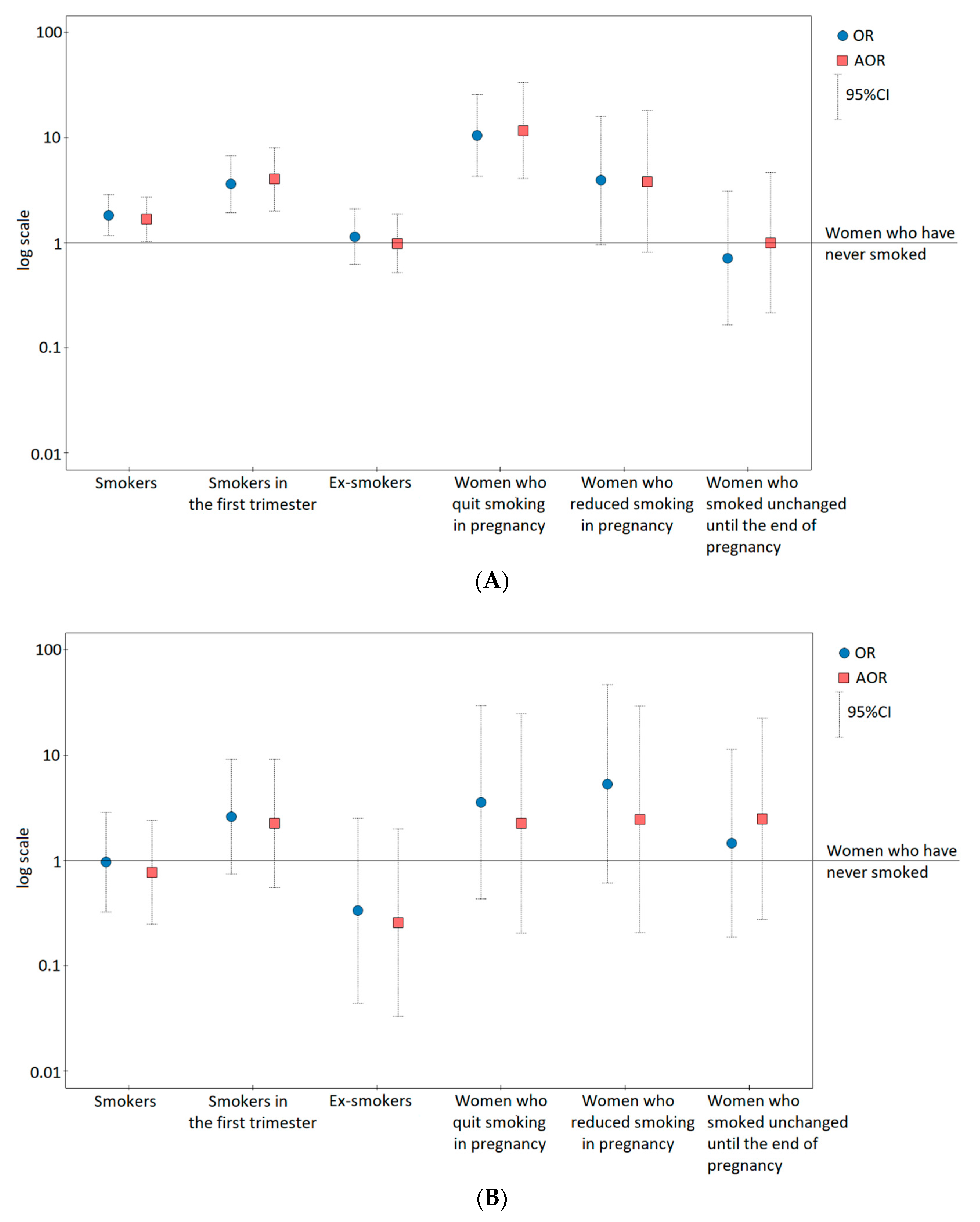
| Smokers ** | 31/133 | 1.83 (1.16–2.87); 0.009 | 1.68 (1.02–2.78); 0.043 |
| Smokers in the first trimester | 17/37 | 3.60 (1.94–6.68); <0.001 | 4.75 (2.34–9.65); <0.001 |
| Ex-smokers | 14/96 | 1.14 (0.62–2.09); 0.668 | 0.83 (0.41–1.66); 0.596 |
| Women who have never smoked | 82/642 | 1 | 1 |
| Women who quit smoking in pregnancy *** | 12/9 | 10.44 (4.27–25.53); <0.0001 | 11.63 (4.07–33.24); <0.0001 |
| Women who reduced smoking in pregnancy | 3/6 | 3.91 (0.96–15.95); 0.057 | 3.81 (0.81–18.05); 0.092 |
| Women who smoked unchanged until the end of pregnancy | 2/22 | 0.71 (0.16–3.08); 0.649 | 1.00 (0.21–4.64); 0.999 |
| Women who have never smoked | 82/642 | 1 | 1 |
| Pre-eclampsia (PE) risk | |||
| Smokers ** | 4/133 | 0.97 (0.33–2.87); 0.950 | 0.91 (0.29–2.88); 0.872 |
| Smokers in the first trimester | 3/37 | 2.60 (0.74–9.16); 0.136 | 2.51 (0.60–10.54); 0.208 |
| Ex-smokers | 1/96 | 0.33 (0.04–2.52); 0.288 | 0.31 (0.04–2.40); 0.260 |
| Women who have never smoked | 20/642 | 1 | 1 |
| Women who quit smoking in pregnancy *** | 1/9 | 3.57 (0.43–29.52); 0.238 | 2.25 (0.2–24.66); 0.508 |
| Women who reduced smoking in pregnancy | 1/6 | 5.35 (0.62–46.54); 0.129 | 2.47 (0.21–29.33); 0.474 |
| Women who smoked unchanged until the end of pregnancy | 1/22 | 1.46 (0.19–11.37); 0.718 | 2.49 (0.27–22.51); 0.418 |
| Women who have never smoked | 20/642 | 1 | 1 |
| Odds Ratios of PIH Forms for Continuous Variables | ||
|---|---|---|
| OR (95% CI:); p | AOR * (95% CI:); p | |
| Gestational hypertension (GH) risk ** | ||
| For smokers (ever before pregnancy): | ||
| Number of years of smoking (for 1 year) | 1.06 (1.02–1.1); 0.006 | 1.05 (1.01–1.10); 0.031 |
| Number of cigarettes per day (for 1 cigarette) | 1.01(1.0–1.03); 0.148 | 1.01 (0.99–1.03); 0.299 |
| Value of pack-years (for 1) | 1.05 (1.0–1.10); 0.034 | 1.04 (0.99–1.09); 0.113 |
| Pre-eclampsia (PE) risk *** | ||
| For smokers (ever before pregnancy): | ||
