Follicle-Stimulating Hormone Treatment and Male Idiopathic Infertility: Effects on Sperm Parameters and Oxidative Stress Indices according to FSHR c. 2039 A/G and c. -29 G/A Genotypes
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Semen Analysis and Bio-Functional Sperm Parameter Evaluation
2.3. Evaluation of Sperm Apoptosis/Vitality
2.4. Evaluation of the Mitochondrial Membrane Potential
2.5. Assessment of DNA Fragmentation
2.6. Degree of Chromatin Compactness Assessment
2.7. Sperm Membrane Lipoperoxidation Evaluation
2.8. Measurement of Mitochondrial Superoxide Levels
2.9. DNA Extraction
2.10. Statistical Analysis
3. Results
4. Discussion
4.1. Conventional Sperm Parameters
4.2. Bio-Functional Sperm Parameters
4.3. Oxidative Stress Indexes
4.4. FSHR Polymorphisms
5. Conclusions
6. Strengths and Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Acosta, A.A.; Oehninger, S.; Ertunc, H.; Philput, C. Possible role of pure human follicle-stimulating hormone in the treatment of severe male-factor infertility by assisted reproduction: Preliminary report. Fertil. Steril. 1991, 55, 1150–1156. [Google Scholar] [CrossRef]
- Acosta, A.A.; Khalifa, E.; Oehninger, S. Pure human follicle stimulating hormone has a role in the treatment of severe male infertility by assisted reproduction: Norfolk’s total experience. Hum. Reprod. 1992, 7, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Granata, A.R.; Simoni, M. FSH treatment of male idiopathic infertility improves pregnancy rate: A meta-analysis. Endocr. Connect. 2015, 4, R46–R58. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Calogero, A.E.; Balercia, G.; Garolla, A.; Krausz, C.; La Vignera, S.; Lombardo, F.; Jannini, E.A.; Maggi, M.; Lenzi, A.; et al. The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2018, 41. [Google Scholar] [CrossRef]
- Baccetti, B.; Strehler, E.; Capitani, S.; Collodel, G.; De Santo, M.; Moretti, E.; Piomboni, P.; Wiedeman, R.; Sterzik, K. The effect of follicle stimulating hormone therapy on human sperm structure (Notulaeseminologicae 11). Hum. Reprod. 1997, 12, 1955–1968. [Google Scholar] [CrossRef][Green Version]
