Matching between Donors and Ulcerative Colitis Patients Is Important for Long-Term Maintenance after Fecal Microbiota Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Patient and Public Involvement
2.3. Patients
2.4. Donors
2.5. Protocol for AFM Monotherapy
2.6. Protocol for A-FMT
2.7. Clinical Evaluation
2.8. Microbial Analysis
2.9. Statistical Analysis
2.10. Data Availability Statement
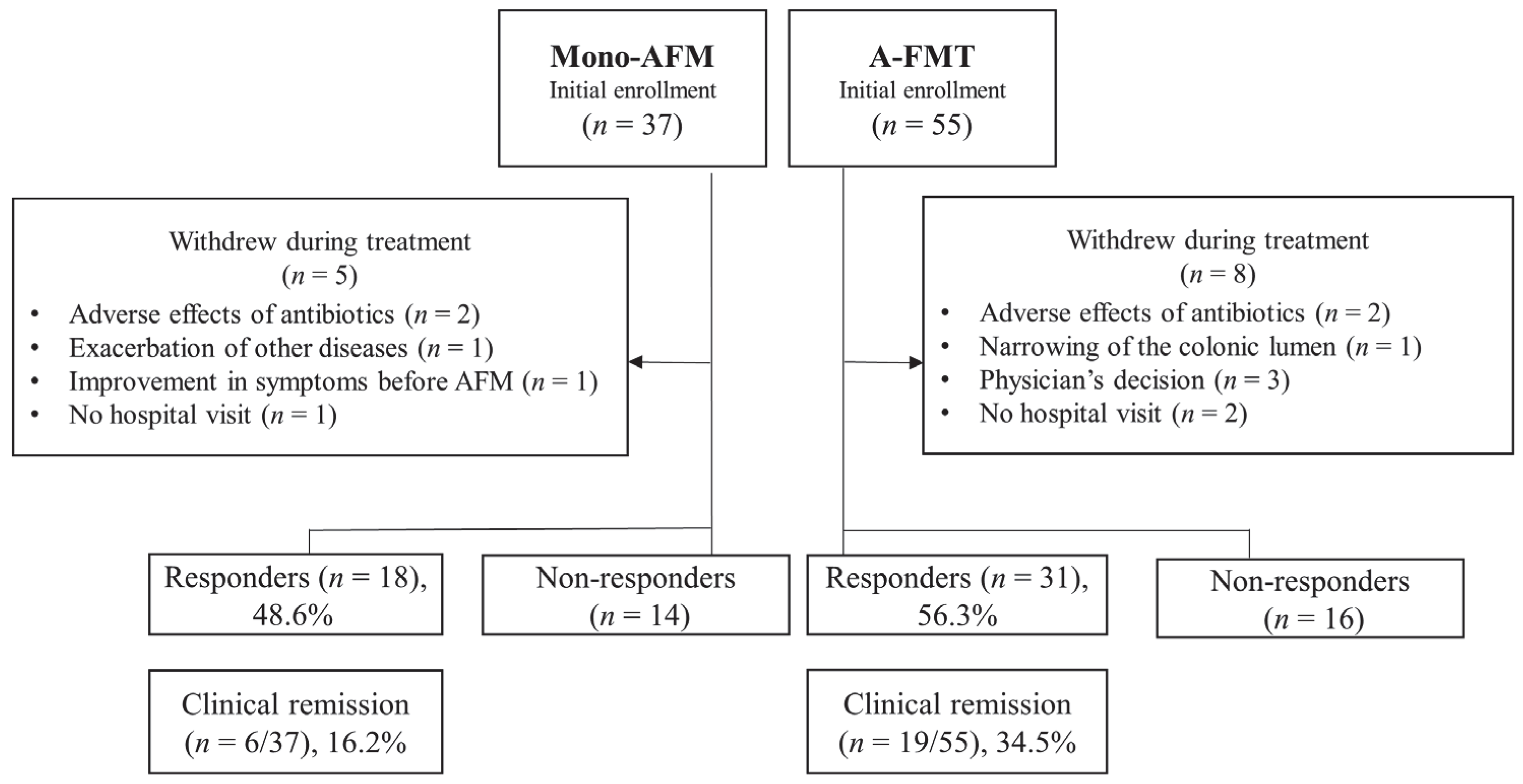
3. Results
3.1. Adverse Events
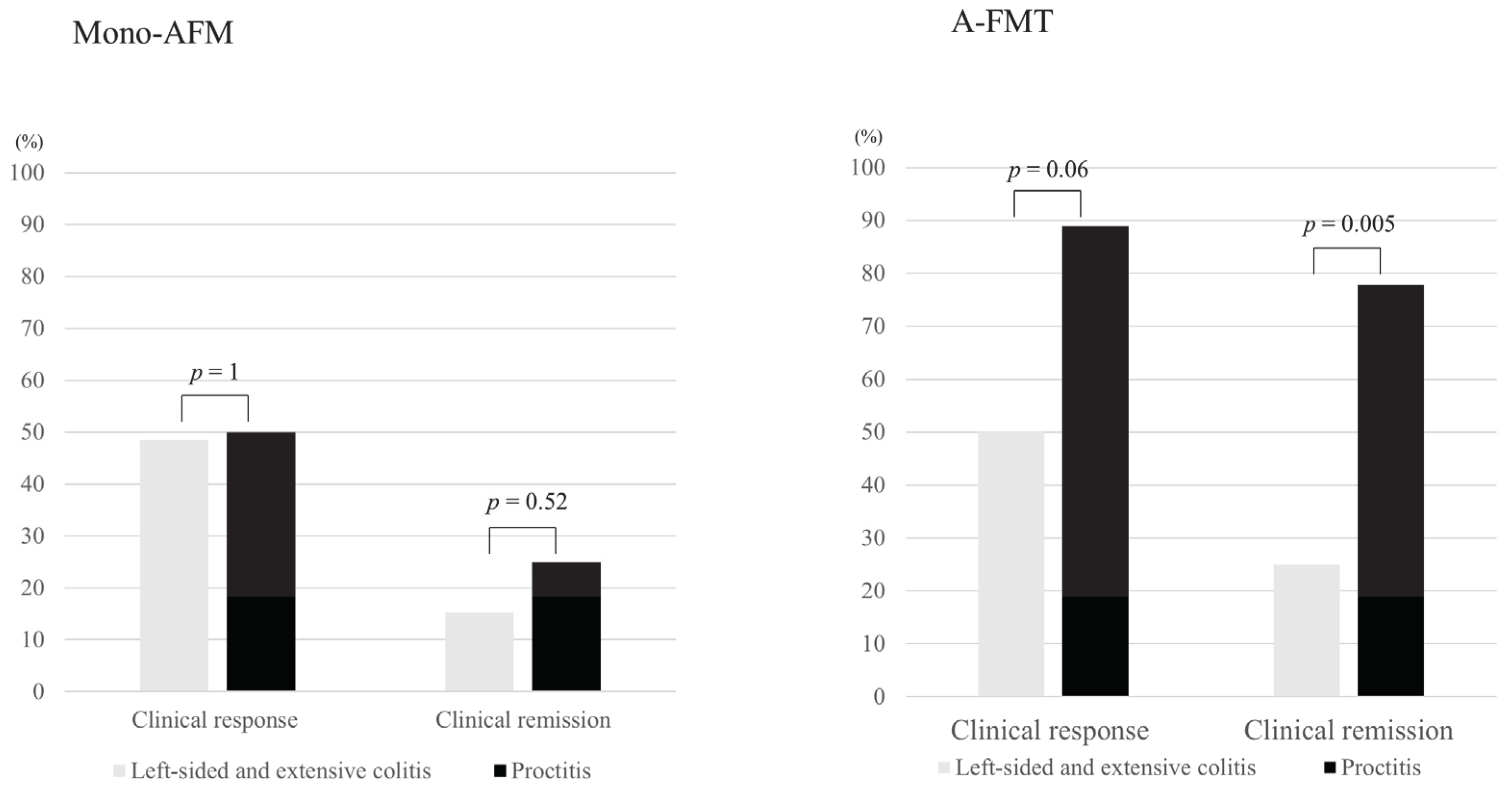
3.2. Short-Term Clinical Evaluation
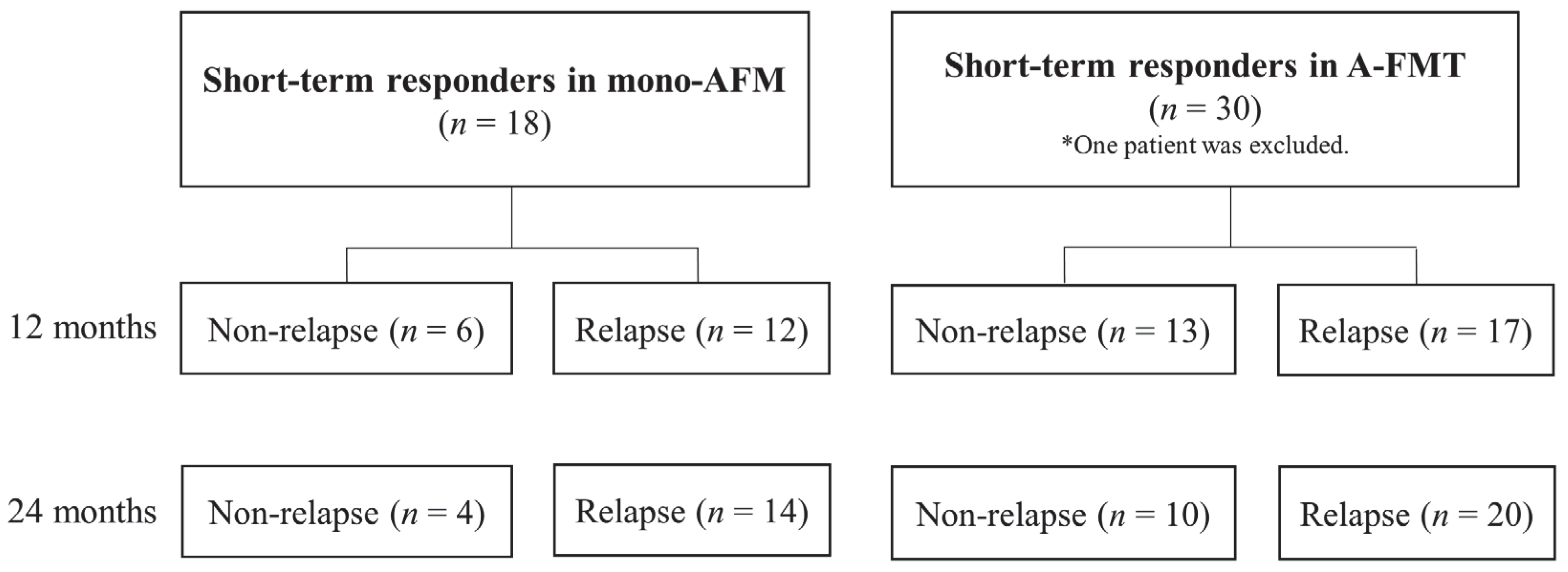
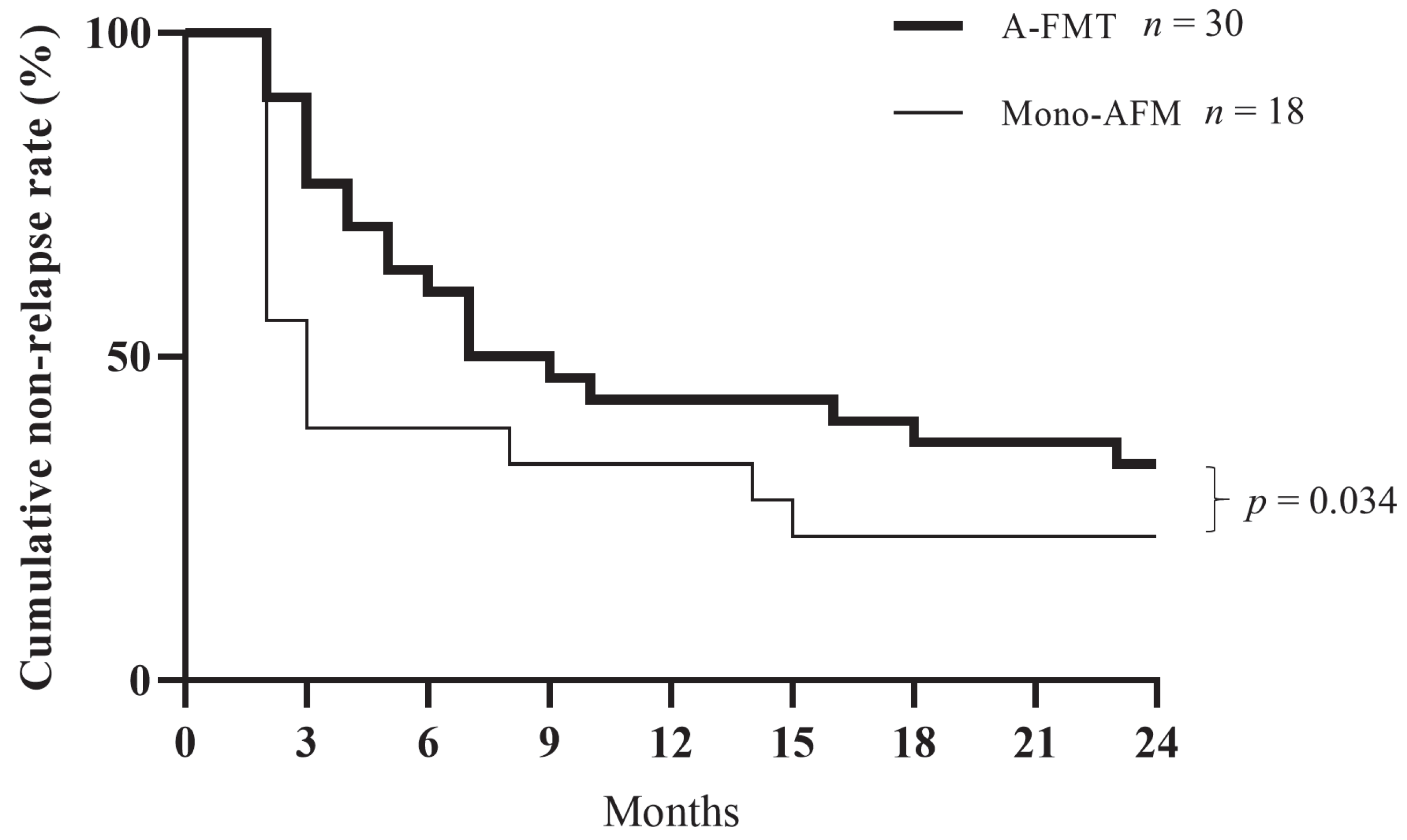
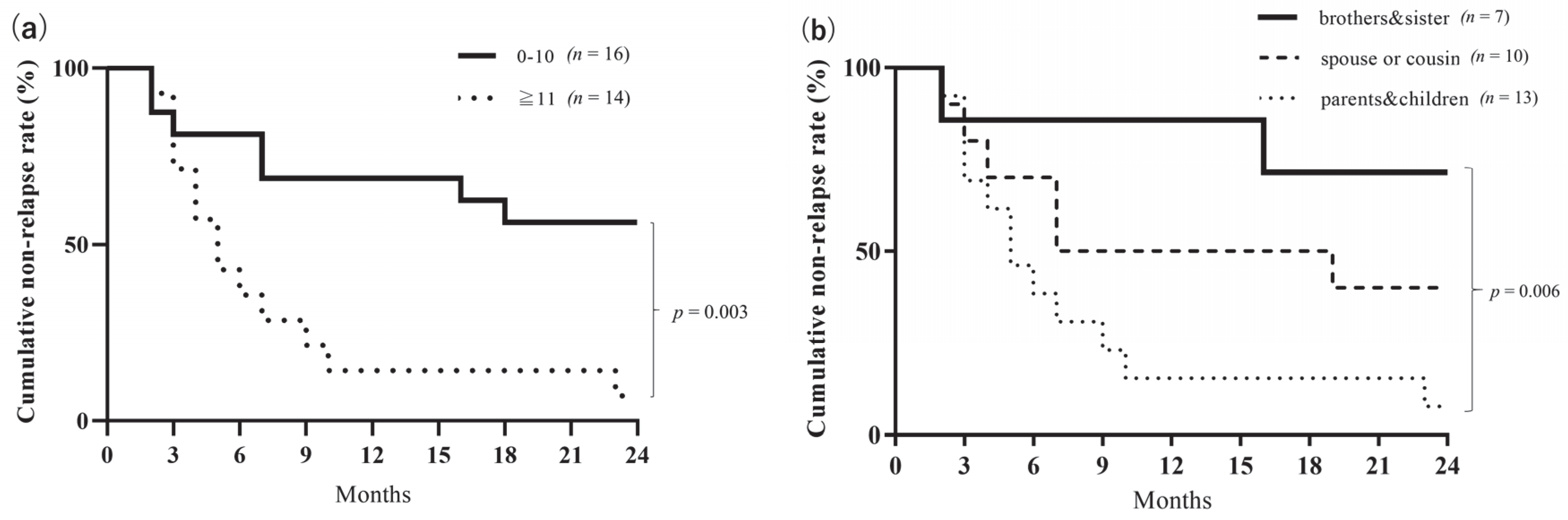
3.3. Long-Term Clinical Evaluation
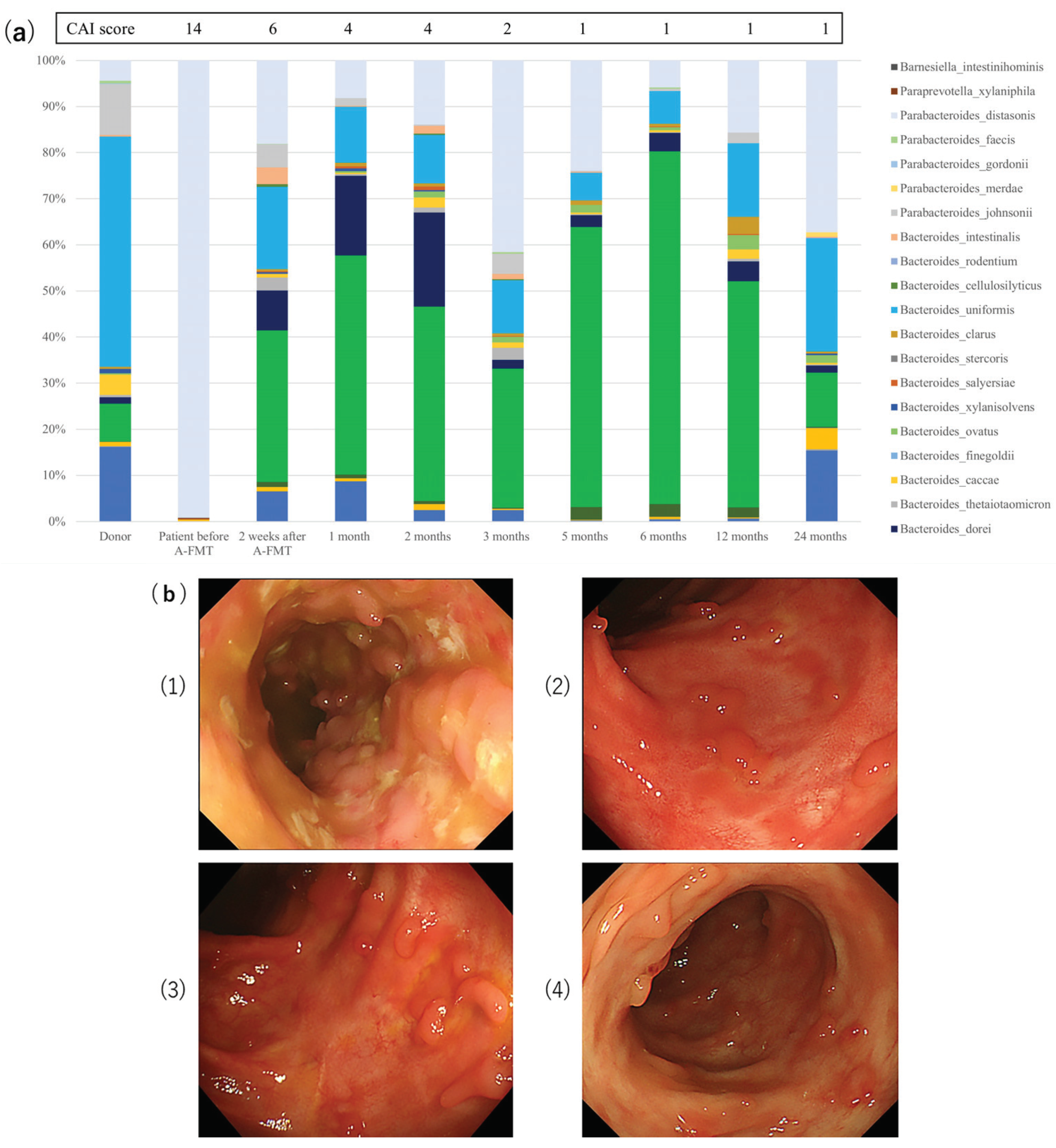
3.4. Microbial Analysis for Long-Term Study
- (1)
- Baseline endoscopic appearance of the descending colon with active colitis; clinical activity index (CAI) score = 14, endoscopic subscore = 3.
- (2)
- Endoscopic appearance at the end of 1 month after A-FMT; CAI score = 4, endoscopic subscore = 1.
- (3)
- Endoscopic appearance at the end of 6 months after A-FMT; CAI score = 1, endoscopic subscore = 1.
- (4)
- Endoscopic appearance at the end of 24 months after A-FMT; CAI score = 1, endoscopic subscore = 1.
4. Discussion
5. Conclusions
- 15th Congress of ECCO2020 on 12–15 February 2020, in Vienna, Austria. This study won the prize for “Top Ten Best DOPs” during the ECCO2020 conference.
- 2020 Crohn’s & Colitis Congress on 23–25 January 2020, in Austin, Texas, USA.
- 61st Annual Meeting of the Japanese Society of Gastroenterology on 21–24 November 2019, in Kobe, Japan.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, U.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.D.; Günther, S.; Hansen, L.H.; Petersen, A. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-Blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Dy, A.B.