Short-Term Effects of PENS versus Dry Needling in Subjects with Unilateral Mechanical Neck Pain and Active Myofascial Trigger Points in Levator Scapulae Muscle: A Randomized Controlled Trial
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Subjects
2.3. Sample Size
2.4. Measurements
2.4.1. Pain and Disability
2.4.2. Myofascial Trigger Point Diagnosis
2.4.3. Range of Motion of the Cervical Spine
2.4.4. Pressure Pain Threshold
2.4.5. Side-Bending Strength
2.5. Interventions
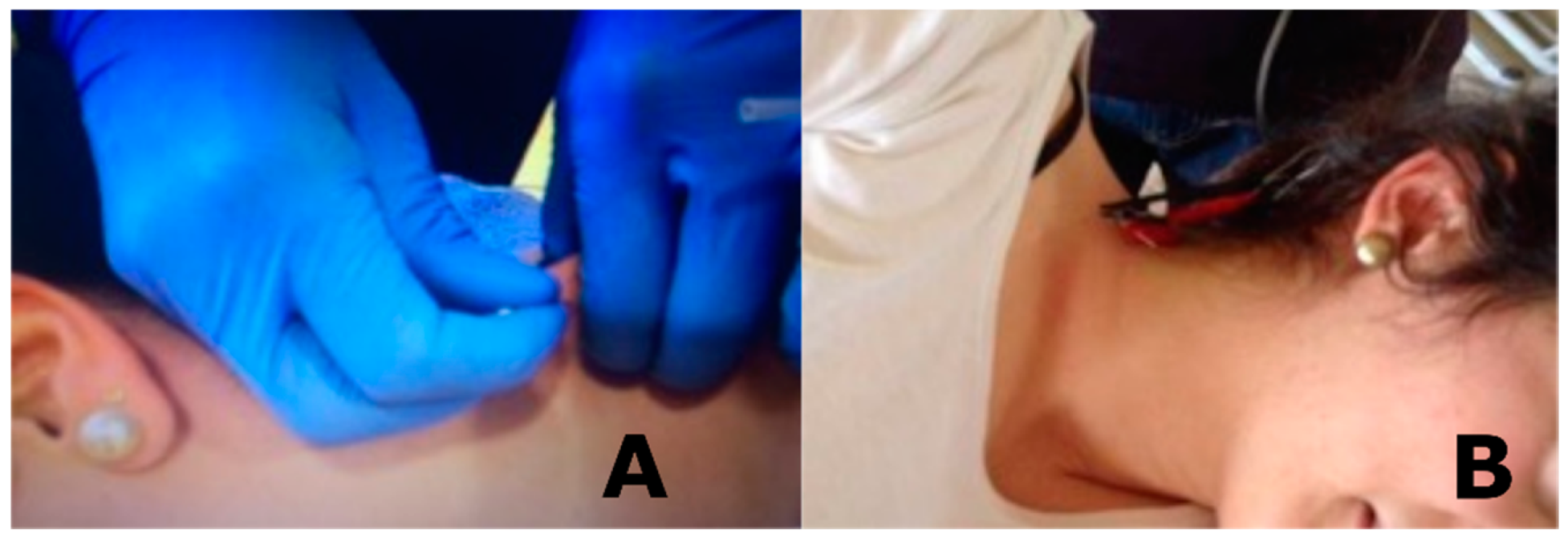
2.5.1. Dry Needling
2.5.2. Percutaneous Electrical Nerve Stimulation
2.6. Statistical Analysis
3. Results
3.1. Pain and Disability
3.2. Pressure Pain Threshold
3.3. Side-Bending Strength
3.4. Range of Motion of the Cervical Spine
4. Discussion
4.1. Pain and Disability
4.2. Pressure Pain Threshold
4.3. Strength
4.4. Cervical Range of Movement
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gattie, E.; Cleland, J.A.; Snodgrass, S. The effectiveness of trigger point dry needling for musculoskeletal conditions by physical therapists: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Griswold, D.; Wilhelm, M.; Donaldson, M.; Learman, K.; Cleland, J. The effectiveness of superficial versus deep dry needling or acupuncture for reducing pain and disability in individuals with spine-related painful conditions: A systematic review with meta-analysis. J. Man. Manip. Ther. 2019, 27, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Dunning, J.; Butts, R.; Young, I.; Mourad, F.; Galante, V.; Bliton, P.; Tanner, M.; Fernández-De-Las-Peñas, C. Periosteal Electrical Dry Needling as an Adjunct to Exercise and Manual Therapy for Knee Osteoarthritis. Clin. J. Pain 2018, 34, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Krey, D.; Borchers, J.; McCamey, K. Tendon needling for treatment of tendinopathy: A systematic review. Phys. Sportsmed. 2015, 43, 80–86. [Google Scholar] [CrossRef]
- Shah, J.P.; Thaker, N.; Heimur, J.; Aredo, J.V.; Sikdar, S.; Gerber, L. Myofascial trigger points then and now: A historical and scientific perspective. PM R 2015, 7, 746–761. [Google Scholar] [CrossRef]
- Cummings, M.; Baldry, P. Regional myofascial pain: Diagnosis and management. Best Pract. Res. Clin. Rheumatol. 2007, 21, 367–387. [Google Scholar] [CrossRef]
- Niddam, D.M.; Chan, R.C.; Lee, S.H.; Yeh, T.C.; Hsieh, J.C. Central modulation of pain evoked from myofascial trigger point. Clin. J. Pain 2007, 23, 440–448. [Google Scholar] [CrossRef]
- Tesch, R.D.S.; Macedo, L.C.D.S.P.; Fernandes, F.S.; De Goffredo Filho, G.S.; Goes, C.P.D.Q.F. Effectiveness of dry needling on the local pressure pain threshold in patients with masticatory myofascial pain. Systematic review and preliminary clinical trial. Cranio J. Craniomandib. Pract. 2019. [Google Scholar] [CrossRef]
- Núñez-Cortés, R.; Cruz-Montecinos, C.; Vásquez-Rosel, Á.; Paredes-Molina, O.; Cuesta-Vargas, A. Dry needling combined with physical therapy in patients with chronic postsurgical pain following total knee arthroplasty: A case series. J. Orthop. Sports Phys. Ther. 2017, 47, 209–216. [Google Scholar] [CrossRef]
