Retinal Vascular Changes in Radiation Maculopathy after Intravitreal Ranibizumab by Optical Coherence Tomography Angiography
Abstract
1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Optical Coherence Tomography Angiography
2.3. Statistical Analysis
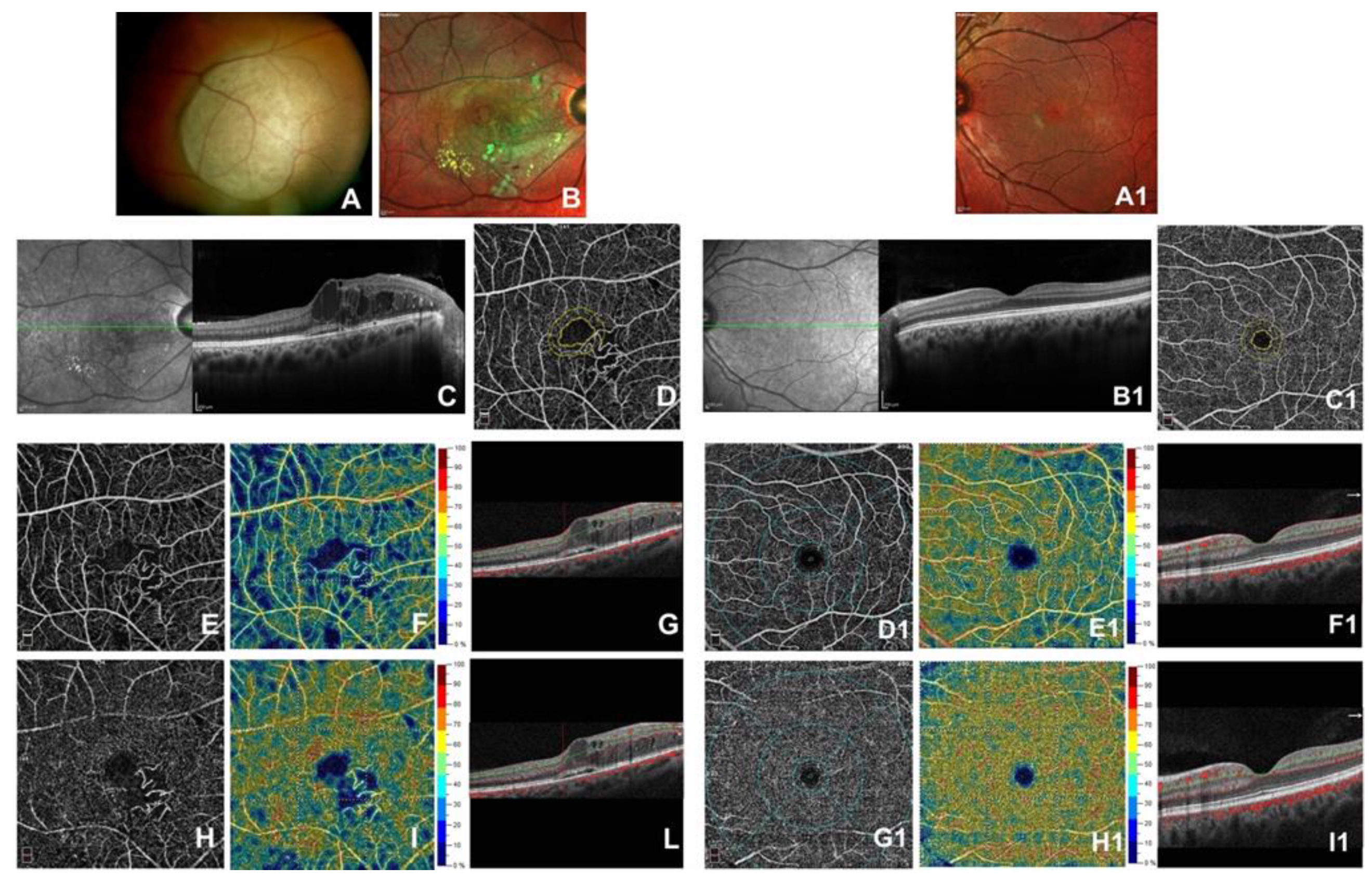
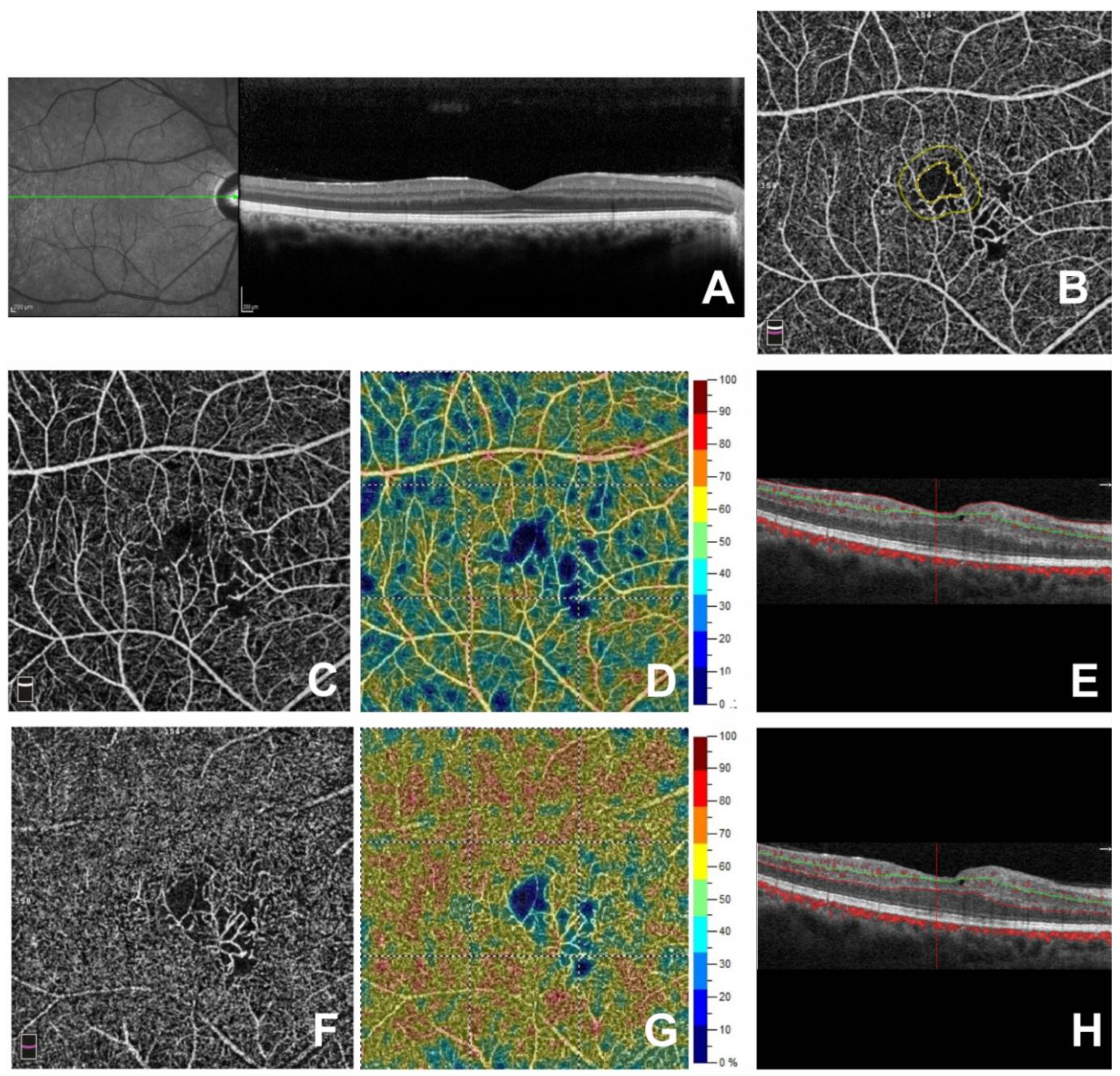
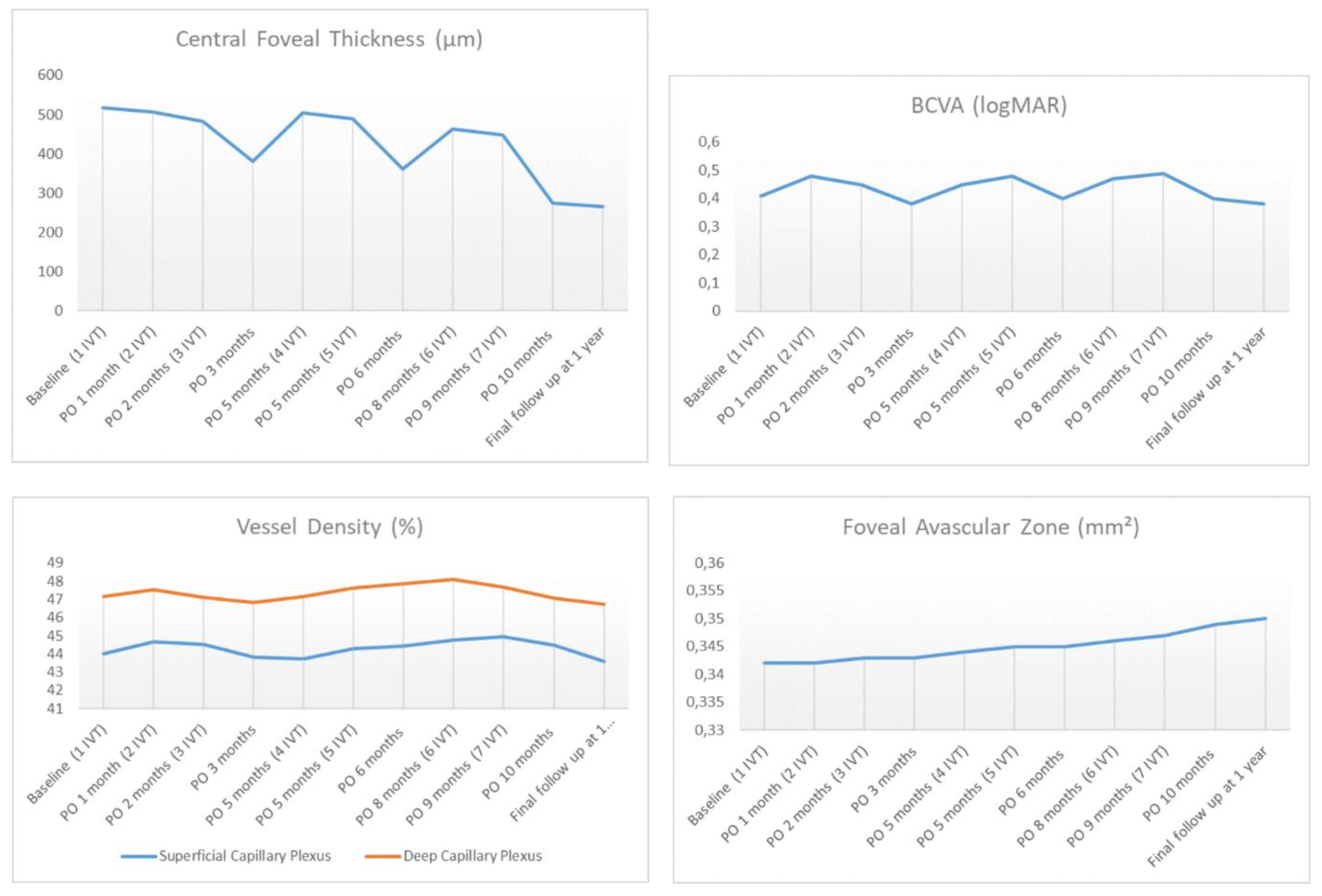
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Murray, T.G.; Latiff, A.; Villegas, V.M.; Gold, A.S. Aflibercept for Radiation Maculopathy Study: A Prospective, Randomized Clinical Study. Ophthalmol. Retin. 2019, 3, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Daruich, A.; Matet, A.; Schalenbourg, A.; Zografos, L. Intravitreal anti–vascular endothelial growth factor treatment at 2-month intervals reduces foveal avascular zone enlargement and vision loss in radiation maculopathy. Retina 2019, 39, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Cater, J.; Gunduz, K.; Miyamoto, C.; Micaily, B.; Brady, L.W. Plaque Radiotherapy for Uveal MelanomaLong-term Visual Outcome in 1106 Consecutive Patients. Arch. Ophthalmol. 2000, 118, 1219. [Google Scholar] [CrossRef] [PubMed]
- Horgan, N.; Shields, C.L.; Mashayekhi, A.; Teixeira, L.F.; Materin, M.A.; Shields, J.A. Early macular morphological changes following plaque radiotherapy for uveal melanoma. Retina 2008, 28, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.R.; Tan, D.; Bunce, C.; Gittos, A.; Neale, M.H.; Hungerford, J.L.; Charnock-Jones, S.; A Cree, I.; Charnock-Jones, D.S. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: Identification of a potential therapeutic window. Br. J. Ophthalmol. 2002, 86, 448–452. [Google Scholar] [CrossRef]
- Mashayekhi, A.; Schönbach, E.; Shields, C.L.; Shields, J.A. Early Subclinical Macular Edema in Eyes with Uveal Melanoma: Association with Future Cystoid Macular Edema. Ophthalmology 2015, 122, 1023–1029. [Google Scholar] [CrossRef]