- Colacurci, N.; Monti, M.G.; Fornaro, F.; Izzo, G.; Izzo, P.; Trotta, C.; Mele, D.; De Franciscis, P. Recombinant human FSH reduces sperm DNA fragmentation in men with idiopathic oligoasthenoteratozoospermia. J. Androl. 2012, 33, 588–593. [Google Scholar] [CrossRef]
- Garolla, A.; Ghezzi, M.; Cosci, I.; Sartini, B.; Bottacin, A.; Engl, B.; Di Nisio, A.; Foresta, C. FSH treatment in infertile males candidate to assisted reproduction improved sperm DNA fragmentation and pregnancy rate. Endocrine 2017, 56, 416–425. [Google Scholar] [CrossRef]
- Tamburino, L.; La Vignera, S.; Tomaselli, V.; Condorelli, R.A.; Cannarella, R.; Mongioì, L.M.; Calogero, A.E. The -29G/A FSH receptor gene polymorphism is associated with higher FSH and LH levels in normozoospermic men. J. Assist. Reprod. Genet. 2017, 34, 1289–1294. [Google Scholar] [CrossRef]
- Tamburino, L.; La Vignera, S.; Tomaselli, V.; Condorelli, R.A.; Mongioì, L.M.; Calogero, A.E. Impact of the FSHB gene -211G/T polymorphism on male gonadal function. J. Assist. Reprod. Genet. 2017, 34, 671–676. [Google Scholar] [CrossRef][Green Version]
- Palomba, S.; Falbo, A.; Espinola, S.; Rocca, M.; Capasso, S.; Cappiello, F.; Zullo, F. Effects of highly purified follicle-stimulating hormone on sperm DNA damage in men with male idiopathic subfertility: A pilot study. J. Endocrinol. Investig. 2011, 34, 747–752. [Google Scholar]
- WHO. Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Barresi, V.; Signorelli, S.S.; Musso, N.; Anzaldi, M.; Fiore, V.; Alberghina, M.; Condorelli, D.F. ICAM-1 and SRD5A1 gene polymorphisms in symptomatic peripheral artery disease. Vasc. Med. 2014, 19, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Bettella, A.; Garolla, A.; Ambrosini, G.; Ferlin, A. Treatment of male idiopathic infertility with recombinant human follicle-stimulating hormone: A prospective, controlled, randomized clinical study. Fertil. Steril. 2005, 84, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.M.; Abou-Setta, A.M.; Al-Inany, H.G. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst. Rev. 2013, 23, CD005071. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Selice, R.; Engl, B.; Bertoldo, A.; Menegazzo, M.; Finos, L.; Lenzi, A.; Foresta, C. Spermatid count as a predictor of response to FSH therapy. Reprod. Biomed. Online 2014, 29, 102–112. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; Mongioi’, L.; Burgio, G.; Cannarella, R.; Giacone, F.; Iacoviello, L.; Morgia, G.; Favilla, V.; et al. Reduced Seminal Concentration of CD45pos Cells after Follicle-Stimulating Hormone Treatment in Selected Patients with Idiopathic Oligoasthenoteratozoospermia. Int. J. Endocrinol. 2014, 2014, 372060. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Bettella, A.; Merico, M.; Garolla, A.; Ferlin, A.; Rossato, M. Use of recombinant human follicle-stimulating hormone in the treatment of male factor infertility. Fertil. Steril. 2002, 77, 238–244. [Google Scholar] [CrossRef]
- Fernández-Arjona, M.; Díaz, J.; Cortes, I.; González, J.; Rodriguez, J.M.; Alvarez, E. Relationship between gonadotrophin secretion, inhibin B and spermatogenesis in oligozoospermic men treated with highly purified urinary follicle-stimulating hormone (uFSH-HP): A preliminary report. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 107, 7–51. [Google Scholar]
- Casamonti, E.; Vinci, S.; Serra, E.; Fino, M.G.; Brilli, S.; Lotti, F.; Maggi, M.; Coccia, M.E.; Forti, G.; Krausz, C. Short-term FSH treatment and sperm maturation: A prospective study in idiopathic infertile men. Andrology 2017, 5, 414–422. [Google Scholar] [CrossRef]
- Cannarella, R.; La Vignera, S.; Condorelli, R.A.; Mongioì, L.M.; Calogero, A.E. FSH dosage effect on conventional sperm parameters: A meta-analysis of randomized controlled studies. Asian J. Androl. 2020, 22, 309–316. [Google Scholar] [CrossRef]
- Bartoov, B.; Eltes, F.; Lunenfeld, E.; Har-Even, D.; Lederman, H.; Lunenfeld, B. Sperm quality of subfertile males before and after treatment with human follicle-stimulating hormone. Fertil. Steril. 1994, 61, 727–734. [Google Scholar] [CrossRef]