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Wei, Y.; Li, Y.; Chen, W.; Chen, H.; Wang, Q.; Yang, F.; Miao, Q.; Xiao, X.; Zhang, H.; et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut 2017, 67, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ge, X.; Nie, Y.; Yang, L.; Ding, C.; McFarland, L.V.; Zhang, X.; Chen, Q.; Gong, J.; Li, N. Fecal microbiota transplantation in patients with slow-transit constipation: A randomized, clinical trial. PLoS ONE 2017, 12, e0171308. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga–Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-Term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal Infusion of Donor Feces for RecurrentClostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Borody, T.J.; Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 88–96. [Google Scholar] [CrossRef]
- Hedin, C.; Van Der Gast, C.J.; Rogers, G.B.; Cuthbertson, L.; McCartney, S.; Stagg, A.J.; Lindsay, J.O.; Whelan, K. Siblings of patients with Crohn’s disease exhibit a biologically relevant dysbiosis in mucosal microbial metacommunities. Gut 2015, 65, 944–953. [Google Scholar] [CrossRef]
- Braun, J.M.; Wei, B. Body Traffic: Ecology, Genetics, and Immunity in Inflammatory Bowel Disease. Annu. Rev. Pathol. Mech. Dis. 2007, 2, 401–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kump, P.; Wurm, P.; Gröchenig, H.P.; Wenzl, H.; Petritsch, W.; Halwachs, B.; Wagner, M.; Stadlbauer, V.; Eherer, A.; Hoffmann, K.M.; et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 47, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Reiff, C.; Kelly, D.; Meharg, C. Inflammatory bowel disease, gut bacteria and probiotic therapy. Int. J. Med. Microbiol. 2010, 300, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.; Shanahan, F. The Gut Microbiota in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; Bogaerde, J.V.D.; Samuel, D.; Leong, R.W.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Rossen, N.G.; Fuentes, S.; Van Der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Löwenberg, M.; Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Y.; Huang, S.; Li, J.; Zhao, K.; Li, X.; Wen, X.; Li, X.-A. Fecal microbiota transplantation for ulcerative colitis: A prospective clinical study. BMC Gastroenterol. 2019, 19, 116. [Google Scholar] [CrossRef]