- Horgan, N.; Shields, C.L.; Mashayekhi, A.; A Shields, J. Classification and treatment of radiation maculopathy. Curr. Opin. Ophthalmol. 2010, 21, 233–238. [Google Scholar] [CrossRef]
- Cennamo, G.; Montorio, D.; Mirra, F.; Comune, C.; D’Alessandro, A.; Tranfa, F. Study of vessel density in adult-onset foveomacular vitelliform dystrophy with Optical Coherence Tomography Angiography. Photodiagnosis Photodyn. Ther. 2020, 29, 101702. [Google Scholar] [CrossRef]
- Huang, D.; Jia, Y.; Gao, S.S.; Lumbroso, B.; Rispoli, M. Optical Coherence Tomography Angiography Using the Optovue Device. Dev. Ophthalmol. 2016, 56, 6–12. [Google Scholar] [CrossRef]
- Cennamo, G.; Montorio, D.; Comune, C.; Clemente, L.; Iovino, C.; Carandente, R.; Tranfa, F. Study of vessel density by optical coherence tomography angiography in patients with central serous chorioretinopathy after low-fluence photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 17, 101742. [Google Scholar] [CrossRef]
- Moein, H.-R.; Novais, E.A.; Rebhun, C.B.; Cole, E.D.; Louzada, R.N.; Witkin, A.J.; Baumal, C.R.; Duker, J.S.; Waheed, N.K. Optical coherence tomography angiography to detect macular capillary ischemia in patients with inner retinal changes after resolved diabetic macular edema. Retina 2017, 38, 1. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, C.; Konstantinidis, L.; Damato, B. Effects of radiotherapy on uveal melanomas and adjacent tissues. Eye 2012, 27, 163–171. [Google Scholar] [CrossRef]
- Archerz, D.B.; Gardiner, T.A. Ionizing radiation and the retina. Curr. Opin. Ophthalmol. 1994, 5, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Guyer, D.R.; Mukai, S.; Egan, K.M.; Seddon, J.M.; Walsh, S.M.; Gragoudas, E. Radiation Maculopathy after Proton Beam Irradiation for Choroidal Melanoma. Ophthalmology 1992, 99, 1278–1285. [Google Scholar] [CrossRef]
- Thomas, K.A. Vascular Endothelial Growth Factor, a Potent and Selective Angiogenic Agent. J. Boil. Chem. 1996, 271, 603–606. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor. Eur. J. Cancer 1996, 32, 2413–2422. [Google Scholar] [CrossRef]
