Activated Phosphoinositide 3-Kinase Delta Syndrome 1: Clinical and Immunological Data from an Italian Cohort of Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Genetic and Flow Cytometry Analysis
3. Results
3.1. Patients
3.2. Infections in APDS-1 Patients
3.3. Lymphoproliferation in APDS-1 Patients
3.4. Other Clinical Features
3.5. Immunological Features at Onset
3.6. PIK3CD Genetic Analysis and Phospo-S6 Kinase Assays
3.7. Therapeutic Strategies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lucas, C.L.; Kuehn, H.S.; Zhao, F.; Niemela, J.E.; Deenick, E.K.; Palendira, U.; Avery, D.T.; Moens, L.; Cannons, J.L.; Biancalana, M.; et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 2014, 15, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Angulo, I.; Vadas, O.; Garçon, F.; Banham-Hall, E.; Plagnol, V.; Leahy, T.R.; Baxendale, H.; Coulter, T.; Curtis, J.; Wu, C.; et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science 2013, 342, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Santos, C.J.; Uzel, G.; Rosenzweig, S.D. PI3K pathway defects leading to immunodeficiency and immune dysregulation. J. Allergy Clin. Immunol. 2019, 143, 1676–1687. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Meyers, R.E.; Cantley, L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998, 67, 481–507. [Google Scholar] [CrossRef] [PubMed]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular function of phosphoinositide 3-Kinase: Implications for development, immunity, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, K.; Vanhaesebroeck, B. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 2003, 3, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Michalovich, D.; Nejentsev, S. Activated PI3 Kinase Delta Syndrome: From Genetics to Therapy. Front. Immunol. 2018, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Coulter, T.I.; Chandra, A.; Bacon, C.M.; Babar, J.; Curtis, J.; Screaton, N.; Goodlad, J.R.; Farmer, G.; Steele, C.L.; Leahy, T.R.; et al. Clinical spectrum and features of activated phosphoinositide 3-kinase δ syndrome: A large patient cohort study. J. Allergy Clin. Immunol. 2017, 139, 597–606. [Google Scholar] [CrossRef]
- Maccari, M.E.; Abolhassani, H.; Aghamohammadi, A.; Aiuti, A.; Aleinikova, O.; Bangs, C.; Baris, S.; Barzaghi, F.; Baxendale, H.; Buckland, M.; et al. Disease Evolution and Response to Rapamycin in Activated Phosphoinositide 3-kinase δ Syndrome: The European Society for Immunodeficiencies-Activated Phosphoinositide 3-kinase δ Syndrome Registry. Front. Immunol. 2018, 9, 543. [Google Scholar] [CrossRef]
- Carpier, J.-M.; Lucas, C.L. Epstein–Barr Virus Susceptibility in Activated PI3Kδ Syndrome (APDS) Immunodeficiency. Front. Immunol. 2018, 8, 5. [Google Scholar] [CrossRef]