- Youngster, I.; Sauk, J.; Pindar, C.; Wilson, R.G.; Kaplan, J.L.; Smith, M.B.; Alm, E.J.; Gevers, D.; Russell, G.H.; Hohmann, E.L. Fecal Microbiota Transplant for Relapsing Clostridium difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin. Infect. Dis. 2014, 58, 1515–1522. [Google Scholar] [CrossRef]
- Ding, X.; Li, Q.; Li, P.; Zhang, T.; Cui, B.; Ji, G.; Lu, X.; Zhang, F. Long-Term Safety and Efficacy of Fecal Microbiota Transplant in Active Ulcerative Colitis. Drug Saf. 2019, 42, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Ishikawa, D.; Haga, K.; Shibuya, T.; Sasaki, T.; Osada, T.; Kuwahara-Arai, K.; Hiramatsu, K.; Watanabe, S. Changes in Intestinal Microbiota Following Combination Therapy with Fecal Microbial Transplantation and Antibiotics for Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, D.; Sasaki, T.; Takahashi, M.; Kuwahara-Arai, K.; Haga, K.; Ito, S.; Okahara, K.; Nakajima, A.; Shibuya, T.; Osada, T.; et al. The Microbial Composition of Bacteroidetes Species in Ulcerative Colitis Is Effectively Improved by Combination Therapy With Fecal Microbiota Transplantation and Antibiotics. Inflamm. Bowel Dis. 2018, 24, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Nakase, H.; Ueno, S.; Uza, N.; Inoue, S.; Mikami, S.; Matsuura, M.; Ohmori, K.; Sakurai, T.; Nagayama, S.; et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm. Bowel Dis. 2007, 13, 1516–1521. [Google Scholar] [CrossRef]
- Owens, C.; Broussard, E.; Surawicz, C. Fecal microbiota transplantation and donor standardization. Trends Microbiol. 2013, 21, 443–445. [Google Scholar] [CrossRef]
- Rossen, N.G.; Macdonald, J.K.; De Vries, E.M.; D’Haens, G.R.; De Vos, W.M.; Zoetendal, E.G.; Ponsioen, C.Y. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J. Gastroenterol. 2015, 21, 5359–5371. [Google Scholar] [CrossRef]