- Frizziero, L.; Parrozzani, R.; Midena, G.; Miglionico, G.; Vujosevic, S.; Pilotto, E.; Midena, E. Hyperreflective intraretinal spots in radiation macular edema on spectral domain optical coherence tomography. Retina 2016, 36, 1664–1669. [Google Scholar] [CrossRef]
- Cennamo, G.; Breve, M.A.; Velotti, N.; Sparnelli, F.; Iovino, C.; Farella, A.; Liuzzi, R.; De Crecchio, G.; Cennamo, G. Evaluation of Vascular Changes with Optical Coherence Tomography Angiography after Plaque Radiotherapy of Choroidal Melanoma. Ophthalmic Res. 2018, 60, 238–242. [Google Scholar] [CrossRef]
- Shields, C.L.; Say, E.A.T.; Samara, W.A.; Khoo, C.T.L.; Mashayekhi, A.; Shields, J.A. Optical coherence tomography angiography of the macula after plaque radiotherapy of choroidal melanoma. Retina 2016, 36, 1493–1505. [Google Scholar] [CrossRef]
- Matet, A.; Daruich, A.; Zografos, L. Radiation Maculopathy After Proton Beam Therapy for Uveal Melanoma: Optical Coherence Tomography Angiography Alterations Influencing Visual Acuity. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3851. [Google Scholar] [CrossRef]
- Sellam, A.; Coscas, F.; Rouic, L.L.-L.; Dendale, R.; Lupidi, M.; Coscas, G.; Desjardins, L.; Cassoux, N. Optical Coherence Tomography Angiography of Macular Features After Proton Beam Radiotherapy for Small Choroidal Melanoma. Am. J. Ophthalmol. 2017, 181, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Chu, Z.; Shahidzadeh, A.; Wang, R.K.; Puliafito, C.A.; Kashani, A.H. Quantifying Microvascular Density and Morphology in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T.; Chin, K.; Semenova, E.A. Intravitreal Anti-VEGF Therapy for Macular Radiation Retinopathy: A 10-Year Study. Eur. J. Ophthalmol. 2015, 26, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.; Reibaldi, M.; Avitabile, T.; Longo, A.; Bonfiglio, V.; Chronopoulos, A.; Caltabiano, R.; Spatola, C.; Russo, A. Intravitreal aflibercept for the treatment of radiation-induced macular edema after ruthenium 106 plaque radiotherapy for choroidal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1547–1554. [Google Scholar] [CrossRef]