- Matorras, R.; Pérez, C.; Corcóstegui, B.; Pijoan, J.I.; Ramón, O.; Delgado, P.; Rodríguez-Escudero, F.J. Treatment of the male with follicle-stimulating hormone in intrauterine insemination with husband’s spermatozoa: A randomized study. Hum. Reprod. 1997, 12, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, G.; Roccheri, M.C.; Brucculeri, A.M.; Longobardi, S.; Cittadini, E.; Bosco, L. Lower sperm DNA fragmentation after r-FSH administration in functional hypogonadotropic hypogonadism. J. Assist. Reprod. Genet. 2013, 30, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Santi, D.; Negri, L.; Hoffmann, I.; Muratori, M.; Baldi, E.; Cambi, M.; Marcou, M.; Greither, T.; Baraldi, E.; et al. Treatment with human, recombinant FSH improves sperm DNA fragmentation in idiopathic infertile men depending on the FSH receptor polymorphism p.N680S: A pharmacogenetic study. Hum. Reprod. 2016, 31, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A.; Cho, C.L.; Esteves, S.C. Sperm DNA fragmentation testing: A cross sectional survey on current practices of fertility specialists. Transl. Androl. Urol. 2017, 6 (Suppl. 4), S710–S719. [Google Scholar] [CrossRef]
- Gosalvez, J.; Tvrda, E.; Agarwal, A. Free radical and superoxide reactivity detection in semen quality assessment: Past, present, and future. J. Assist. Reprod. Genet. 2017, 34, 697–707. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Vicari, E.; D’Agata, R.; Salemi, M.; Calogero, A.E. High levels of lipid peroxidation in semen of diabetic patients. Andrologia 2012, 44 (Suppl. 1), 565–570. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum. Reprod. 1995, 10 (Suppl. 1), 15–21. [Google Scholar] [CrossRef]
- Griveau, J.F.; Renard, P.; Le Lannou, D. Superoxide anion production by human spermatozoa as a part of the ionophore-induced acrosome reaction process. Int. J. Androl. 1995, 18, 67–74. [Google Scholar] [CrossRef]
- Grigorova, M.; Punab, M.; Poolamets, O.; Sõber, S.; Vihljajev, V.; Žilaitienė, B.; Erenpreiss, J.; Matulevičius, V.; Tsarev, I.; Laan, M. Study in 1790 Baltic men: FSHR Asn680Ser polymorphism affects total testes volume. Andrology 2013, 1, 293–300. [Google Scholar] [CrossRef]