- Preite, S.; Gomez-Rodriguez, J.; Cannons, J.L.; Schwartzberg, P.L. T and B-cell signaling in activated PI3K delta syndrome: From immunodeficiency to autoimmunity. Immunol. Rev. 2019, 291, 154–173. [Google Scholar] [CrossRef] [PubMed]
- Wentink, M.; Dalm, V.; Lankester, A.C.; van Schouwenburg, P.A.; Schölvinck, L.; Kalina, T.; Zachova, R.; Sediva, A.; Lambeck, A.; Pico-Knijnenburg, I.; et al. Genetic defects in PI3Kδ affect B-cell differentiation and maturation leading to hypogammaglobulineamia and recurrent infections. Clin. Immunol. 2017, 176, 77–86. [Google Scholar] [CrossRef]
- Goto, F.; Uchiyama, T.; Nakazawa, Y.; Imai, K.; Kawai, T.; Onodera, M. Persistent Impairment of T-Cell Regeneration in a Patient with Activated PI3K δ Syndrome. J. Clin. Immunol. 2017, 37, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.S.J.; Bier, J.; Cole, T.S.; Wong, M.; Hsu, P.; Berglund, L.J.; Boztug, K.; Lau, A.; Gostick, E.; Price, D.A.; et al. Activating PIK3CD mutations impair human cytotoxic lymphocyte differentiation and function and EBV immunity. J. Allergy Clin. Immunol. 2019, 143, 276–291.e6. [Google Scholar] [CrossRef] [PubMed]
- Bier, J.; Rao, G.; Payne, K.; Brigden, H.; French, E.; Pelham, S.J.; Lau, A.; Lenthall, H.; Edwards, E.S.J.; Smart, J.M.; et al. Activating mutations in PIK3CD disrupt the differentiation and function of human and murine CD4+ T cells. J. Allergy Clin. Immunol. 2019, 144, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Wentink, M.W.J.; Mueller, Y.M.; Dalm, V.A.S.H.; Driessen, G.J.; van Hagen, P.M.; van Montfrans, J.M.; van der Burg, M.; Katsikis, P.D. Exhaustion of the CD8+ T Cell Compartment in Patients with Mutations in Phosphoinositide 3-Kinase Delta. Front. Immunol. 2018, 9, 446. [Google Scholar] [CrossRef]
- Jamee, M.; Moniri, S.; Zaki-Dizaji, M.; Olbrich, P.; Yazdani, R.; Jadidi-Niaragh, F.; Aghamahdi, F.; Abolhassani, H.; Condliffe, A.M.; Aghamohammadi, A.; et al. Clinical, Immunological, and Genetic Features in Patients with Activated PI3Kδ Syndrome (APDS): A Systematic Review. Clin. Rev. Allergy Immunol. 2019. [Google Scholar] [CrossRef]
- Coulter, T.I.; Cant, A.J. The Treatment of Activated PI3Kδ Syndrome. Front. Immunol. 2018, 9, 2043. [Google Scholar] [CrossRef]
- Rae, W.; Ramakrishnan, K.A.; Gao, Y.; Ashton-Key, M.; Pengelly, R.J.; Patel, S.V.; Ennis, S.; Williams, A.P.; Faust, S.N. Precision treatment with sirolimus in a case of activated phosphoinositide 3-kinase δ syndrome. Clin. Immunol. 2016, 171, 38–40. [Google Scholar] [CrossRef]
- Rao, V.K.; Webster, S.; Dalm, V.A.S.H.; Šedivá, A.; van Hagen, P.M.; Holland, S.; Rosenzweig, S.D.; Christ, A.D.; Sloth, B.; Cabanski, M.; et al. Effective “activated PI3Kδ syndrome”–targeted therapy with the PI3Kδ inhibitor leniolisib. Blood 2017, 130, 2307–2316. [Google Scholar] [CrossRef]