- Zarei, H.; Bervis, S.; Piroozi, S.; Motealleh, A. Added Value of Gluteus Medius and Quadratus Lumborum Dry Needling in Improving Knee Pain and Function in Female Athletes With Patellofemoral Pain Syndrome: A Randomized Clinical Trial. Arch. Phys. Med. Rehabil. 2020, 101, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Laita, L.; Jiménez-del-Barrio, S.; Marín-Zurdo, J.; Moreno-Calvo, A.; Marín-Boné, J.; Albarova-Corral, M.I.; Estébanez-de-Miguel, E. Effects of dry needling in HIP muscles in patients with HIP osteoarthritis: A randomized controlled trial. Musculoskelet. Sci. Pract. 2019, 43, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, Q.M.; Liu, Q.G.; Thitham, N.; Li, L.H.; Ma, Y.T.; Zhao, J.M. Evidence for Dry Needling in the Management of Myofascial Trigger Points Associated With Low Back Pain: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 144–152.e2. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaiee, A.; Takamjani, I.E.; Sarrafzadeh, J.; Salehi, R.; Ahmadi, M. Ultrasound-guided dry needling decreases pain in patients with piriformis syndrome. Muscle Nerve 2019, 60, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Cagnie, B.; Dewitte, V.; Barbe, T.; Timmermans, F.; Delrue, N.; Meeus, M. Physiologic effects of dry needling. Curr. Pain Headache Rep. 2013, 17, 348. [Google Scholar] [CrossRef]
- Hsieh, Y.-L.; Yang, S.-A.; Yang, C.-C.; Chou, L.-W. Dry Needling at Myofascial Trigger Spots of Rabbit Skeletal Muscles Modulates the Biochemicals Associated With Pain, Inflammation, and Hypoxia. Evid. Based Complement. Altern. Med. 2012, 2012, 342165. [Google Scholar] [CrossRef]
- Chae, Y.; Chang, D.S.; Lee, S.H.; Jung, W.M.; Lee, I.S.; Jackson, S.; Kong, J.; Lee, H.; Park, H.J.; Lee, H.; et al. Inserting needles into the body: A meta-analysis of brain activity associated with acupuncture needle stimulation. J. Pain 2013, 14, 215–222. [Google Scholar] [CrossRef]
- Aranha, M.F.M.; Müller, C.E.E.; Gavião, M.B.D. Pain intensity and cervical range of motion in women with myofascial pain treated with acupuncture and electroacupuncture: A double-blinded, randomized clinical trial. Braz. J. Phys. Ther. 2015, 19, 34–43. [Google Scholar] [CrossRef]
- Hollis, S.; McClure, P. Intramuscular electrical stimulation for muscle activation of the tibialis anterior after surgical repair: A case report. J. Orthop. Sports Phys. Ther. 2017, 47, 965–969. [Google Scholar] [CrossRef]
- Dunning, J.; Butts, R.; Henry, N.; Mourad, F.; Brannon, A.; Rodriguez, H.; Young, I.; Arias-Buría, J.L.; Fernándezde-Las-Peñas, C. Electrical dry needling as an adjunct to exercise, manual therapy and ultrasound for plantar fasciitis: A multi-center randomized clinical trial. PLoS ONE 2018, 13, e0205405. [Google Scholar] [CrossRef]
- Ahmed, S.; Haddad, C.; Subramaniam, S.; Khattab, S.; Kumbhare, D. The Effect of Electric Stimulation Techniques on Pain and Tenderness at the Myofascial Trigger Point: A Systematic Review. Pain Med. 2019, 20, 1774–1788. [Google Scholar] [CrossRef]