- Selice, R.; Garolla, A.; Pengo, M.; Caretta, N.; Ferlin, A.; Foresta, C. The response to FSH treatment in oligozoospermic men depends on FSH receptor gene polymorphisms. Int. J. Androl. 2011, 34, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Moriondo, V.; Marino, M.; Adversi, F.; Capodanno, F.; Grisolia, C.; La Marca, A.; La Sala, G.B.; Simoni, M. FSHR polymorphism p.N680S mediates different responses to FSH in vitro. Mol. Cell. Endocrinol. 2014, 393, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Gromoll, J.; Pekel, E.; Nieschlag, E. The structure and organization of the human follicle-stimulating hormone receptor (FSHR) gene. Genomics 1996, 35, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Kuroi, N.; Sano, M.; Tabara, Y.; Katsuya, T.; Ogihara, T.; Makita, Y.; Hata, A.; Yamada, M.; Takahashi, N.; et al. Mutation of the follicle-stimulating hormone receptor gene 5′-untranslated region associated with female hypertension. Hypertension 2006, 48, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Colpi, G.M.; Francavilla, S.; Haidl, G.; Link, K.; Behre, H.M.; Goulis, D.G.; Krausz, C.; Giwercman, A. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology 2018, 6, 513–524. [Google Scholar] [CrossRef]
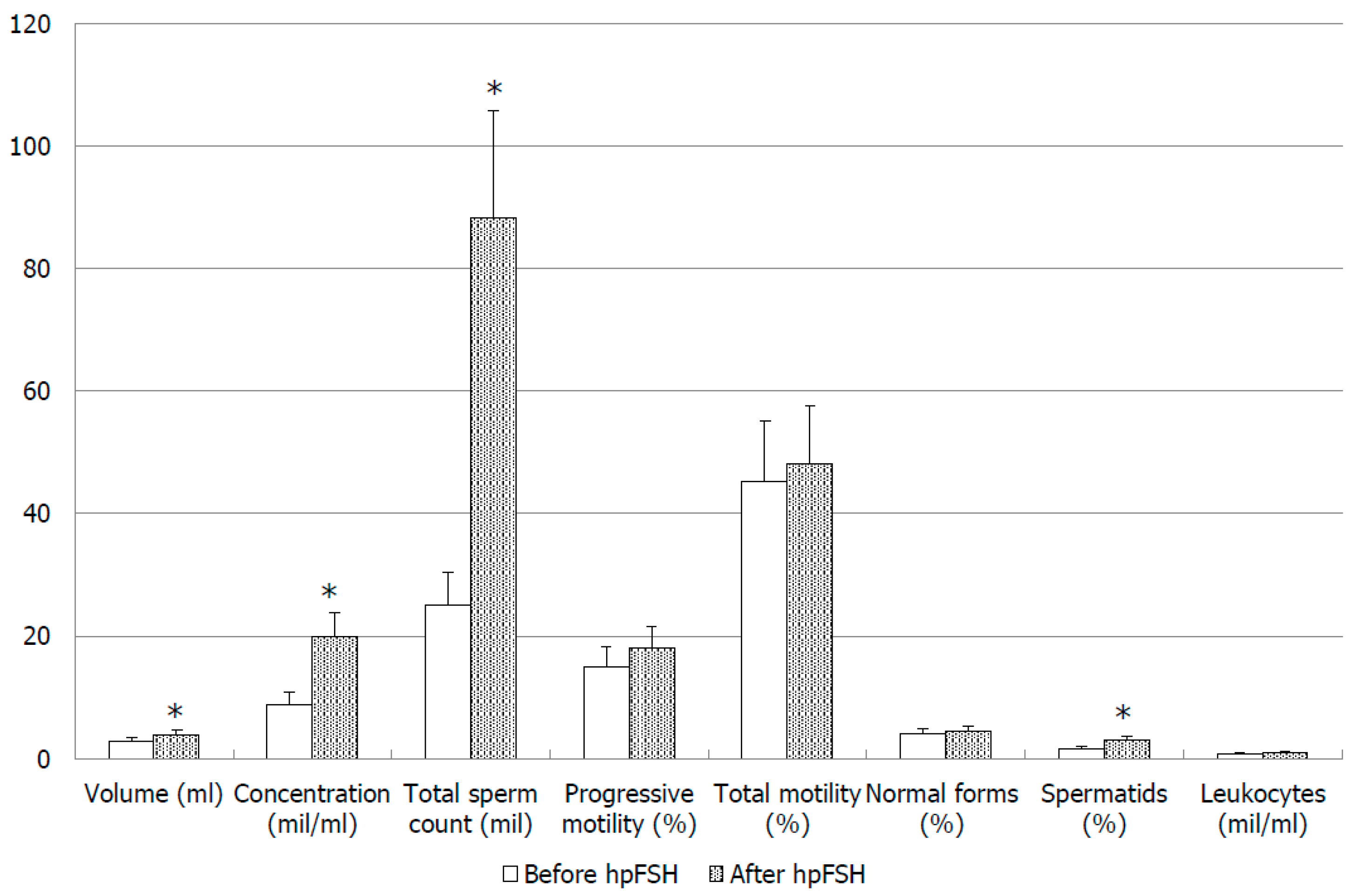

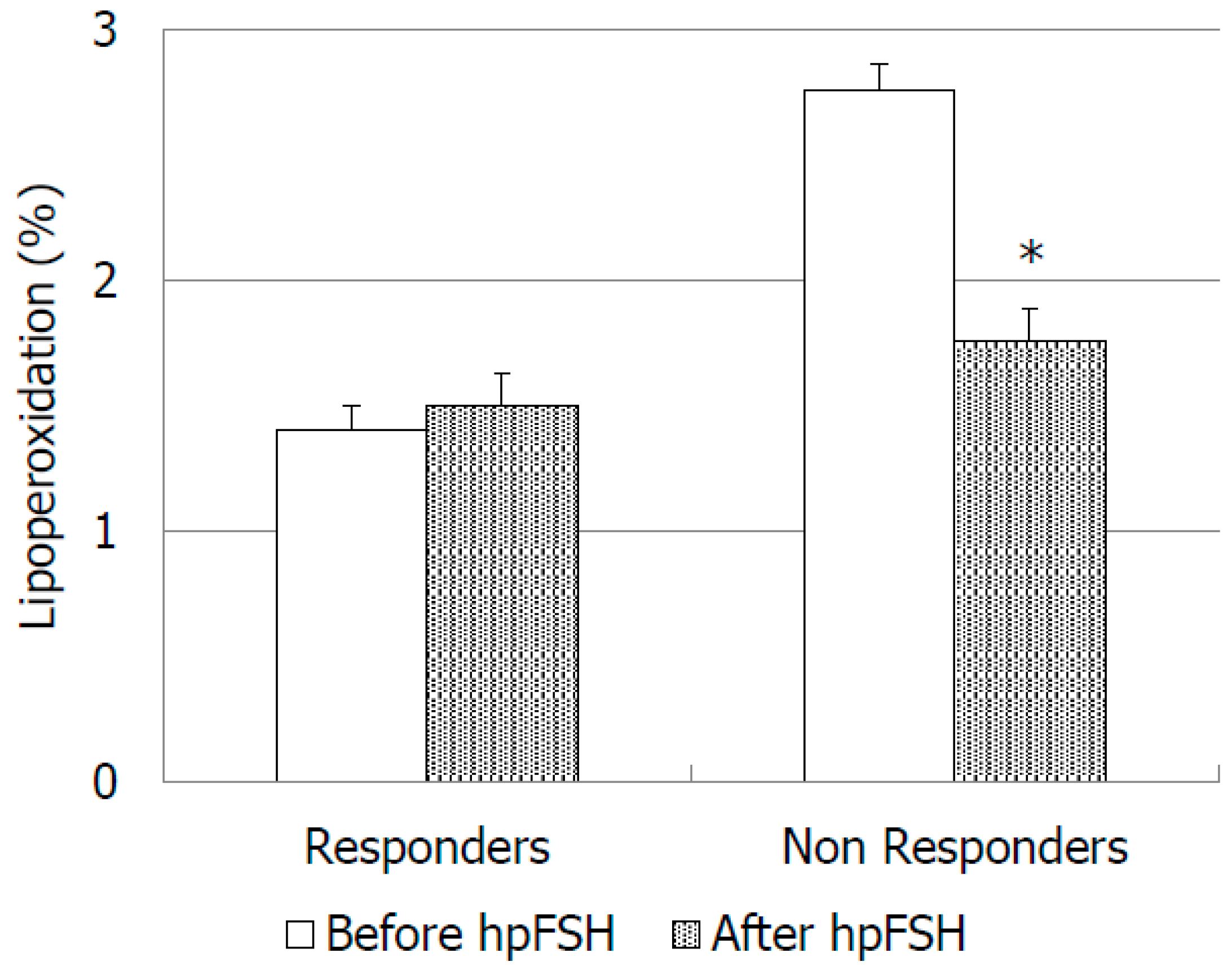

| Parameter | Before hpFSH | After hpFSH | p-Value |
|---|---|---|---|
| Volume (mL) | 2.77 ± 0.21 | 2.83 ± 0.27 | >0.05 |
| Concentration (mil/mL) | 12.20 ± 2.13 | 14.60 ± 0.46 | >0.05 |
| Total sperm count (min/ejaculate) | 30.87 ± 5.97 | 53.26 ± 13.72 | >0.05 |
| Progressive motility (%) | 14.47 ± 2.03 | 14.51 ± 2.28 | >0.05 |
| Total motility (%) | 47.52 ± 2.60 | 45.69 ± 3.17 | >0.05 |
| Normal forms (%) | 4.14 ± 0.43 | 4.03 ± 0.41 | >0.05 |
| Spermatids (%) | 1.67 ± 0.20 | 3.66 ± 1.20 | >0.05 |
| Leukocytes (mil/mL) | 0.49 ± 0.24 | 0.63 ± 0.19 | >0.05 |
| Parameter | Before hpFSH | After hpFSH | p-Value |
|---|---|---|---|
| Volume (mL) | 2.56 ± 0.28 | 2.64 ± 0.36 | >0.05 |
| Concentration (mil/mL) | 14.05 ± 3.11 | 10.17 ± 2.26 | >0.05 |
| Total sperm count (mil/ejaculate) | 31.66 ± 6.87 | 24.15 ± 4.61 | >0.05 |
| Progressive motility (%) | 10.94 ± 1.63 | 11.61 ± 1.54 | >0.05 |
| Total motility (%) | 43.8 ± 3.379 | 43.72 ± 4.04 | >0.05 |
| Normal forms (%) | 3.72 ± 0.52 | 3.72 ± 0.41 | >0.05 |
| Spermatids (%) | 1.79 ± 0.24 | 4.22 ± 2.13 | >0.05 |
| Leukocytes (mil/mL) | 0.28 ± 0.06 | 0.29 ± 0.97 | >0.05 |
