Inadequate Weight Gain According to the Institute of Medicine 2009 Guidelines in Women with Gestational Diabetes: Frequency, Clinical Predictors, and the Association with Pregnancy Outcomes
Abstract
1. Introduction
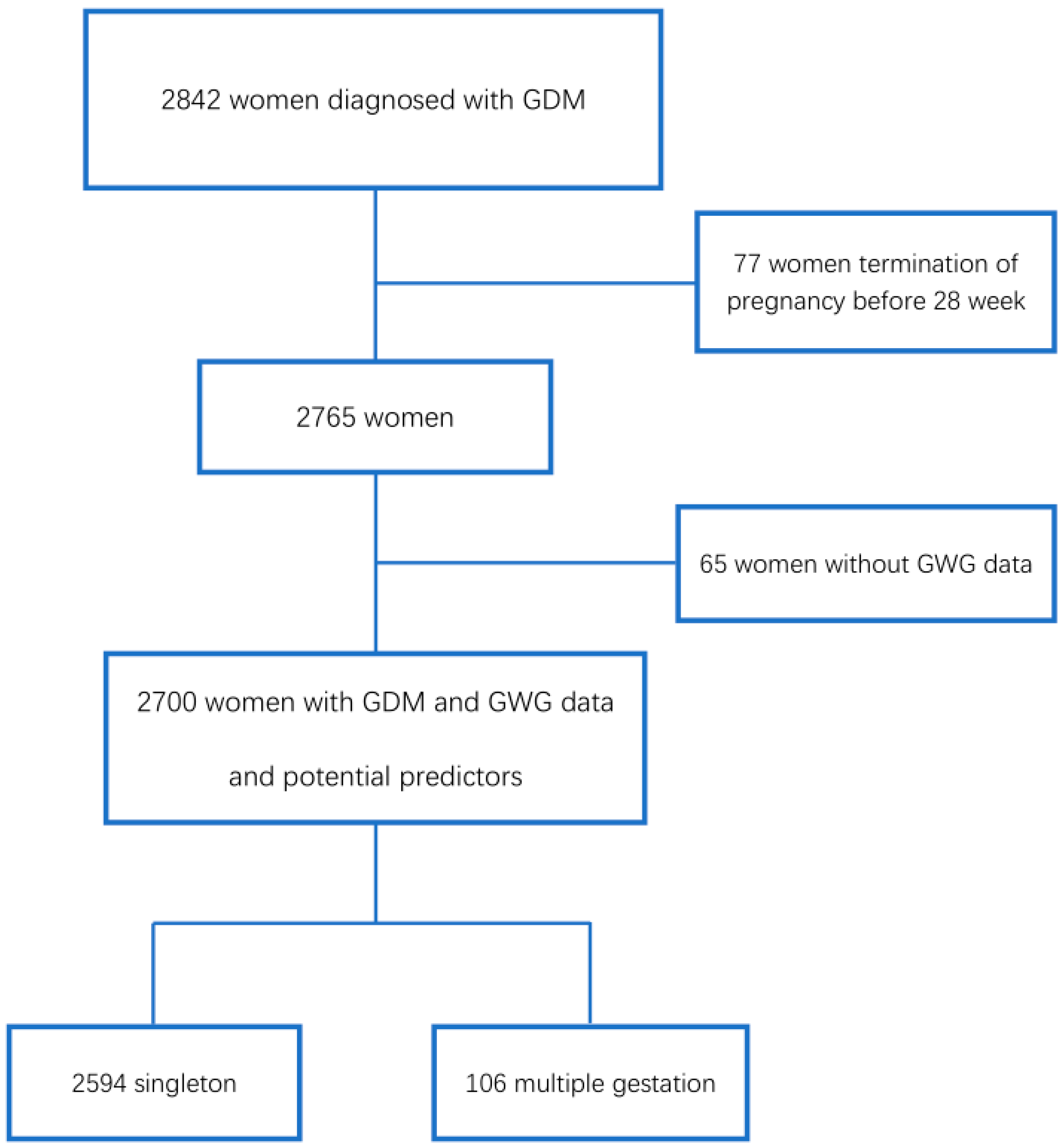
2. Materials and Methods
2.1. Study Design
2.2. Variables Collected
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Prevalence and of Inadequate GWG