- León-Hernández, J.V.; Martín-Pintado-Zugasti, A.; Frutos, L.G.; Alguacil-Diego, I.M.; De La Llave-Rincón, A.I.; Fernandez-Carnero, J. Immediate and short-term effects of the combination of dry needling and percutaneous TENS on post-needling soreness in patients with chronic myofascial neck pain. Braz. J. Phys. Ther. 2016, 20, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.; De Souza, I.C.C.; Torres, I.L.S.; Fregni, F.; Caumo, W. Paraspinal stimulation combined with trigger point needling and needle rotation for the treatment of myofascial pain: A randomized sham-controlled clinical trial. Clin. J. Pain 2014, 30, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Chassot, M.; Dussan-Sarria, J.A.; Sehn, F.C.; Deitos, A.; de Souza, A.; Vercelino, R.; Torres, I.S.; Fregni, F.; Caumo, W. Electroacupuncture analgesia is associated with increased serum brain-derived neurotrophic factor in chronic tension-type headache: A randomized, sham controlled, crossover trial. BMC Complement. Altern. Med. 2015, 15, 144. [Google Scholar] [CrossRef]
- Leitch, M.; Brown, R.; Macefield, V.G. Intramuscular stimulation of tibialis anterior in human subjects: The effects of discharge variability on force production and fatigue. Physiol. Rep. 2017, 5, e13326. [Google Scholar] [CrossRef] [PubMed]
- Botelho, L.; Angoleri, L.; Zortea, M.; Deitos, A.; Brietzke, A.; Torres, I.L.S.; Fregni, F.; Caumo, W. Insights about the neuroplasticity state on the effect of intramuscular electrical stimulation in pain and disability associated with chronic myofascial pain syndrome (MPS): A double-blind, randomized, sham-controlled trial. Front. Hum. Neurosci. 2018, 12, 388. [Google Scholar] [CrossRef]
- Da Graca-Tarragó, M.; Deitos, A.; Brietzke, A.P.; Torres, I.L.S.; Stefani, L.C.; Fregni, F.; Caumo, W. Electrical intramuscular stimulation in osteoarthritis enhances the inhibitory systems in pain processing at cortical and cortical spinal system. Pain Med. (U.S.) 2016, 17, 877–891. [Google Scholar] [CrossRef][Green Version]
- Aranha, M.F.M.; Alves, M.C.; Bérzin, F.; Gavião, M.B.D. Eficácia da eletroacupuntura para dor miofascial do músculo trapézio: Uma série de casos. Rev. Bras. Fisioter. 2011, 15, 371–379. [Google Scholar] [CrossRef][Green Version]
- Rainey, C.E. The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: A case report. Int. J. Sports Phys. Ther. 2013, 8, 145–161. [Google Scholar]
- Rock, J.M.; Rainey, C.E. Treatment of nonspecific thoracic spine pain with trigger point dry needling and intramuscular electrical stimulation: A case series. Int. J. Sports Phys. Ther. 2014, 9, 699–711. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, 698–702. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63, S240–S252. [Google Scholar]
- Kahl, C.; Cleland, J.A. Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: An overview of psychometric properties. Phys. Ther. Rev. 2005, 10, 123–128. [Google Scholar] [CrossRef]
- Andrade Ortega, J.A.; Delgado Martínez, A.D.; Ruiz, R.A. Validation of the Spanish version of the Neck Disability Index. Spine (Phila. Pa. 1976). 2010, 35, E114–E118. [Google Scholar] [CrossRef]