- Turner, S.; Pryer, K.M.; Miao, V.P.W.; Palmer, J.D. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef]
- Osada, T.; Ohkusa, T.; Okayasu, I.; Yoshida, T.; Hirai, S.; Beppu, K.; Shibuya, T.; Sakamoto, N.; Kobayashi, O.; Nagahara, A.; et al. Correlations among total colonoscopic findings, clinical symptoms, and laboratory markers in ulcerative colitis. J. Gastroenterol. Hepatol. 2008, 23, S262–S267. [Google Scholar] [CrossRef]
- Morisita, M. Measuring of interspecific association and similarity between communities. Mem. Fac. Sci. Kyushu Univ. Ser. E 1959, 3, 65–80. [Google Scholar]
- Kimoto, S. Some quantitative analysis on the Chrysomelid fauna of the Ryukyu Archipelago. Esakia 1967, 6, 27–54. [Google Scholar]
- Horn, H.S. Measurement of "Overlap" in Comparative Ecological Studies. Am. Nat. 1966, 100, 419–424. [Google Scholar] [CrossRef]
- Burrello, C.; Giuffrè, M.R.; Macandog, A.D.; Diaz-Basabe, A.; Cribiù, F.M.; Lopez, G.; Borgo, F.C.; Nezi, L.; Caprioli, F.; Vecchi, M.; et al. Fecal Microbiota Transplantation Controls Murine Chronic Intestinal Inflammation by Modulating Immune Cell Functions and Gut Microbiota Composition. Cells 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Okai, S.; Usui, F.; Ohta, M.; Mori, H.; Kurokawa, K.; Matsumoto, S.; Kato, T.; Miyauchi, E.; Ohno, H.; Shinkura, R. Intestinal IgA as a modulator of the gut microbiota. Gut Microbes 2017, 8, 486–492. [Google Scholar] [CrossRef]
- Kassam, Z.; Lee, C.H.; Yuan, Y.; Hunt, R.H. Fecal microbiota transplantation for Clostridium difficile infection: Systematic review and meta-analysis. Am. J. Gastroenterol. 2013, 108, 500–508. [Google Scholar] [CrossRef]
- Osman, M.; Stoltzner, Z.; O’Brien, K.; Ling, K.; Koelsch, E.; Dubois, N.; Amaratunga, K.; Smith, M.; Kassam, Z. Donor Efficacy in Fecal Microbiota Transplantation for Recurrent Clostridium difficile: Evidence from a 1,999-Patient Cohort. Open Forum Infect. Dis. 2016, 3, 841. [Google Scholar] [CrossRef]