- Caminal, J.M.; Flores-Moreno, I.; Arias, L.; Gutierrez, C.; Piulats, J.M.; Catala, J.; Rubio, M.J.; Cobos, E.; Garcia, P.; Pera, J.; et al. Intravitreal Dexamethasone Implant for Radiation Maculopathy Secondary to Plaque Brachytherapy in Choroidal Melanoma. Retina 2015, 35, 1890–1897. [Google Scholar] [CrossRef]
- Baillif, S.; Maschi, C.; Gastaud, P.; Caujolle, J.-P. Intravitreal dexamethasone 0.7-mg implant for radiation macular edema after proton beam therapy for choroidal melanoma. Retina 2013, 33, 1784–1790. [Google Scholar] [CrossRef]
- Shields, C.L.; Demirci, H.; Dai, V.; Marr, B.P.; Mashayekhi, A.; Materin, M.A.; Manquez, M.E.; Shields, J.A. Intravitreal Triamcinolone Acetonide for Radiation Maculopathy after Plaque Radiotherapy for Choroidal Melanoma. Retina 2005, 25, 868–874. [Google Scholar] [CrossRef]
- Russo, A.; Reibaldi, M.; Avitabile, T.; Uva, M.G.; Franco, L.M.; Gagliano, C.; Bonfiglio, V.; Spatola, C.; Privitera, G.; Longo, A. Dexamethasone intravitreal implant vs ranibizumab in the treatment of macular edema secondary to brachytherapy for choroidal melanoma. Retina 2018, 38, 788–794. [Google Scholar] [CrossRef]
- Say, E.A.T.; Samara, W.A.; Khoo, C.T.L.; Magrath, G.; Sharma, P.; Ferenczy, S.; Shields, C.L. Parafoveal Capillary Density after Plaque Radiotherapy for Choroidal Melanoma. Retina 2016, 36, 1–1678. [Google Scholar] [CrossRef]
- Balaratnasingam, C.; Inoue, M.; Ahn, S.; McCann, J.; Dhrami-Gavazi, E.; Yannuzzi, L.A.; Freund, K.B. Visual Acuity Is Correlated with the Area of the Foveal Avascular Zone in Diabetic Retinopathy and Retinal Vein Occlusion. Ophthalmology 2016, 123, 2352–2367. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.C.; Kvanta, A.; Amrén, U.; Epstein, D. Optical Coherence Tomography Angiography in Central Retinal Vein Occlusion: Correlation Between the Foveal Avascular Zone and Visual Acuity. Investig. Ophthalmol. Vis. Sci. 2016, 57, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Samara, W.A.; Shahlaee, A.; Sridhar, J.; Khan, M.A.; Ho, A.C.; Hsu, J. Quantitative Optical Coherence Tomography Angiography Features and Visual Function in Eyes With Branch Retinal Vein Occlusion. Am. J. Ophthalmol. 2016, 166, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Sato, T.; Hara-Ueno, C.; Fukushima, Y.; Sayanagi, K.; Shiraki, N.; Sawa, M.; Ikuno, Y.; Sakaguchi, H.; Nishida, K. Retinal Microvasculature and Visual Acuity in Eyes With Branch Retinal Vein Occlusion: Imaging Analysis by Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2087–2094. [Google Scholar] [CrossRef]
| Eyes (n) | 40 |
|---|---|
| Sex (Male/Female) | 22/18 |
| Age (years; mean ± SD) | 51.9 ± 11 |
| Tumor Thickness before brachytheraphy (mm) | 3.22 ± 0.5 |
| Tumor Thickness 1 months after brachytheraphy (mm) | 1.87 ± 0.5 |
| Mean Radiation Zone (Gy) | |
| Tumor apex | 100 |
| Tumor base | 152.93 ± 53.85 |
| Fovea | 11.73 ± 16.39 |
| Optic disc | 9.68 ± 9.10 |
| Time plaque-RM (years) | 1.9 ± 0.3 |
| Intravitreal injections (n.) | 7 ± 1 |
| Follow up (months) | 11 ± 2 |
| Eyes with RM | Fellow Eyes | p-Value | |
|---|---|---|---|
| OCT Parameters (%) | |||
| Superficial Capillary Plexus | |||
| Whole | 44.03 ± 2.43 | 50.47 ± 3.28 | <0.001 |
| Parafovea (Inner ring) | 46.36 ± 2.34 | 52.27 ± 3.80 | <0.001 |
| Fovea (Center ring) | 24.12 ± 3.03 | 33.17 ± 3.73 | <0.001 |
| Deep Capillary Plexus | |||
| Whole | 47.15 ± 3.03 | 56.72 ± 3.59 | <0.001 |
| Parafovea (Inner ring) | 49.66 ± 3.59 | 58.02 ± 3.46 | <0.001 |
| Fovea (Center ring) | 31.09 ± 2.58 | 41.37 ± 3.87 | <0.001 |
| Foveal Avascular Zone (mm²) | 0.341 ± 0.07 | 0.211 ± 0.04 | <0.001 |
| CFT (µm) | 517.40 ± 43.96 | 264.05 ± 49.27 | <0.001 |
| BCVA (logMAR) | 0.41 ± 0.08 | 0.10 ± 0.08 | <0.001 |
| Baseline | 1-Year Follow Up | p-Value | |
|---|---|---|---|