| Parameter | Before hpFSH | After hpFSH | p-Value |
|---|---|---|---|
| Alive spermatozoa (%) | 53.57 ± 16.49 | 53.25 ± 10.93 | >0.05 |
| Spermatozoa in early apoptosis (%) | 2.87 ± 1.33 | 2.8 ± 2.05 | >0.05 |
| Spermatozoa in late apoptosis (%) | 17.55 ± 20.79 | 13.67 ± 8.03 | >0.05 |
| Spermatozoa with low MMP (%) | 51.02 ± 16.874 | 46.14 ± 25.37 | >0.05 |
| Sperm chromatin compactness (%) | 27.41 ± 10.92 | 25.73 ± 5.83 | >0.05 |
| Sperm DNA fragmentation (%) | 10.56 ± 4.85 | 4.87 ± 3.01 | >0.05 |
| FSHR 2039A/G | FSHR -29 G/A | ||||||
|---|---|---|---|---|---|---|---|
| AA | GG | GG | AA | ||||
| Parameter | Before hpFSH | After hpFSH | Before hpFSH | After hpFSH | Before hpFSH | After hpFSH | Before hpFSH |
| Volume (mL) | 2.8 ± 0.5 | 3.0 ± 0.5 | 2.9 ± 0.4 | 3.4 ± 0.4 | 2.6 ± 1.1 | 2.8 ± 1.0 | 1.9 ± 0.5 |
| Concentration (mil/mL) | 12.3 ± 3.4 | 11.0 ± 3.7 | 12.2 ± 6.2 | 20.3 ± 11.5 | 16.7 ± 15.3 | 17.0 ± 17.7 | 4.8 ± 4.6 |
| Total sperm count (min/ejaculate) | 33.6 ± 9.1 | 36.0 ± 14.1 | 31.0 ± 15.5 | 85.6 ± 60.2 | 36.2 ± 32.1 | 53.2 ± 84.7 | 7.7 ± 6.2 |
| Progressive motility (%) | 18.0 ± 3.7 | 12.0 ± 2.9 | 17.7 ± 3.9 | 16.8 ± 5.3 | 15.7 ± 8.6 | 15.2 ± 9.8 | 20.0 ± 14.1 |
| Total motility (%) | 49.0 ± 6.2 | 46.0 ± 7.6 | 50.8 ± 4.1 | 55.2 ± 4.2 | 50.3 ± 13.3 | 48.2 ± 15.7 | 52.5 ± 10.6 |
| Normal forms (%) | 4.3 ± 0.6 | 4.5 ± 1.4 | 6.3 ± 1.6 | 4.4 ± 1.2 | 3.9 ± 2.2 | 4.5 ± 2.5 | 7.0 ± 7.1 |
| Spermatids (%) | 1.6 ± 0.5 | 3.0 ± 1.6 | 2.3 ± 0.4 | 3.6 ± 1.8 | 2.0 ± 1.6 | 2.9 ± 3.0 | 1.0 ± 0.0 |
| Leukocytes (mil/mL) | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.3 | 0.6 ± 0.5 | 0.2 ± 0.1 |
| Alive spermatozoa (%) | 57.5 ± 6.9 | 47.4 ± 4.6 | 51.6 ± 9.2 | 44.6 ± 5.2 | 51.2 ± 18.9 | 47.9 ± 12.8 | --- |
| Spermatozoa in early apoptosis (%) | 4.1 ± 2.6 | 1.7 ± 0.6 | 2.3 ± 0.6 | 2.3 ± 1.2 | 2.0 ± 1.2 | 2.7 ± 2.5 | --- |
| Spermatozoa in late apoptosis (%) | 11.0 ± 5.1 | 12.0 ± 1.6 | 12.3 ± 6.1 | 7.5 ± 2.9 | 18.3 ± 21.0 | 12.9 ± 10.4 | --- |
| Spermatozoa with low MMP (%) | 26.3 ± 10.9 | 10.3 ± 0.9 | 43.9 ± 13.6 | 50.6 ± 14.3 | 30.7 ± 22.0 | 31.3 ± 24.6 | --- |
| Sperm chromatin compactness (%) | 18.8 ± 9.9 | 23.9 ± 0.7 | 18.6 ± 2.0 | 15.2 ± 1.3 | 20.8 ± 13.1 | 19.2 ± 5.0 | --- |