4.2. Variables Independently Associated with Inadequate GWG
4.2.1. Ethnicity
4.2.2. Maternal Anthropometry
4.2.3. Unfavorable Obstetric History
4.2.4. Smoking
4.2.5. Gestational Age at Delivery and Length of Follow-up
4.3. Inadequate GWG and Pregnancy Outcomes
4.3.1. PIH and Preeclampsia
4.3.2. Cesarean Delivery
4.3.3. LGA, Macrosomia and SGA
4.4. Is iGWG Satisfactory in Women with GDM?
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Pre-Pregnancy BMI Category (kg/m2) | Recommended Range of GWG During Pregnancy (kg) | |
|---|---|---|
| Singleton Pregnancy | Multiple Pregnancy | |
| Underweight (BMI < 18.5) | 12.5–18.0 | |
| Normal weight (BMI = 18.5–24.9) | 11.5–16.0 | 16.8–24.5 |
| Overweight (BMI = 25.0–29.9) | 7.0–11.5 | 14.1–22.7 |
| Obesity (BMI ≥ 30) | 5.0–9.0 | 11.3–19.1 |
Appendix B

References
- ADA. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes 2020. Diabetes Care 2020, 43, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Albareda, M.; Caballero, A.; Badell, G.; Piquer, S.; Ortiz, A.; De Leiva, A.; Corcoy, R. Diabetes and abnormal glucose tolerance in women with previous gestational diabetes. Diabetes Care 2003, 26, 1199–1205. [Google Scholar] [CrossRef]
- Wendland, E.M.; Torloni, M.R.; Falavigna, M.; Trujillo, J.; Dode, M.A.; Campos, M.A.; Duncan, B.B.; Schmidt, M.I. Gestational diabetes and pregnancy outcomes—A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Sendag, F.; Terek, M.C.; Itil, I.M.; Oztekin, K.; Bilgin, O. Maternal and perinatal outcomes in women with gestational diabetes mellitus as compared to nondiabetic controls. J. Reprod. Med. 2001, 46, 1057–1062. [Google Scholar]
- Abenhaim, H.A.; Kinch, R.A.; Morin, L.; Benjamin, A.; Usher, R. Effect of prepregnancy body mass index categories on obstetrical and neonatal outcomes. Arch. Gynecol. Obstet. 2007, 275, 39–43. [Google Scholar] [CrossRef]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Ricart, W.; López, J.; Mozas, J.; Pericot, A.; Sancho, M.A.; González, N.; Balsells, M.; Luna, R.; Cortázar, A.; Navarro, P.; et al. Body mass index has a greater impact on pregnancy outcomes than gestational hyperglycaemia. Diabetologia 2005, 48, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Durmuş, B.; Hofman, A.; MacKenbach, J.P.; Steegers, E.A.P.; Jaddoe, V.W.V. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity 2013, 21, 1046–1055. [Google Scholar] [CrossRef]
- Haugen, M.; Brantsæter, A.L.; Winkvist, A.; Lissner, L.; Alexander, J.; Oftedal, B. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: A prospective observational cohort study. BMC Pregnancy Childbirth 2014, 201. [Google Scholar] [CrossRef]
- Viswanathan, M.; Siega-Riz, A.M.; Moos, M.K.; Deierlein, A.; Mumford, S.; Knaack, J.; Thieda, P.; Lux, L.J.; Lohr, K.N. Outcomes of maternal weight gain. Evid Rep. Technol. Assess. Full. Rep. 2008, 1–223. [Google Scholar]
- Siega-Riz, A.M.; Viswanathan, M.; Moos, M.K.; Deierlein, A.; Mumford, S.; Knaack, J.; Thieda, P.; Lux, L.J.; Lohr, K.N. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: Birthweight, fetal growth, and postpartum weight retention. Am. J. Obstet. Gynecol. 2009, 201, 339.e1–339.e14. [Google Scholar] [CrossRef] [PubMed]