| OCT Parameters (%) | |||
| Superficial Capillary Plexus | |||
| Whole | 44.03 ± 2.43 | 43.58 ± 2.85 | 0.402 |
| Parafovea (Inner ring) | 46.36 ± 2.34 | 46.03 ± 2.73 | 0.363 |
| Fovea (Center ring) | 24.12 ± 3.03 | 23.89 ± 2.93 | 0.740 |
| Deep Capillary Plexus | |||
| Whole | 47.15 ± 3.03 | 46.75 ± 3.24 | 0.282 |
| Parafovea (Inner ring) | 49.66 ± 3.59 | 49.71 ± 3.37 | 0.935 |
| Fovea (Center ring) | 31.09 ± 2.58 | 30.12 ± 2.83 | 0.109 |
| Foveal Avascular Zone (mm²) | 0.341 ± 0.07 | 0.354 ± 0.04 | 0.255 |
| CFT (µm) | 517.40 ± 43.96 | 265.7 ± 31.55 | <0.001 |
| BCVA (logMAR) | 0.41 ± 0.08 | 0.42 ± 0.07 | 0.210 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cennamo, G.; Montorio, D.; Bernardo, R.; Farella, A.; Liuzzi, R.; Breve, M.A.; Reibaldi, M.; Cennamo, G. Retinal Vascular Changes in Radiation Maculopathy after Intravitreal Ranibizumab by Optical Coherence Tomography Angiography. J. Clin. Med. 2020, 9, 1618. https://doi.org/10.3390/jcm9061618
Cennamo G, Montorio D, Bernardo R, Farella A, Liuzzi R, Breve MA, Reibaldi M, Cennamo G. Retinal Vascular Changes in Radiation Maculopathy after Intravitreal Ranibizumab by Optical Coherence Tomography Angiography. Journal of Clinical Medicine. 2020; 9(6):1618. https://doi.org/10.3390/jcm9061618
Chicago/Turabian StyleCennamo, Gilda, Daniela Montorio, Roberta Bernardo, Antonio Farella, Raffaele Liuzzi, Maria Angelica Breve, Michele Reibaldi, and Giovanni Cennamo. 2020. "Retinal Vascular Changes in Radiation Maculopathy after Intravitreal Ranibizumab by Optical Coherence Tomography Angiography" Journal of Clinical Medicine 9, no. 6: 1618. https://doi.org/10.3390/jcm9061618
APA StyleCennamo, G., Montorio, D., Bernardo, R., Farella, A., Liuzzi, R., Breve, M. A., Reibaldi, M., & Cennamo, G. (2020). Retinal Vascular Changes in Radiation Maculopathy after Intravitreal Ranibizumab by Optical Coherence Tomography Angiography. Journal of Clinical Medicine, 9(6), 1618. https://doi.org/10.3390/jcm9061618