- Vermeire, S.; Joossens, M.; Verbeke, K.; Wang, J.; Machiels, K.; Sabino, J.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Raes, J. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 10, 387–394. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef]
- Hedin, C.; Van Der Gast, C.J.; Stagg, A.J.; Lindsay, J.O.; Whelan, K. The gut microbiota of siblings offers insights into microbial pathogenesis of inflammatory bowel disease. Gut Microbes 2017, 8, 359–365. [Google Scholar] [CrossRef]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.; et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Vatanen, T.; Cutfield, W.S.; O’Sullivan, J.M. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Microbiol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pena, C.; Alvarez-Cisneros, T.; Quiroz-Baez, R.; Friedland, R.P. Microbiota and aging. A review and commentary. Arch. Med. Res. 2017, 48, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Girotra, M.; Garg, S.; Anand, R.; Song, Y.; Dutta, S.K. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection in the Elderly: Long-Term Outcomes and Microbiota Changes. Dig. Dis. Sci. 2016, 61, 3007–3015. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.S.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef]
- Schirmer, M.; Franzosa, E.A.; Lloyd-Price, J.; McIver, L.J.; Schwager, R.; Poon, T.W.; Ananthakrishnan, A.N.; Andrews, E.; Barron, G.; Lake, K.; et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat. Microbiol. 2018, 3, 337–346. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Zimmer, J.; Lange, B.; Frick, J.-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2011, 66, 53–60. [Google Scholar] [CrossRef]
- Papanicolas, L.E.; Choo, J.M.; Wang, Y.; Leong, L.E.; Costello, S.P.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Bacterial viability in faecal transplants: Which bacteria survive? EBioMedicine 2019, 41, 509–516. [Google Scholar] [CrossRef]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; Bogaerde, J.V.D.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated With Response to Fecal Microbiota Transplantation in Patients With Ulcerative Colitis. Gastroenterology 2018, 156, 1440–1454.e2. [Google Scholar] [CrossRef] [PubMed]