- Viecceli, C.; Remonti, L.R.; Hirakata, V.N.; Mastella, L.S.; Gnielka, V.; Oppermann, M.L.R.; Silveiro, S.P.; Reichelt, A.J. Weight gain adequacy and pregnancy outcomes in gestational diabetes: A meta-analysis. Obes. Rev. 2017, 18, 567–580. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Weight gain during pregnancy. Obstet. Gynecol. 2013, 121, 171–173. [Google Scholar]
- Stewart, Z.A.; Wallace, E.M.; Allan, C. A Patterns of weight gain in pregnant women with and without gestational diabetes mellitus: An observational study. Aust. N. Z. J. Obstet. Gynaecol. 2012. [Google Scholar] [CrossRef]
- Egan, A.M.; Dennedy, M.C.; Al-Ramli, W.; Heerey, A.; Avalos, G.; Dunne, F. ATLANTIC-DIP: Excessive gestational weight gain and pregnancy outcomes in women with gestational or pregestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2014, 99, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Barquiel, B.; Herranz, L.; Meneses, D.; Moreno, Ó.; Hillman, N.; Burgos, M.Á.; Bartha, J.L. Optimal Gestational Weight Gain for Women with Gestational Diabetes and Morbid Obesity. Matern. Child. Health J. 2018, 22, 1297–1305. [Google Scholar] [CrossRef]
- Berglund, S.K.; García-Valdés, L.; Torres-Espinola, F.J.; Segura, M.T.; Martínez-Zaldívar, C.; Aguilar, M.J.; Agil, A.; Lorente, J.A.; Florido, J.; Padilla, C.; et al. Maternal, fetal and perinatal alterations associated with obesity, overweight and gestational diabetes: An observational cohort study (PREOBE). BMC Public Health 2016, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Barquiel, B.; Herranz, L.; Hillman, N.; Burgos, M.Á.; Grande, C.; Tukia, K.M.; Bartha, J.L.; Pallardo, L.F. HbA1c and gestational weight gain are factors that influence neonatal outcome in mothers with gestational diabetes. J. Women’s Health 2016, 25, 579–585. [Google Scholar] [CrossRef]
- Ricart, W.; López, J.; Mozas, J.; Pericot, A.; Sancho, M.A.; González, N.; Balsells, M.; Luna, R.; Cortázar, A.; Navarro, P.; et al. Potential impact of American Diabetes Association (2000) criteria for diagnosis of gestational diabetes mellitus in Spain. Diabetologia 2005, 48, 1135–1141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acosta, D.; Balsells, M.; Ballesteros, M.; Bandres, M.O.; Bartha, J.L.; Bellart, J.; Chico, A.I.; Codina, M.; Corcoy, R.; Cortázar, A.; et al. Asistencia a la gestante con diabetes. Guía de práctica clínica actualizada en 2014. Av. Diabetol. 2015, 31, 45–59. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. NICE Intrapartum Care: Care of Healthy Women and Their Babies During Childbirth Guidance and Guidelines. 2014. Available online: www.nice.org.uk/guidance/cg190 (accessed on 15 December 2014).
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 644: The Apgar Score. Obstet. Gynecol. 2015, 126, e52–e55. [CrossRef] [PubMed]
- Yeh, P.; Emary, K.; Impey, L. The relationship between umbilical cord arterial pH and serious adverse neonatal outcome: Analysis of 51 519 consecutive validated samples. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Santamaria Lozano, R.; Verdú Martín, L.; Martín Caballero, C.; García López, G. Tablas Españolas de Pesos Neonatales Según Edad Gestacional; Ed. Artes Gráficas Beatulo: Badalona, Spain, 1998; Available online: https//www.menarini.es/aviso-legal/509-salud/areas-terapeuticas/ginecologia/3073-tablas-espanolas-de-pesos-neonatales.html (accessed on 15 June 2018).