| Number of years of smoking (for 1 year) | 1.05 (0.97–1.14); 0.256 | 1.04 (0.95–1.14); 0.352 |
| Number of cigarettes per day (for 1 cigarette) | 1.00 (0.96–1.05); 0.909 | 1.00 (0.95–1.04); 0.866 |
| Value of pack-years (for 1) | 1.02 (0.94–1.10); 0.616 | 1.01 (0.93–1.09); 0.891 |
| Odds Ratios of PIH for Smoking in the First Trimester, in the Pre-Pregnancy BMI Categories | ||
|---|---|---|
| Cases/Controls | PIH Risk OR * (95% CI:); p | |
| Whole cohort | ||
| Smoking in the first trimester | 20/37 | 3.40 (1.90–6.09); <0.001 |
| Women who have never smoked | 102/642 | 1 |
| Underweight | ||
| Smoking in the first trimester | 2/3 | 22.00 (1.52–319.48); 0.024 |
| Women who have never smoked | 1/33 | 1 |
| Normal BMI | ||
| Smoking in the first trimester | 7/23 | 2.96 (1.21–7.26); 0.018 |
| Women who have never smoked | 47/457 | 1 |
| Overweight | ||
| Smoking in the first trimester | 6/7 | 3.89 (1.2–12.63); 0.024 |
| Women who have never smoked | 24/109 | 1 |
| Obesity | ||
| Smoking in the first trimester | 5/4 | 1.79 (0.44–7.23); 0.413 |
| Women who have never smoked | 30/43 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, M.; Więckowska, B. The Influence of Various Smoking Categories on The Risk of Gestational Hypertension and Pre-Eclampsia. J. Clin. Med. 2020, 9, 1743. https://doi.org/10.3390/jcm9061743
Lewandowska M, Więckowska B. The Influence of Various Smoking Categories on The Risk of Gestational Hypertension and Pre-Eclampsia. Journal of Clinical Medicine. 2020; 9(6):1743. https://doi.org/10.3390/jcm9061743
Chicago/Turabian StyleLewandowska, Małgorzata, and Barbara Więckowska. 2020. "The Influence of Various Smoking Categories on The Risk of Gestational Hypertension and Pre-Eclampsia" Journal of Clinical Medicine 9, no. 6: 1743. https://doi.org/10.3390/jcm9061743
APA StyleLewandowska, M., & Więckowska, B. (2020). The Influence of Various Smoking Categories on The Risk of Gestational Hypertension and Pre-Eclampsia. Journal of Clinical Medicine, 9(6), 1743. https://doi.org/10.3390/jcm9061743