| All (n = 92) | Mono-AFM (n = 37) | A-FMT (n = 55) | P Value | |
|---|---|---|---|---|
| Age (years) | 41.1 ± 13.9 | 42.5 ± 14.7 | 40.1 ± 13.3 | 0.42 |
| Sex (M/F) | 56/36 | 18/19 | 38/17 | 0.049 |
| Duration of disease (years) | 8.7 ± 8.1 | 8.9 ± 9.0 | 8.6 ± 7.4 | 0.83 |
| Disease location | ||||
| Proctitis | 13 | 4 | 9 | 0.66 |
| Left-sided colitis | 27 | 11 | 16 | 0.95 |
| Extensive colitis | 52 | 22 | 30 | 0.64 |
| Ongoing/past treatment | ||||
| 5-ASA | 78/86 | 32/35 | 46/51 | 0.94/1 |
| Corticosteroid | 30/67 | 14/30 | 16/37 | 0.38/0.22 |
| Apheresis | 1/38 | 0/19 | 1/19 | 0/0.11 |
| Azathioprine | 20/38 | 7/16 | 13/22 | 0.78/0.76 |
| Tacrolimus | 6/16 | 2/5 | 4/11 | 0.47/0.60 |
| Anti-TNF | 16/35 | 6/12 | 10/23 | 0.97/0.36 |
| CAI score | 10.1 ± 2.7 | 9.6 ± 2.3 | 10.3 ± 2.9 | 0.21 |
| ≥11 | 36 | 11 | 25 | 0.13 |
| 6–10 | 53 | 25 | 28 | 0.11 |
| ≤5 | 3 | 1 | 2 | 0.06 |
| Endoscopic evaluation | ||||
| UCEIS | 4.4 ± 2.4 | 3.8 ± 2.0 | 4.7 ± 2.5 | 0.12 |
| Mayo endoscopic score | 1.9 ± 0.7 | 1.8 ± 0.8 | 1.9 ± 0.7 | 0.86 |
| Sum of Mayo endoscopic scores | 6.3 ± 4.2 | 6.3 ± 4.3 | 6.3 ± 4.1 | 0.99 |
| Case | Age & Sex | Duration of UC (years) | Type of UC | Donor | Donor Age & Sex | Age Difference (years) | Decrease in CAI Score | Sum of Mayo Endoscopic Scores | Duration of Maintenance (months) |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 27M | 9 | Extensive | Mother | 59F | 32 | 4 | 9 | 3 |
| #2 | 24M | 6 | Extensive | Mother | 48F | 24 | 5 | 9 | 10 |
| #3 | 26F | 0.5 | Left-sided | Father | 58M | 32 | 4 | 4 | 9 |
| #4 | 62M | 1 | Proctitis | Spouse | 58F | 4 | 8 | 1 | 24 * |
| #5 | 32M | 10 | Proctitis | Spouse | 29F | 3 | 7 | 1 | 3 |
| #6 | 36M | 3 | Proctitis | Spouse | 30F | 6 | 9 | 1 | 24 * |