- Cornblath, M.; Schwartz, R. Hypoglycemia in the neonate. J. Pediatr. Endocrinol. 1993, 6, 113–129. [Google Scholar] [CrossRef]
- Maisels, M.J. Bilirubin: On understanding and influencing its metabolism in the newborn infant. Pediatr. Clin. N. Am. 1972, 19, 447–501. [Google Scholar] [CrossRef]
- Edwards, M.O.; Kotecha, S.J.; Kotecha, S. Respiratory Distress of the Term Newborn Infant. Paediatr. Respir. Rev. 2013, 14, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Loughead, J.L.; Mimouni, F.; Tsang, R.C. Serum Ionized Calcium Concentrations in Normal Neonates. Am. J. Dis. Child. 1988, 142, 516–518. [Google Scholar] [CrossRef]
- Kates, E.H.; Kates, J.S. Anemia and polycythemia in the newborn. Pediatr. Rev. 2007, 28, 33–34. [Google Scholar] [CrossRef]
- Wong, T.; Barnes, R.A.; Ross, G.P.; Cheung, N.W.; Flack, J.R. Are the Institute of Medicine weight gain targets applicable in women with gestational diabetes mellitus? Diabetologia 2017, 60, 416–423. [Google Scholar] [CrossRef]
- Headen, I.E.; Davis, E.M.; Mujahid, M.S.; Abrams, B. Racial-Ethnic Differences in Pregnancy-Related Weight. Adv. Nutr. 2012, 3, 83–94. [Google Scholar] [CrossRef]
- Cheng, H.R.; Walker, L.O.; Brown, A.; Lee, J.Y. Gestational Weight Gain and Perinatal Outcomes of Subgroups of Asian-American Women, Texas, 2009. Women’s Health Issues 2015, 25, 303–311. [Google Scholar] [CrossRef]
- Straube, S.; Voigt, M.; Briese, V.; Schneider, K.T.M.; Voigt, M. Weight gain in pregnancy according to maternal height and weight. J. Perinat. Med. 2008, 36, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Khanolkar, A.R.; Hanley, G.E.; Koupil, I.; Janssen, P.A. 2009 IOM guidelines for gestational weight gain: How well do they predict outcomes across ethnic groups? Ethn. Health 2017, 25, 1–16. [Google Scholar] [CrossRef]
- Lindberg, S.; Anderson, C.; Pillai, P.; Tandias, A.; Arndt, B.; Hanrahan, L. Prevalence and predictors of unhealthy weight gain in pregnancy. Wis. Med. J. 2016, 115, 233–237. [Google Scholar]
- Chiolero, A.; Faeh, D.; Paccaud, F.; Cornuz, J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am. J. Clin. Nutr. 2008, 87, 801–809. [Google Scholar] [CrossRef]
- Hulman, A.; Lutsiv, O.; Park, C.K.; Krebs, L.; Beyene, J.; McDonald, S.D. Are women who quit smoking at high risk of excess weight gain throughout pregnancy? BMC Pregnancy Childbirth 2016, 16, 263. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Kasai, J.; Nakanishi, S.; Saigusa, Y.; Miyagi, E.; Aoki, S. Should the IADPSG criteria be applied when diagnosing early-onset gestational diabetes? Diabetes Res. Clin. Pract. 2018, 140, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Hillier, T.A.; Ogasawara, K.K.; Pedula, K.L.; Vesco, K.K.; Oshiro, C.E.S.; Van Marter, J.L. Timing of Gestational Diabetes Diagnosis by Maternal Obesity Status: Impact on Gestational Weight Gain in a Diverse Population. J. Women’s Health 2020, 29, 1–9. [Google Scholar] [CrossRef]
- Fortner, R.T.; Pekow, P.; Solomon, C.G.; Markenson, G.; Chasan-Taber, L. Prepregnancy body mass index, gestational weight gain, and risk of hypertensive pregnancy among Latina women. Am. J. Obstet. Gynecol. 2009, 200, 167.e1–167.e7. [Google Scholar] [CrossRef]