- Rathbone, A.T.L.; Grosman-Rimon, L.; Kumbhare, D.A. Interrater Agreement of Manual Palpation for Identification of Myofascial Trigger Points: A Systematic Review and Meta-Analysis. Clin. J. Pain 2017, 33, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Dommerholt, J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: A delphi study. Pain Med. (U.S.) 2018, 19, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Simons, D.; Travell, J. Travell, Simons & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual, 3th ed.; Wolters Kluwer: Philadelpia, PA, USA, 2019. [Google Scholar]
- Shadmehr, A.; Bagheri, H.; Ansari, N.N.; Sarafraz, H. The reliability measurements of lateral scapular slide test at three different degrees of shoulder joint abduction. Br. J. Sports Med. 2010, 44, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; McCarthy, C.J.; Chorti, A.; Cooke, M.W.; Gates, S. A Systematic Review of Reliability and Validity Studies of Methods for Measuring Active andPassive Cervical Range of Motion. J. Manip. Physiol. Ther. 2010, 33, 138–155. [Google Scholar] [CrossRef]
- Walton, D.; MacDermid, J.; Nielson, W.; Teasell, R.; Chiasson, M.; Brown, L. Reliability, Standard Error, and Minimum Detectable Change of Clinical Pressure Pain Threshold Testing in People With and Without Acute Neck Pain. J. Orthop. Sport. Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef]
- Versteegh, T.H.; Beaudet, D.; Greenbaum, M.; Hellyer, L.; Tritton, A.; Walton, D. Evaluating the reliability of a novel neck-strength assessment protocol for healthy adults using self- generated resistance with a hand-held dynamometer. Physiother. Canada 2015, 67, 58–64. [Google Scholar] [CrossRef]
- McCutcheon, L.; Yelland, M. Iatrogenic pneumothorax: Safety concerns when using acupuncture or dry needling in the thoracic region. Phys. Ther. Rev. 2011, 16, 126–132. [Google Scholar] [CrossRef]
- Hong, C.Z. Lidocaine injection versus dry needling to myofascial trigger point: The importance of the local twitch response. Am. J. Phys. Med. Rehabil. 1994, 73, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Pecos-Martín, D.; Montañez-Aguilera, F.J.; Gallego-Izquierdo, T.; Urraca-Gesto, A.; Gómez-Conesa, A.; Romero-Franco, N.; Plaza-Manzano, G. Effectiveness of dry needling on the lower trapezius in patients with mechanical neck pain: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Vance, C.G.T.; Lisi, T.L. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. J. Neurochem. 2005, 95, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Field, A.P. Discovering Statistics Using IBM SPSS Statistics, 5th ed.; SAGE Publications Ltd.: London, UK, 2018. [Google Scholar]
- Plaza-Manzano, G.; Gómez-Chiguano, G.F.; Cleland, J.A.; Arías-Buría, J.L.; Fernández-de-las-Peñas, C.; Navarro-Santana, M.J. Effectiveness of percutaneous electrical nerve stimulation for musculoskeletal pain: A systematic review and meta-analysis. Eur. J. Pain (U.K.) 2020. [Google Scholar] [CrossRef]