- Nademi, Z.; Slatter, M.A.; Dvorak, C.C.; Neven, B.; Fischer, A.; Suarez, F.; Booth, C.; Rao, K.; Laberko, A.; Rodina, J.; et al. Hematopoietic stem cell transplant in patients with activated PI3K delta syndrome. J. Allergy Clin. Immunol. 2017, 139, 1046–1049. [Google Scholar] [CrossRef]
- Okano, T.; Imai, K.; Tsujita, Y.; Mitsuiki, N.; Yoshida, K.; Kamae, C.; Honma, K.; Mitsui-Sekinaka, K.; Sekinaka, Y.; Kato, T.; et al. Hematopoietic stem cell transplantation for progressive combined immunodeficiency and lymphoproliferation in patients with activated phosphatidylinositol-3-OH kinase δ syndrome type 1. J. Allergy Clin. Immunol. 2019, 143, 266–275. [Google Scholar] [CrossRef]
- Plebani, A.; Lougaris, V.; Soresina, A.; Meini, A.; Zunino, F.; Losi, C.G.; Gatta, R.; Cattaneo, G.; Nespoli, L.; Marinoni, M.; et al. A novel immunodeficiency characterized by the exclusive presence of transitional B cells unresponsive to CpG. Immunology 2007, 121, 183–188. [Google Scholar] [CrossRef]
- Lougaris, V.; Baronio, M.; Moratto, D.; Tampella, G.; Gazzurelli, L.; Facchetti, M.; Martire, B.; Cardinale, F.; Lanzarotto, F.; Bondioni, M.P.; et al. A novel monoallelic gain of function mutation in p110δ causing atypical activated phosphoinositide 3-kinase δ syndrome (APDS-1). Clin. Immunol. 2019, 200, 31–34. [Google Scholar] [CrossRef]
- Lougaris, V.; Baronio, M.; Castagna, A.; Tessarin, G.; Rossi, S.; Gazzurelli, L.; Benvenuto, A.; Moratto, D.; Chiarini, M.; Cattalini, M.; et al. Paediatric MAS/HLH caused by a novel monoallelic activating mutation in p110δ. Clin. Immunol. 2020, 219, 108543. [Google Scholar] [CrossRef] [PubMed]
- Saettini, F.; Pelagatti, M.A.; Sala, D.; Moratto, D.; Giliani, S.; Badolato, R.; Biondi, A. Early diagnosis of PI3Kδ syndrome in a 2 years old girl with recurrent otitis and enlarged spleen. Immunol. Lett. 2017, 190, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, Y.; Mitsui-Sekinaka, K.; Imai, K.; Yeh, T.W.; Mitsuiki, N.; Asano, T.; Ohnishi, H.; Kato, Z.; Sekinaka, Y.; Zaha, K.; et al. Phosphatase and tensin homolog (PTEN) mutation can cause activated phosphatidylinositol 3-kinase δ syndrome–like immunodeficiency. J. Allergy Clin. Immunol. 2016, 138, 1672–1680. [Google Scholar] [CrossRef]
- Odnoletkova, I.; Kindle, G.; Quinti, I.; Grimbacher, B.; Knerr, V.; Gathmann, B.; Ehl, S.; Mahlaoui, N.; Van Wilder, P.; Bogaerts, K.; et al. The burden of common variable immunodeficiency disorders: A retrospective analysis of the European Society for Immunodeficiency (ESID) registry data. Orphanet J. Rare Dis. 2018, 13, 201. [Google Scholar] [CrossRef]
- Cansever, M.; Zietara, N.; Chiang, S.C.C.; Ozcan, A.; Yilmaz, E.; Karakukcu, M.; Rohlfs, M.; Somekh, I.; Canoz, O.; Abdulrezzak, U.; et al. A Rare Case of Activated Phosphoinositide 3-Kinase Delta Syndrome (APDS) Presenting With Hemophagocytosis Complicated With Hodgkin Lymphoma. J. Pediatr. Hematol. Oncol. 2019, 42, 1. [Google Scholar] [CrossRef] [PubMed]