- Macdonald-Wallis, C.; Tilling, K.; Fraser, A.; Nelson, S.M.; Lawlor, D.A. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2013, 209, 327.e1–327.e17. [Google Scholar] [CrossRef]
- Gonzalez-Ballano, I.; Saviron-Cornudella, R.; Esteban, L.M.; Sanz, G.; Castán, S. Pregestational body mass index, trimester-specific weight gain and total gestational weight gain: How do they influence perinatal outcomes? J. Matern. Neonatal Med. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Zhou, A.; Cao, Z.; Zhang, Y.; Qiu, L.; Yao, C.; Wang, Y.; Zhang, B. Association of pre-pregnancy body mass index, gestational weight gain with cesarean section in term deliveries of China. Sci. Rep. 2016, 6, 37168. [Google Scholar] [CrossRef] [PubMed]
- Drehmer, M.; Duncan, B.B.; Kac, G.; Schmidt, M.I. Association of Second and Third Trimester Weight Gain in Pregnancy with Maternal and Fetal Outcomes. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Harvey, M.W.; Braun, B.; Ertel, K.A.; Pekow, P.S.; Markenson, G.; Chasan-Taber, L. Prepregnancy Body Mass Index, Gestational Weight Gain, and Odds of Cesarean Delivery in Hispanic Women. Obesity 2018, 26, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA J. Am. Med. Assoc. 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Vuralli, D. Clinical Approach to Hypocalcemia in Newborn Period and Infancy: Who Should Be Treated? Int. J. Pediatr. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gou, B.; Guan, H.; Bi, Y.; Ding, B. Gestational diabetes: Weight gain during pregnancy and its relationship to pregnancy outcomes. Chin. Med. J. (Engl.) 2019, 6–11. [Google Scholar] [CrossRef]
- Jiang-nan, W.; Wei-rong, G.; Xi-rong, X.; Yi, Z.; Xiao-tian, L.; Chuan-Min, Y. Gestational weight gain targets during the second and third trimesters of pregnancy for women with gestational diabetes mellitus in China. Eur. J. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Rizzo, T.; Metzger, B.; Burns, W.; Burns, K. Correlations between antepartum maternal metabolism and intelligence of offspring. N. Engl. J. Med. 1991, 325, 911–916. [Google Scholar] [CrossRef]
- Metzger, B.E.; Buchanan, T.A.; Coustan, D.R.; De Leiva, A.; Dunger, D.B.; Hadden, D.R.; Hod, M.; Kitzmiller, J.L.; Kjos, S.L.; Oats, J.N.; et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007, 30. [Google Scholar] [CrossRef] [PubMed]
- Corcoy, R.; Codina, M.; Cerqueira, M.J.; Rectoret, G.; Cervera, T.; Cabero, L.; de Leiva, A. Intensive treatment of pregnancy diabetes: Clinical course in 100 patients. Rev. Clin. Esp. 1988, 183, 344–348. [Google Scholar] [PubMed]
- Montaner, P.; Ripollés, J.; Pamies, C.; Corcoy, R. Measurement of fasting ketonuria and capillary blood glucose after main meals in women with gestational diabetes mellitus: How well is the metabolic picture captured? J. Obstet. Gynaecol. Res. 2011, 37, 722–728. [Google Scholar] [CrossRef]