| #7 | 43M | 2.5 | Left-sided | Spouse | 43F | 0 | 9 | 2 | 7 |
| #8 | 22F | 1.5 | Left-sided | Father | 56M | 34 | 7 | 2 | 24 * |
| #9 | 46M | 26 | Extensive | Spouse | 49F | 3 | 13 | 10 | 2 |
| #10 | 61F | 24 | Proctitis | Daughter | 32F | 29 | 3 | 1 | 5 |
| #11 | 30M | 3.5 | Extensive | Brother | 27M | 3 | 8 | 12 | 24 * |
| #12 | 37M | 6 | Extensive | Spouse | 35F | 2 | 4 | 8 | 18 |
| #13 | 60F | 5 | Extensive | Daughter | 26F | 34 | 5 | 5 | 23 |
| #14 | 55F | 6 | Proctitis | Sister | 53F | 2 | 3 | 1 | 16 |
| #15 | 65M | 23 | Extensive | Daughter | 32F | 33 | 3 | 12 | 4 |
| #16 | 47M | 27 | Proctitis | Spouse | 46F | 1 | 3 | 4 | 24 * |
| #17 | 46F | 5 | Extensive | Sister | 49F | 3 | 5 | 2 | 24 * |
| #18 | 31M | 14 | Proctitis | Father | 66M | 33 | 4 | 1 | 6 |
| #19 | 55F | 34 | Left-sided | Cousin | 20F | 35 | 13 | 2 | 4 |
| #20 | 30M | 12 | Extensive | Mother | 53F | 23 | 6 | 8 | 3 |
| #21 | 29M | 5 | Extensive | Mother | 56F | 27 | 7 | 6 | 7 |
| #22 | 40M | 3 | Left-sided | Mother | 65F | 25 | 8 | 3 | 5 |
| #23 | 24F | 11 | Left-sided | Mother | 48F | 24 | 8 | 3 | 3 |
| #24 | 36M | 6 | Extensive | Spouse | 43F | 7 | 3 | 11 | 7 |
| #25 | 64F | 13 | Left-sided | Sister | 58F | 6 | 11 | 5 | 24 * |
| #26 | 73F | 14 | Extensive | Sister | 65F | 8 | 6 | 7 | 24 * |
| #27 | 32M | 12 | Extensive | Sister | 30F | 2 | 12 | 8 | 2 |
| #28 | 26M | 5 | Left-sided | Sister | 25F | 1 | 4 | 2 | 24 * |
| #29 | 43F | 7 | Left-sided | Spouse | 46M | 3 | 12 | 5 | 24 * |
| #30 | 33M | 15 | Proctitis | Father | 66M | 33 | 6 | 3 | 2 |
| Bacteroidetes Species | |
|---|---|
| Case #4 | Bacteroides uniformis**, Parabacteroides distasonis**, Bacteroides dorei*, Prevotella copri |
| Case #6 | Bacteroides uniformis**, Parabacteroides distasonis**, Bacteroides dorei*, Alistipes putredinis, Alistipes onderdonkii, Bacteroides plebeius, Bacteroides coprocola, Bacteroides massiliensis, Bacteroides vulgatus, Bacteroides thetaiotaomicron, Bacteroides caccae, Bacteroides stercoris, Bacteroides rodentium, Parabacteroides merdae, Paraprevotella xylaniphila |