- Lougaris, V.; Ravelli, A.; Villanacci, V.; Salemme, M.; Soresina, A.; Fuoti, M.; Lanzarotto, F.; Lanzini, A.; Plebani, A.; Bassotti, G. Gastrointestinal Pathologic Abnormalities in Pediatric- and Adult-Onset Common Variable Immunodeficiency. Dig. Dis. Sci. 2015, 60, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Lougaris, V.; Baronio, M.; Gazzurelli, L.; Lorenzini, T.; Fuoti, M.; Moratto, D.; Bozzola, A.; Ricci, C.; Bondioni, M.P.; Ravelli, A.; et al. A de novo monoallelic CTLA-4 deletion causing pediatric onset CVID with recurrent autoimmune cytopenias and severe enteropathy. Clin. Immunol. 2018, 197, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Elgizouli, M.; Lowe, D.M.; Speckmann, C.; Schubert, D.; Hülsdünker, J.; Eskandarian, Z.; Dudek, A.; Schmitt-Graeff, A.; Wanders, J.; Jørgensen, S.F.; et al. Activating PI3Kδ mutations in a cohort of 669 patients with primary immunodeficiency. Clin. Exp. Immunol. 2016, 183, 221–229. [Google Scholar] [CrossRef]
- Warnatz, K.; Wehr, C.; Dräger, R.; Schmidt, S.; Eibel, H.; Schlesier, M.; Peter, H.H. Expansion of CD19hiCD21lo/neg B cells in common variable immunodeficiency (CVID) patients with autoimmune cytopenia. Immunobiology 2002, 206, 502–513. [Google Scholar] [CrossRef]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Chatila, T.; Cunningham-Rundles, C.; Etzioni, A.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; et al. Human Inborn Errors of Immunity: 2019 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2020, 40, 24–64. [Google Scholar] [CrossRef]
- Cunningham-Rundles, C. How I treat common variable immune deficiency. Blood 2010, 116, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.; Novosad, S.A.; Winthrop, K.L. Infection Risk and Safety of Corticosteroid Use. Rheum. Dis. Clin. N. Am. 2016, 42, 157–176. [Google Scholar] [CrossRef] [PubMed]

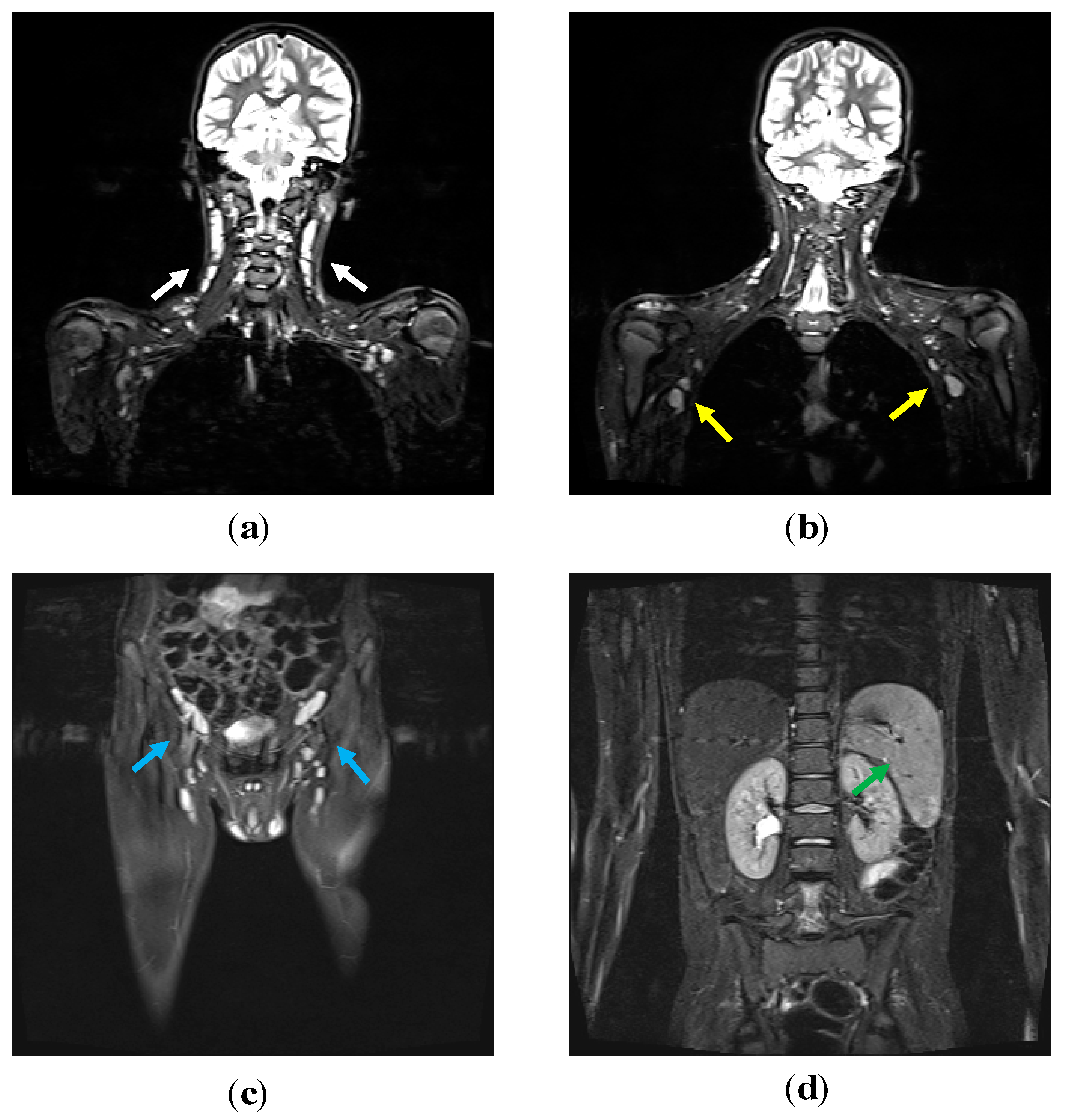

| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | |
|---|---|---|---|---|---|---|---|---|
| Gender | Male | Male | Male | Female | Male | Male | Female | Female |
| Status at last clinical visit | Alive | Alive | Alive | Alive | Alive | Alive | Alive | Alive |
| Current age * | 20.7 | 29.7 | 14.0 | 48.8 | 12.8 | 9.3 | 15.8 | 6.5 |
| Follow-up time † | 1.0 | 16.7 | 7.0 | 21.7 | 1.0 | 4.0 | 3.0 | 2.5 |
| Onset of infection * | 4.5 | 0.5 | 0.7 | 20.5 | – | 3.4 | 8.0 | 1.0 |
| Onset of lymphoproliferation * | 19.0 | 27.7 | 11.0 | – | 12.0 | 5.2 | 8.0 | 1.5 |
| Onset of autoimmunity * | – | 12.0 | 4.7 | – | 11.2 | – | 13.0 | – |
| First immunological evaluation § | 2019 | 2002 | 2011 | 1998 | 2019 | 2016 | 2017 | 2016 |