- Tam, C.H.T.; Ma, R.C.W.; Yuen, L.Y.; Ozaki, R.; Li, A.M.; Hou, Y.; Chan, M.H.M.; Ho, C.S.; Yang, X.; Chan, J.C.N.; et al. The impact of maternal gestational weight gain on cardiometabolic risk factors in children. Diabetologia 2018, 61, 2539–2548. [Google Scholar] [CrossRef]
- Jane, L.T.; Aiken, C.E.; Ozanne, S.E. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002848. [Google Scholar]
- Salomaki, H.; Vahatalo, L.H.; Kirsti, L.; Norma, T.J.; Penttinen, A.-M.; Ailanen, L.; Ilyasizadeh, J.; Pesonen, U.; Koulu, M. Prenatal Metformin Exposure in Mice Programs the Metabolic Phenotype of the Offspring during a High Fat Diet at Adulthood. PLoS ONE 2013, 8, e56594. [Google Scholar] [CrossRef]
- Grotenfelt, N.E.; Wasenius, N.; Eriksson, J.G.; Huvinen, E.; Stach-lempinen, B.; Koivusalo, S.B.; Rönö, K. Effect of maternal lifestyle intervention on metabolic health and adiposity of offspring: Findings from the Finnish Gestational Diabetes Prevention Study (RADIEL). Diabetes Metab. 2019, 46, 46–53. [Google Scholar] [CrossRef]
- Rasmussen, K.; Yaktine, A. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain Pregnancy Reexamining Guidel. 2009. [Google Scholar] [CrossRef]

| Characteristic | % or P50 (P25, P75) in Each Weight Gain Category | p within IOM Categories * | |||
|---|---|---|---|---|---|
| Overall | Weight Gain < IOM | Weight Gain within IOM | Weight Gain > IOM | ||
| Non-Caucasian ethnicity (%) | 5.5 | 4.4 | 3.8 | 11.3 | <0.001 |
| Age (years) | 33.0 (29.0; 36.0) | 33.0 (29.0; 36.0) | 33.0 (30.0; 36.0) | 33.0 (29.0; 36.0) | 0.694 |
| Height (cm) | 160 (155; 164) | 159 (155; 163) | 160 (156; 164) | 160 (156; 164) | 0.002 |
Prepregnancy BMI category (%)
| 2.6 63.1 23.5 10.7 | 2.7 77.9 12.3 7.1 | 3.4 58.5 28.8 9.3 | 1.0 30.1 45.5 23.4 | <0.001 |
| Family history of diabetes (%) | 56.4 | 54.4 | 57.7 | 59.8 | 0.083 |
| Prior history of abnormal glucose tolerance/gestational diabetes mellitus (%) | 13.7 | 13.5 | 13.5 | 14.7 | 0.783 |
| Prior pregnancy (%) | 62.3 | 60.0 | 64.6 | 65.0 | 0.041 |
| Unfavorable obstetric history (%) | 12.8 | 9.3 | 15.3 | 18.4 | <0.001 |
| Multiple pregnancy (%) | 3.9 | 5.7 | 2.3 | 1..6 | <0.001 |
Smoking habit during pregnancy
| 11.5 23.4 | 9.5 20.8 | 12.2 24.7 | 15.8 28.2 | <0.001 |
Season at gestational diabetes mellitus diagnosis
| 31.0 25.2 19.3 | 30.6 24.6 20.1 | 30.7 26.7 17.6 | 32.4 24.2 20.1 | 0.681 |
| Gestational age at diagnosis of gestational diabetes mellitus (weeks) | 29 (26; 33) | 29 (25; 33) | 30 (26; 33) | 29 (26; 34) | 0.008 |
Glycemic values (mmol/L) at diagnosis
| 4.7 (4.3; 5.1) 11.6 (10.9; 12.5) 10.2 (9.6; 11.1) 7.8 (6.6; 8.8) | 4.6 (4.3; 5.0) 11.6 (10.9; 12.5) 10.2 (9.6; 11.1) 7.9 (6.8; 8.8) | 4.7 (4.31; 5.1) 11.6 (10.8; 12.4) 10.2 (9.6; 11.1) 7.9 (6.6; 8.8) | 4.9 (4.5; 5.4) 11.5 (10.9; 12.6) 10.2 (9.5; 11.1) 7.5 (6.1; 8.7) | <0.001 0.562 0.183 0.002 |
| Number of abnormal glucose values | 2 (2; 3) | 2 (2; 3) | 2 (2; 3) | 2 (2; 3) | 0.594 |
| Autoimmunity against beta cells (%) | 9.2 | 9.0 | 9.4 | 9.6 | 0.904 |
| Gestational age at delivery (weeks) | 39 (38;40) | 39 (38;40) | 39 (38;40) | 39 (38;40) | <0.001 |
| Length of follow-up (weeks) | 7 (3; 11) | 7 (4; 11) | 7 (4;10) | 6 (3;10) | 0.032 |