| Case #11 | Bacteroides uniformis**, Parabacteroides distasonis**, Bacteroides dorei*, Alistipes putredinis, Alistipes onderdonkii, Bacteroides massiliensis, Bacteroides vulgatus, Bacteroides thetaiotaomicron Bacteroides caccae, Bacteroides clarus |
| Case #25 | Bacteroides uniformis**, Parabacteroides distasonis**, Bacteroides dorei*, Bacteroides massiliensis, Bacteroides vulgatus, Bacteroides thetaiotaomicron |
| Case #29 | Bacteroides uniformis**, Parabacteroides distasonis**, Alistipes putredinis, Alistipes onderdonkii*, Parabacteroides merdae |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okahara, K.; Ishikawa, D.; Nomura, K.; Ito, S.; Haga, K.; Takahashi, M.; Shibuya, T.; Osada, T.; Nagahara, A. Matching between Donors and Ulcerative Colitis Patients Is Important for Long-Term Maintenance after Fecal Microbiota Transplantation. J. Clin. Med. 2020, 9, 1650. https://doi.org/10.3390/jcm9061650
Okahara K, Ishikawa D, Nomura K, Ito S, Haga K, Takahashi M, Shibuya T, Osada T, Nagahara A. Matching between Donors and Ulcerative Colitis Patients Is Important for Long-Term Maintenance after Fecal Microbiota Transplantation. Journal of Clinical Medicine. 2020; 9(6):1650. https://doi.org/10.3390/jcm9061650
Chicago/Turabian StyleOkahara, Koki, Dai Ishikawa, Kei Nomura, Shoko Ito, Keiichi Haga, Masahito Takahashi, Tomoyoshi Shibuya, Taro Osada, and Akihito Nagahara. 2020. "Matching between Donors and Ulcerative Colitis Patients Is Important for Long-Term Maintenance after Fecal Microbiota Transplantation" Journal of Clinical Medicine 9, no. 6: 1650. https://doi.org/10.3390/jcm9061650
APA StyleOkahara, K., Ishikawa, D., Nomura, K., Ito, S., Haga, K., Takahashi, M., Shibuya, T., Osada, T., & Nagahara, A. (2020). Matching between Donors and Ulcerative Colitis Patients Is Important for Long-Term Maintenance after Fecal Microbiota Transplantation. Journal of Clinical Medicine, 9(6), 1650. https://doi.org/10.3390/jcm9061650