- MacDowall, A.; Skeppholm, M.; Robinson, Y.; Olerud, C. Validation of the visual analog scale in the cervical spine. J. Neurosurg. Spine 2018, 28, 227–235. [Google Scholar] [CrossRef]
- Ziaeifar, M.; Arab, A.M.; Nourbakhsh, M.R. Clinical Effectiveness of Dry Needling Immediately After Application on Myofascial Trigger Point in Upper Trapezius Muscle. J. Chiropr. Med. 2016, 15, 252–258. [Google Scholar] [CrossRef]
- Martín-Pintado-Zugasti, A.; Fernández-Carnero, J.; León-Hernández, J.V.; Calvo-Lobo, C.; Beltran-Alacreu, H.; Alguacil-Diego, I.; Gallego-Izquierdo, T.; Pecos-Martin, D. Postneedling Soreness and Tenderness After Different Dosages of Dry Needling of an Active Myofascial Trigger Point in Patients With Neck Pain: A Randomized Controlled Trial. PM R 2018, 10, 1311–1320. [Google Scholar] [CrossRef]
- Martín-Pintado-Zugasti, A.; Pecos-Martin, D.; Rodríguez-Fernández, Á.L.; Alguacil-Diego, I.M.; Portillo-Aceituno, A.; Gallego-Izquierdo, T.; Fernandez-Carnero, J. Ischemic Compression After Dry Needling of a Latent Myofascial Trigger Point Reduces Postneedling Soreness Intensity and Duration. PM R 2015, 7, 1026–1034. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Linton, S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000, 85, 317–332. [Google Scholar] [CrossRef]
- Leeuw, M.; Goossens, M.E.J.B.; Linton, S.J.; Crombez, G.; Boersma, K.; Vlaeyen, J.W.S. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J. Behav. Med. 2007, 30, 77–94. [Google Scholar] [CrossRef]
- Demirbüken, I.; Özgül, B.; Kuru Çolak, T.; Aydoʇdu, O.; Sari, Z.; Yurdalan, S.U. Kinesiophobia in relation to physical activity in chronic neck pain. J. Back Musculoskelet. Rehabil. 2016, 29, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Luque-Suarez, A.; Martinez-Calderon, J.; Falla, D. Role of kinesiophobia on pain, disability and quality of life in people suffering from chronic musculoskeletal pain: A systematic review. Br. J. Sports Med. 2019, 53, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Alacreu, H.; López-de-Uralde-Villanueva, I.; Calvo-Lobo, C.; La Touche, R.; Cano-de-la-cuerda, R.; Gil-Martínez, A.; Fernández-Ayuso, D.; Fernández-Carnero, J. Prediction models of health-related quality of life in different neck pain conditions: A cross-sectional study. Patient Prefer. Adherence 2018, 12, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Phillips, T.M.; Danoff, J.V.; Gerber, L.H. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J. Appl. Physiol. 2005, 99, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Mejuto-Vázquez, M.J.; Salom-Moreno, J.; Ortega-Santiago, R.; Truyols-Domínguez, S.; Fernández-De-Las-peñas, C. Short- Term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2014, 44, 252–260. [Google Scholar] [CrossRef]
- Cerezo-Téllez, E.; Torres-Lacomba, M.; Fuentes-Gallardo, I.; Perez-Muñoz, M.; Mayoral-Del-Moral, O.; Lluch-Girbés, E.; Prieto-Valiente, L.; Falla, D. Effectiveness of dry needling for chronic nonspecific neck pain: A randomized, single-blinded, clinical trial. Pain 2016, 157, 1905–1917. [Google Scholar] [CrossRef]