| Weight gain (kg) | 10.2 (7.7; 13.0) | 8.2 (5.7; 10.0) | 12.2 (9.5; 13.9) | 16.0 (13.0; 18.2) | <0.001 |
| Insufficient Weight Gain | Excessive Weight Gain | ||||||
|---|---|---|---|---|---|---|---|
| OR | p | 95% CI | Overall p | OR | p | 95% CI | |
| Non-Caucasian ethnicity (yes) | 1.182 | 0.500 | 0.727–1.922 | <0.001 | 3.283 | <0.001 | 1.984–5.433 |
| Height (cm) | 0.979 | 0.008 | 0.963–0.994 | 0.001 | 1.020 | 0.062 | 0.999–1.041 |
Prepregnancy BMI category
| 0.659 1 0.301 0.603 | 0.137 <0.001 0.005 | 0.381–1.142 0.235–0.387 0.425–0.857 | <0.001 | 0.487 1 3.190 5.060 | 0.191 < 0.001 < 0.001 | 0.165–1.433 2.403–4.234 3.472–7.375 |
| Unfavorable obstetric history (yes) | 0.520 | <0.001 | 0.387–0.700 | <0.001 | 0.865 | 0.399 | 0.618–1.211 |
Smoking habit
| 1 0.668 0.727 | 0.011 0.007 | 0.490–0.910 0.576–0.918 | <0.001 | 1 1.614 1.382 | 0.010 0.030 | 1.121–2.325 1.031–1.852 |
| Gestational age at delivery (weeks) | 0.855 | <0.001 | 0.806–0.907 | <0.001 | 1.026 | 0.527 | 0.947–1.113 |
| Length of follow-up (weeks) | 1.035 | <0.001 | 1.018–1.052 | <0.001 | 0.980 | 0.066 | 0.958–1.001 |
| Outcome | Prevalence (%) in Each GWG Category | Overall p | |||
|---|---|---|---|---|---|
| Overall | iGWG | aGWG | eGWG | ||
| Pregnancy-induced hypertension | 5.3 | 3.4 | 5.5 | 10.1 | <0.001 |
| Preeclampsia | 2.9 | 1.3 | 1.7 | 6.5 | 0.031 |
| Insulin treatment | 46.8 | 45.4 | 46.5 | 51.4 | 0.068 |
| Cesarean delivery | 24.1 | 19.9 | 23.4 | 36.8 | <0.001 |
| Fetal scalp blood pH < 7.25 | 3.8 | 3.1 | 4.0 | 5.3 | 0.077 |
| Preterm birth | 9.8 | 13.3 | 6.9 | 4.8 | <0.001 |
| Apgar at 5 min < 7 | 0.5 | 0.6 | 0.3 | 0.4 | 0.735 |
| Arterial pH < 7.1 | 3.8 | 3.5 | 3.1 | 6.0 | 0.035 |
| Obstetric trauma | 2.3 | 2.1 | 2.6 | 2.4 | 0.697 |
| LGA newborn | 11.2 | 6.4 | 10.7 | 26.1 | <0.001 |
| Macrosomia (≥ 4000 g) | 5.7 | 2.4 | 5.7 | 14.9 | <0.001 |
| SGA newborn | 9.9 | 11.7 | 9.3 | 5.9 | 0.001 |
| Neonatal hypoglycemia | 2.5 | 2.4 | 2.5 | 2.7 | 0.932 |
| Jaundice requiring treatment | 5.1 | 6.1 | 4.0 | 4.1 | 0.040 |
| Respiratory distress requiring treatment | 3.3 | 4.7 | 1.9 | 2.0 | <0.001 |
| Neonatal hypocalcemia | 1.6 | 2.5 | 1.0 | 0.0 | 0.009 |
| Neonatal polycythemia | 1.4 | 1.3 | 1.4 | 1.8 | 0.662 |
| Perinatal mortality | 0.5 | 0.6 | 0.5 | 0.2 | 0.603 |
| Outcome | Unadjusted OR Unadjusted CI 95% | Adjusted OR * Adjusted CI 95% | ||||||
|---|---|---|---|---|---|---|---|---|
| iGWG | aGWG | eGWG | Overall p | iGWG | aGWG | eGWG | Overall p | |
| Pregnancy-induced hypertension | 0.604 0.397–0.920 | 1 1 | 1.949 1.282–2.963 | <0.001 | 0.655 0.396–1.085 | 1 1 | 1.357 0.818–2.253 | 0.028 |
| Preeclampsia | 0.785 0.109–5.658 | 1 1 | 4.095 0.832–20.161 | 0.054 | 1.008 0.077–13.163 | 1 1 | 6.519 0.746–56.939 | <0.050 |
| Insulin treatment | 0.955 0.805–1.134 | 1 1 | 1.219 0.976–1.523 | 0.068 | -- | 1 1 | -- | ns |
| Cesarean delivery | 0.812 0.660–0.999 | 1 1 | 1.898 1.488–2.419 | <0.001 | 0.715 0.556–0.920 | 1 1 | 1.641 1.225–2.197 | <0.001 |
| Fetal scalp blood pH < 7.25 | 0.777 0.492–1.225 | 1 1 | 1.367 0.810–2.306 | 0.081 | -- | 1 1 | -- | ns |