| First immunological evaluation * | 19.7 | 12.0 | 4.7 | 27.0 | 12.0 | 5.3 | 13.2 | 1.7 |
| APDS-1 diagnosis * | 19.7 | 29.0 | 7.6 | 43.2 | 12.0 | 5.3 | 13.8 | 2.2 |
| Diagnostic delay † | 0.7 | 15.0 | 2.9 | 16.2 | 0.8 | 0.1 | 0.8 | 0.7 |
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | |
|---|---|---|---|---|---|---|---|---|
| Symptom leading to immunological evaluation | Lymphoproliferation | RRTI | EN, Pneumonia | RRTI | HLH | Lymphoproliferation | Lymphoproliferation | RRTI |
| Initial diagnostic hypothesis | Lymphoma | CVID-like | CVID | CVID | HLH | Lymphoma | Lymphoma | CID |
| Sinopulmonary infections | Otitis, Pneumonia, | Pneumonia | Pneumonia | Otitis, Pneumonia | – | Otitis, Pneumonia | Pneumonia | Otitis, Pneumonia |
| Infections other than URTI | Sepsis, episodic Candidiasis, recurrent | Dental abscess Gastroenteritis | – | – | – | – | Otomastoiditis | – |
| Viral infection | VZV, episodic | EBV, episodic | HSV-1, episodic | – | – | CMV, episodic | – | EBV, persistent |
| Lymphadenopathies | Diffuse | Diffuse | Diffuse | – | Diffuse | Diffuse | Diffuse | Cervical |
| Hepato/splenomegaly | Hepatosplenomegaly | Hepatosplenomegaly | Hepatosplenomegaly | – | Hepatosplenomegaly | Hepatosplenomegaly | Hepatosplenomegaly | Splenomegaly |
| Autoimmunity | – | Psoriatic dermatitis | EN, Leucopenia, Thrombocytopenia, Thyroiditis | – | Arthritis, Rash | – | Hemolytic anemia Recurrent parotiditis | – |
| Allergy and asthma | Asthma | – | Asthma | – | – | Asthma | – | Wheezing |
| Gastrointestinal involvement | – | – | IBD | – | – | – | – | – |
| Neurodevelopmental delay | – | – | – | – | – | – | – | – |
| Others | – | – | – | – | – | – | FTT, Delayed puberty | – |
| Malignancy | – | – | – | – | – | – | – | – |
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | |
|---|---|---|---|---|---|---|---|---|
| Date of biopsy | 2019 | 2019 | 2017 | / | 2019 | 2016 | 2017 | / |
| Lymph node region | Cervical | Cervical | Cervical | / | Inguinal | Cervical | Axillary | / |
| Lymph node histology | Atypical polyclonal lymphoproliferation, EBV-negative | Polyclonal paracortical hyperplasia, EBV-negative | Reactive lymphoproliferation | / | Reactive predominantly paracortical hyperplasia, HSV-1, HSV-2, CMV, EBV negative | Atypical marginal zone hyperplasia with scattered EBV+ cells | Interfollicular hyperplasia | / |
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | ||
|---|---|---|---|---|---|---|---|---|---|
| WBC ×103/µL (4.0–10.8) | 11.25 | 6.90 | 7.18 | 4.18 | 6.14 | 8.8 | 8.51 | 8.06 | |
| Neutrophils ×103/µL (1.5–8.0) | 9.40 | 3.40 | 4.93 | 2.93 | 4.58 | 5.33 | 6.43 | 4.90 | |
| Lymphocytes ×103/µL (0.9–4.0) | 1.51 | 2.30 | 1.42 | 0.785 | 1.21 | 2.38 | 1.27 | 2.12 | |
| IgG, mg/dL (700–1600) | 855 | 1452 | 282 † | 495 | 2119 ߇ | 362 ƒ§ | 2056 ߇ | 725 ∂# | |