- Mansfield, C.J.; Vanetten, L.; Willy, R.; Di Stasi, S.; Magnussen, R.; Briggs, M. The effects of needling therapies on muscle force production: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2019, 49, 154–170. [Google Scholar] [CrossRef]
- Johnson, E.K.; Chiarello, C.M. The slump test: The effects of head and lower extremity position on knee extension. J. Orthop. Sports Phys. Ther. 1997, 26, 310–317. [Google Scholar] [CrossRef]
- Ackland, D.C.; Merritt, J.S.; Pandy, M.G. Moment arms of the human neck muscles in flexion, bending and rotation. J. Biomech. 2011, 44, 475–486. [Google Scholar] [CrossRef]
- Swinkels, R.A.H.M.; Swinkels-Meewisse, I.E.J.C.M. Normal values for cervical range of motion. Spine 2014, 39, 362–367. [Google Scholar] [CrossRef]
- Wager, T.D.; Atlas, L.Y. The neuroscience of placebo effects: Connecting context, learning and health. Nat. Rev. Neurosci. 2015, 16, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Malfliet, A.; Lluch Girbés, E.; Pecos-Martin, D.; Gallego-Izquierdo, T.; Valera-Calero, A. The Influence of Treatment Expectations on Clinical Outcomes and Cortisol Levels in Patients With Chronic Neck Pain: An Experimental Study. Pain Pract. 2019, 19, 370–381. [Google Scholar] [CrossRef] [PubMed]

| Characteristic, Mean (SD) | DN (n = 22) | PENS (n = 22) | p-Value |
|---|---|---|---|
| Age, years | 25.45 (8.53) | 24.14 (9.39) | 0.63 |
| Height, cm | 171.73 (8.86) | 171.59 (6.60) | 0.95 |
| Weight, kg | 65.42 (10.79) | 63.49 (8.58) | 0.51 |
| BMI, kg/m2 | 21.64 (2.67) | 21.60 (2.02) | 0.95 |
| Computer Use, hours/week | 15.77 (10.82) | 16.92 (7.84) | 0.69 |
| MTrP Distance, cm | 8.12 (1.07) | 8.08 (1.13) | 0.93 |
| VAS, cm | 5.01 (1.52) | 4.56 (1.73) | 0.36 |
| NDI | 19.64 (7.32) | 22.09 (9.75) | 0.35 |
| PPT, kg/cm2 | 1.98 (0.56) | 2.11 (0.52) | 0.43 |
| Strength, N | 145.20 (28.70) | 152.40 (32.20) | 0.44 |
| Flexion, Degrees | 44.83 (9.85) | 44.59 (11.25) | 0.94 |
| Extension, Degrees | 50.72 (9.72) | 52.81 (13.90) | 0.57 |
| Rotation, Degrees | |||
| Painful Side | 58.21 (15.46) | 61.59 (10.50) | 0.40 |
| Nonpainful Side | 52.18 (12.78) | 59.47 (14.47) | 0.08 |
| Side Bending, Degrees | |||
| Painful Side | 38.59 (5.81) | 38.50 (5.07) | 0.96 |
| Nonpainful Side | 34.30 (5.31) | 37.45 (3.65) | 0.03 |
| Sex, n (%) | 0.53 | ||
| Male | 9 (40.9) | 7 (31.8) | |
| Female | 13 (59.1) | 15 (68.2) | |
| Painful Side, n (%) | 0.07 | ||
| Right | 16 (72.7) | 10 (45.5) | |
| Left | 6 (27.3) | 12 (54.5) |
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| VAS, cm | ||||
| DN, Mean (SD) | 5.01 (1.52) | 3.30 (1.70) | 2.28 (1.58) | 2.26 (1.55) |
| PENS, Mean (SD) | 4.56 (1.73) | 2.62 (2.11) | 2.04 (1.69) | 1.71 (1.29) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 1.71 (1.02, 2.40) ‡ | 2.73 (1.83, 3.64) ‡ | 2.76 (1.86, 3.65) ‡ | |
| PENS | 1.94 (1.25, 2.63) ‡ | 2.52 (1.61, 3.42) ‡ | 2.85 (1.96, 3.74) ‡ | |
| Between-Group Differences, Mean (95% CI) | −0.23 (−0.94, 0.48) | 0.22 (−0.72, 1.15) | −0.09 (−1.01, 0.83) | |
| NDI | ||||
| DN, Mean (SD) | 19.64 (7.32) | - | - | 13.09 (7.45) |
| PENS, Mean (SD) | 22.09 (9.75) | - | - | 12.27 (8.20) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 6.55 (4.43, 8.66) ‡ | |||