| Preterm birth | 2.029 1.546–2.832 | 1 1 | 0.689 0.424–1.121 | <0.001 | -- | 1 1 | -- | ns |
| Apgar at 5 min < 7 | 1.647 0.436–6.226 | 1 1 | 1.182 0.197–7.097 | 0.739 | -- | 1 1 | -- | ns |
| Arterial pH < 7.1 | 1.138 0.672–1.927 | 1 1 | 2.000 1.113–3.595 | 0.038 | -- | 1 1 | -- | ns |
| Obstetric trauma | 0.793 0.453–1.389 | 1 1 | 0.949 0.466–1.936 | 0.698 | -- | 1 1 | -- | ns |
| LGA newborn | 0.575 0.425–0.777 | 1 1 | 2.952 2.200–3.962 | <0.001 | 0.569 0.400–0.810 | 1 1 | 2.003 1.397–2.871 | <0.001 |
| Macrosomia (≥4000g) | 0.412 0.265–0.640 | 1 1 | 2.893 1.983–4.220 | <0.001 | 0.461 0.282–0.752 | 1 1 | 1.822 1.152–2.881 | <0.001 |
| SGA newborn | 1.289 0.947–1.706 | 1 1 | 0.608 0.392–0.943 | 0.001 | 1.228 0.893–1.689 | 1 1 | 0.515 0.310–0.855 | 0.002 |
| Neonatal hypoglycemia | 0.951 0.547–1.656 | 1 1 | 1.076 0.534–2.168 | 0.932 | -- | 1 1 | -- | ns |
| Jaundice requiring treatment | 1.579 1.052–2.369 | 1 1 | 1.024 0.583–1.800 | 0.041 | -- | 1 1 | -- | ns |
| Respiratory distress requiring treatment | 2.558 1.472–4.447 | 1 1 | 1.086 0.489–2.413 | 0.001 | -- | 1 1 | -- | ns |
| Neonatal hypocalcemia | 2.529 0.952–6.720 | 1 1 | odd coefficient | 0.177 | 4.557 1.037–20.003 | 1 1 | odd coefficient | 0.133 |
| Neonatal polycythemia | 0.904 0.433–1.886 | 1 1 | 1.310 0.548–3.132 | 0.665 | -- | 1 1 | -- | ns |
| Perinatal mortality | 1.213 0.364–4.039 | 1 1 | 0.437 0.049–3.917 | 0.624 | -- | 1 1 | -- | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Liu, J.; Pujol, I.; López, A.; Martínez, M.J.; García-Patterson, A.; Adelantado, J.M.; Ginovart, G.; Corcoy, R. Inadequate Weight Gain According to the Institute of Medicine 2009 Guidelines in Women with Gestational Diabetes: Frequency, Clinical Predictors, and the Association with Pregnancy Outcomes. J. Clin. Med. 2020, 9, 3343. https://doi.org/10.3390/jcm9103343
Xie X, Liu J, Pujol I, López A, Martínez MJ, García-Patterson A, Adelantado JM, Ginovart G, Corcoy R. Inadequate Weight Gain According to the Institute of Medicine 2009 Guidelines in Women with Gestational Diabetes: Frequency, Clinical Predictors, and the Association with Pregnancy Outcomes. Journal of Clinical Medicine. 2020; 9(10):3343. https://doi.org/10.3390/jcm9103343
Chicago/Turabian StyleXie, Xinglei, Jiaming Liu, Isabel Pujol, Alicia López, María José Martínez, Apolonia García-Patterson, Juan M. Adelantado, Gemma Ginovart, and Rosa Corcoy. 2020. "Inadequate Weight Gain According to the Institute of Medicine 2009 Guidelines in Women with Gestational Diabetes: Frequency, Clinical Predictors, and the Association with Pregnancy Outcomes" Journal of Clinical Medicine 9, no. 10: 3343. https://doi.org/10.3390/jcm9103343
APA StyleXie, X., Liu, J., Pujol, I., López, A., Martínez, M. J., García-Patterson, A., Adelantado, J. M., Ginovart, G., & Corcoy, R. (2020). Inadequate Weight Gain According to the Institute of Medicine 2009 Guidelines in Women with Gestational Diabetes: Frequency, Clinical Predictors, and the Association with Pregnancy Outcomes. Journal of Clinical Medicine, 9(10), 3343. https://doi.org/10.3390/jcm9103343