| Sperm DNA fragmentation (%) | 1.7 ± 0.5 | 4.7 ± 2.9 | 3.8 ± 0.6 | 4.2 ± 1.3 | 3.3 ± 2.6 | 3.3 ± 3.3 | --- |
| FSHR 2039AG Genotype | A/G Allele Frequency | FSHR -29 G/A Genotype | G/A Allele Frequency | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | A | G | AA | AG | GG | G | A | |
| Responders | 2/6 33.3% | 2/6 33.3% | 2/6 33.3% | 6/12 50% | 6/12 50% | --- | 0/4 0% | 4/4 100.0 *% | 8/8 100% | 0/8 0.0 *% |
| Non-responders | 3/11 27.3% | 2/11 18.2% | 6/11 54.5% | 12/22 54.5% | 10/22 45.5% | --- | 4/13 30.8% | 9/13 69.2 *% | 22/26 84.6% | 4/26 15.4 *% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongioì, L.M.; Condorelli, R.A.; Alamo, A.; Cannarella, R.; Musso, N.; La Vignera, S.; Calogero, A.E. Follicle-Stimulating Hormone Treatment and Male Idiopathic Infertility: Effects on Sperm Parameters and Oxidative Stress Indices according to FSHR c. 2039 A/G and c. -29 G/A Genotypes. J. Clin. Med. 2020, 9, 1690. https://doi.org/10.3390/jcm9061690
Mongioì LM, Condorelli RA, Alamo A, Cannarella R, Musso N, La Vignera S, Calogero AE. Follicle-Stimulating Hormone Treatment and Male Idiopathic Infertility: Effects on Sperm Parameters and Oxidative Stress Indices according to FSHR c. 2039 A/G and c. -29 G/A Genotypes. Journal of Clinical Medicine. 2020; 9(6):1690. https://doi.org/10.3390/jcm9061690
Chicago/Turabian StyleMongioì, Laura M., Rosita A. Condorelli, Angela Alamo, Rossella Cannarella, Nicolò Musso, Sandro La Vignera, and Aldo E. Calogero. 2020. "Follicle-Stimulating Hormone Treatment and Male Idiopathic Infertility: Effects on Sperm Parameters and Oxidative Stress Indices according to FSHR c. 2039 A/G and c. -29 G/A Genotypes" Journal of Clinical Medicine 9, no. 6: 1690. https://doi.org/10.3390/jcm9061690
APA StyleMongioì, L. M., Condorelli, R. A., Alamo, A., Cannarella, R., Musso, N., La Vignera, S., & Calogero, A. E. (2020). Follicle-Stimulating Hormone Treatment and Male Idiopathic Infertility: Effects on Sperm Parameters and Oxidative Stress Indices according to FSHR c. 2039 A/G and c. -29 G/A Genotypes. Journal of Clinical Medicine, 9(6), 1690. https://doi.org/10.3390/jcm9061690