| PENS | 9.82 (7.70, 11.94) ‡ | |||
| Between-Group Differences, Mean (95% CI) | −3.27 (−6.27, −0.27) † |
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| PPT, kg/cm2 | ||||
| DN, Mean (SD) | 1.98 (0.56) | 2.71 (0.52) | 2.72 (0.46) | 2.70 (0.52) |
| PENS, Mean (SD) | 2.11 (0.52) | 3.71 (0.46) | 3.84 (0.43) | 4.18 (0.46) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 0.73 (0.44, 1.01) ‡ | 0.74 (0.47, 1.01) ‡ | 0.72 (0.39, 1.04) ‡ | |
| PENS | 1.61 (1.32, 1.89) ‡ | 1.73 (1.45, 2.00) ‡ | 2.06 (1.74, 2.39) ‡ | |
| Between-Group Differences, Mean (95% CI) | 0.88 (0.58, 1.17) ‡ | 0.99 (0.71, 1.27) ‡ | 1.35 (1.01, 1.68) ‡ | |
| Strength, N | ||||
| DN, Mean (SD) | 145.30 (28.70) | 155.30 (28.80) | 163.20 (33.00) | 173.00 (37.10) |
| PENS, Mean (SD) | 152.40 (32.20) | 162.70 (32.70) | 167.10 (32.90) | 173.90 (33.60) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 10.00 (−2.80, 22.80) | 18.00 (6.20, 29.80) ‡ | 27.80 (15.30, 40.30) ‡ | |
| PENS | 10.20 (−2.60, 23.00) | 14.70 (2.90, 26.50) ‡ | 21.50 (9.00, 34.00) ‡ | |
| Between-Group Differences, Mean (95% CI) | 0.20 (−13.00, 13.40) | −3.30 (−15.40, 8.90) | −6.30 (−19.20, 6.60) |
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| Cervical Flexion, Degrees | ||||
| DN, Mean (SD) | 44.83 (9.85) | 45.90 (8.61) | 48.36 (9.31) | 47.27 (8.41) |
| PENS, Mean (SD) | 44.59 (11.25) | 52.95 (7.50) | 54.73 (6.39) | 51.27 (7.59) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 1.08 (−4.25, 6.41) | 3.53 (−3.35, 10.32) | 2.44 (−3.74, 8.62) | |
| PENS | 8.36 (3.04, 13.69) ‡ | 10.14 (3.35, 16.92) ‡ | 6.68 (0.50, 12.86) † | |
| Between-Group Differences, Mean (95% CI) | 7.29 (1.79, 12.78) † | 6.60 (−0.39, 13.60) | 4.24 (−2.13, 10.61) | |
| Cervical Extension, Degrees | ||||
| DN, Mean (SD) | 50.72 (9.72) | 53.50 (9.86) | 57.32 (7.49) | 58.05 (7.95) |
| PENS, Mean (SD) | 52.81 (13.90) | 58.41 (10.14) | 56.64 (11.37) | 57.68 (11.26) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | 2.78 (−1.09, 6.65) | 6.60 (1.00, 12.19) † | 7.33 (2.11, 12.54) ‡ | |
| PENS | 5.59 (1.72, 9.46) ‡ | 3.82 (−1.77, 9.41) | 4.86 (−0.36, 10.08) | |
| Between-Group Differences, Mean (95% CI) | 2.81 (−1.18, 6.80) | −2.78 (−8.54, 2.98) | −2.46 (−2.92, 7.84) |
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| DN, Mean (SD) | ||||
| Painful Side | 58.21 (14.46) | 64.41 (8.92) | 68.09 (9.32) | 66.50 (8.95) |
| Nonpainful Side | 52.18 (12.78) | 64.5 (8.95) | 66.27 (12.69) | 66.14 (10.67) |
| PENS, Mean (SD) | ||||
| Painful Side | 61.59 (10.50) | 69.55 (9.38) | 71.50 (8.02) | 70.55 (7.32) |
| Nonpainful Side | 59.48 (14.47) | 67.68 (9.36) | 70.41 (9.02) | 69.68 (9.52) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | ||||
| Painful Side | 6.20 (−0.84, 13.25) | 9.88 (2.15, 17.62) ‡ | 8.29 (0.59, 16.00) † | |
| Nonpainful Side | 12.32 (6.85, 17.80) ‡ | 14.10 (7.52, 20.68) ‡ | 13.96 (7.18, 20.74) ‡ | |
| PENS | ||||
| Painful Side | 7.96 (0.91, 15.00) † | 9.91 (2.18, 17.64) ‡ | 8.96 (1.25, 16.66) † | |
| Nonpainful Side | 8.21 (2.73, 13.68) ‡ | 10.93 (4.35, 17.51) ‡ | 10.21 (3.43, 16.98) ‡ | |