| IgA, mg/dL (70–400) | 227 | 80 | 55 † | 10 | 207 ߇ | 38 ƒ§ | 287 ߇ | 15 ∂# | |
| IgM, mg/dL (40–230) | 2332 | 145 | 170 † | 7 | 127 ߇ | 414 ƒ§ | 155 ߇ | 228 ∂# | |
| Anti-HBs IgG (>10 IU/L) | 30 IU/L | n.a. | absent | absent | 584 IU/L | absent | n.a. | n.a. | |
| Anti-tetanus IgG (>0.1 IU/mL) | 0.5 IU/L | 2.5 IU/L | absent | absent | 0.6 IU/mL | absent | n.a. | 1.0 UI/L | |
| CFSE-base T cells Proliferation assay | |||||||||
| Anti-CD3 | normal | n.a. | normal | n.a. | n.a. | CD4+ normalCD8+ reduced | n.a. | n.a. | |
| Anti-CD3 + IL-2 | normal | n.a. | normal | n.a. | n.a. | normal | n.a. | n.a. | |
| PHA | reduced | n.a. | normal | n.a. | n.a. | CD4+ normalCD8+ reduced | n.a. | n.a. | |
| Patients No. | PIK3CD_MUT | PIK3CD_EFF | pS6K |
|---|---|---|---|
| 1 | c.1570T > G | p.Y524D | Increased |
| 2 | c.3061G > A | p.E1021K | Increased * |
| 3 | c.3061G > A | p.E1021K | Increased * |
| 4 | c.1973C > T | p.P658L | Increased * |
| 5 | c.323C > G | p.R108L | Increased * |
| 6 | c.3061G > A | p.E1021K | Increased * |
| 7 | c.1574A > C | p.E525A | Increased * |
| 8 | c.3061G > A | p.E1021K | Increased * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessarin, G.; Rossi, S.; Baronio, M.; Gazzurelli, L.; Colpani, M.; Benvenuto, A.; Zunica, F.; Cardinale, F.; Martire, B.; Brescia, L.; et al. Activated Phosphoinositide 3-Kinase Delta Syndrome 1: Clinical and Immunological Data from an Italian Cohort of Patients. J. Clin. Med. 2020, 9, 3335. https://doi.org/10.3390/jcm9103335
Tessarin G, Rossi S, Baronio M, Gazzurelli L, Colpani M, Benvenuto A, Zunica F, Cardinale F, Martire B, Brescia L, et al. Activated Phosphoinositide 3-Kinase Delta Syndrome 1: Clinical and Immunological Data from an Italian Cohort of Patients. Journal of Clinical Medicine. 2020; 9(10):3335. https://doi.org/10.3390/jcm9103335
Chicago/Turabian StyleTessarin, Giulio, Stefano Rossi, Manuela Baronio, Luisa Gazzurelli, Michael Colpani, Alessio Benvenuto, Fiammetta Zunica, Fabio Cardinale, Baldassarre Martire, Letizia Brescia, and et al. 2020. "Activated Phosphoinositide 3-Kinase Delta Syndrome 1: Clinical and Immunological Data from an Italian Cohort of Patients" Journal of Clinical Medicine 9, no. 10: 3335. https://doi.org/10.3390/jcm9103335
APA StyleTessarin, G., Rossi, S., Baronio, M., Gazzurelli, L., Colpani, M., Benvenuto, A., Zunica, F., Cardinale, F., Martire, B., Brescia, L., Costagliola, G., Luti, L., Casazza, G., Menconi, M. C., Saettini, F., Palumbo, L., Girelli, M. F., Badolato, R., Lanzi, G., ... Lougaris, V. (2020). Activated Phosphoinositide 3-Kinase Delta Syndrome 1: Clinical and Immunological Data from an Italian Cohort of Patients. Journal of Clinical Medicine, 9(10), 3335. https://doi.org/10.3390/jcm9103335