| Between-Group Differences, Mean (95% CI) | ||||
| Painful Side | 1.75 (−5.51, 9.01) | 0.03 (−7.95, 8.00) | 0.66 (−7.28, 8.61) | |
| Nonpainful Side | −4.12 (−9.76, 1.52) | −3.16 (−9.95, 3.62) | −3.76 (−10.74, 3.23) |
| Variable | Baseline | Post-Treatment | 48 Hours | 1 Week |
|---|---|---|---|---|
| DN, Mean (SD) | ||||
| Painful Side | 38.59 (5.81) | 40.09 (4.00) | 39.73 (5.68) | 40.91 (4.05) |
| Nonpainful Side | 34.30 (5.31) | 41.00 (4.92) | 40.45 (4.93) | 40.50 (5.32) |
| PENS, Mean (SD) | ||||
| Painful Side | 38.50 (5.07) | 42.27 (5.19) | 41.81 (4.15) | 41.41 (5.61) |
| Nonpainful Side | 37.45 (3.65) | 41.82 (5.24) | 40.27 (6.02) | 41.36 (5.52) |
| Within-Group Differences from Baseline, Mean (95% CI) | ||||
| DN | ||||
| Painful Side | 1.50 (−0.67, 3.68) | 1.14 (−1.89, 4.16) | 2.32 (−0.43, 5.07) | |
| Nonpainful Side | 6.70 (4.15, 9.26) ‡ | 6.16 (2.96, 9.35) ‡ | 6.20 (3.16, 9.25) ‡ | |
| PENS | ||||
| Painful Side | 3.77 (1.60, 5.95) ‡ | 3.32 (0.29, 6.34) † | 2.91 (0.16, 5.66) † | |
| Nonpainful Side | 4.36 (1.81, 6.92) ‡ | 2.82 (−0.38, 6.01) | 3.91 (0.87, 6.95) ‡ | |
| Between-Group Differences, Mean (95% CI) | ||||
| Painful Side | 2.27 (0.03, 4.51) † | 2.18 (−0.94, 5.30) | 0.59 (−2.24, 3.42) | |
| Nonpainful Side | −2.34 (−4.97, 0.29) | −3.34 (−6.63, −0.05) † | −2.29 (−5.43, 0.84) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-de-Miguel, S.; Pecos-Martin, D.; Larroca-Sanz, T.; Sanz-de-Vicente, B.; Garcia-Montes, L.; Fernandez-Matias, R.; Gallego-Izquierdo, T. Short-Term Effects of PENS versus Dry Needling in Subjects with Unilateral Mechanical Neck Pain and Active Myofascial Trigger Points in Levator Scapulae Muscle: A Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1665. https://doi.org/10.3390/jcm9061665
Garcia-de-Miguel S, Pecos-Martin D, Larroca-Sanz T, Sanz-de-Vicente B, Garcia-Montes L, Fernandez-Matias R, Gallego-Izquierdo T. Short-Term Effects of PENS versus Dry Needling in Subjects with Unilateral Mechanical Neck Pain and Active Myofascial Trigger Points in Levator Scapulae Muscle: A Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(6):1665. https://doi.org/10.3390/jcm9061665
Chicago/Turabian StyleGarcia-de-Miguel, Santiago, Daniel Pecos-Martin, Tamara Larroca-Sanz, Beatriz Sanz-de-Vicente, Laura Garcia-Montes, Ruben Fernandez-Matias, and Tomas Gallego-Izquierdo. 2020. "Short-Term Effects of PENS versus Dry Needling in Subjects with Unilateral Mechanical Neck Pain and Active Myofascial Trigger Points in Levator Scapulae Muscle: A Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 6: 1665. https://doi.org/10.3390/jcm9061665
APA StyleGarcia-de-Miguel, S., Pecos-Martin, D., Larroca-Sanz, T., Sanz-de-Vicente, B., Garcia-Montes, L., Fernandez-Matias, R., & Gallego-Izquierdo, T. (2020). Short-Term Effects of PENS versus Dry Needling in Subjects with Unilateral Mechanical Neck Pain and Active Myofascial Trigger Points in Levator Scapulae Muscle: A Randomized Controlled Trial. Journal of Clinical Medicine, 9(6), 1665. https://doi.org/10.3390/jcm9061665






