Chronic Microvascular Complications in Prediabetic States—An Overview
Abstract
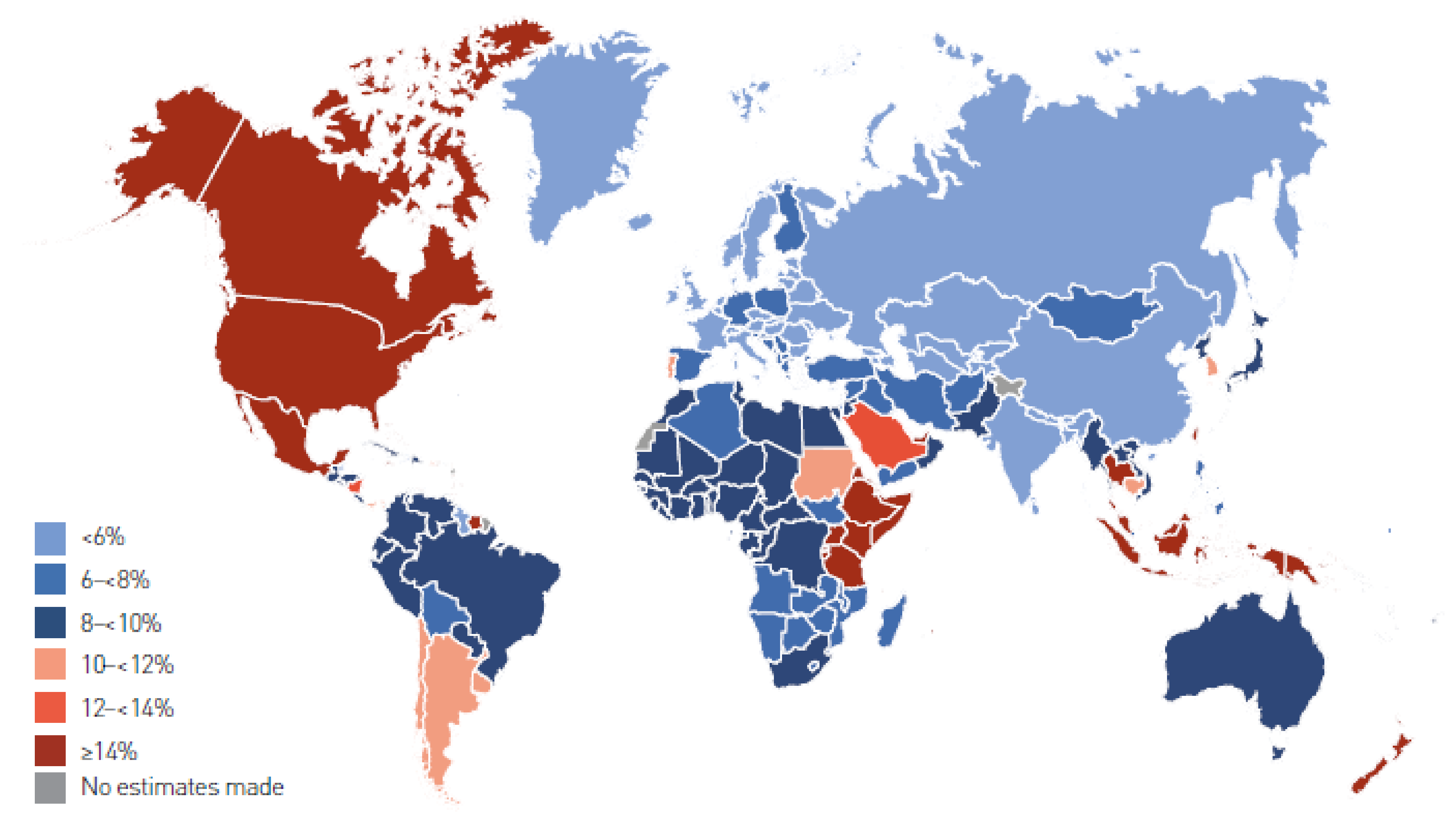
1. Definition and Prevalence
2. Methodology
2.1. Pathophysiology
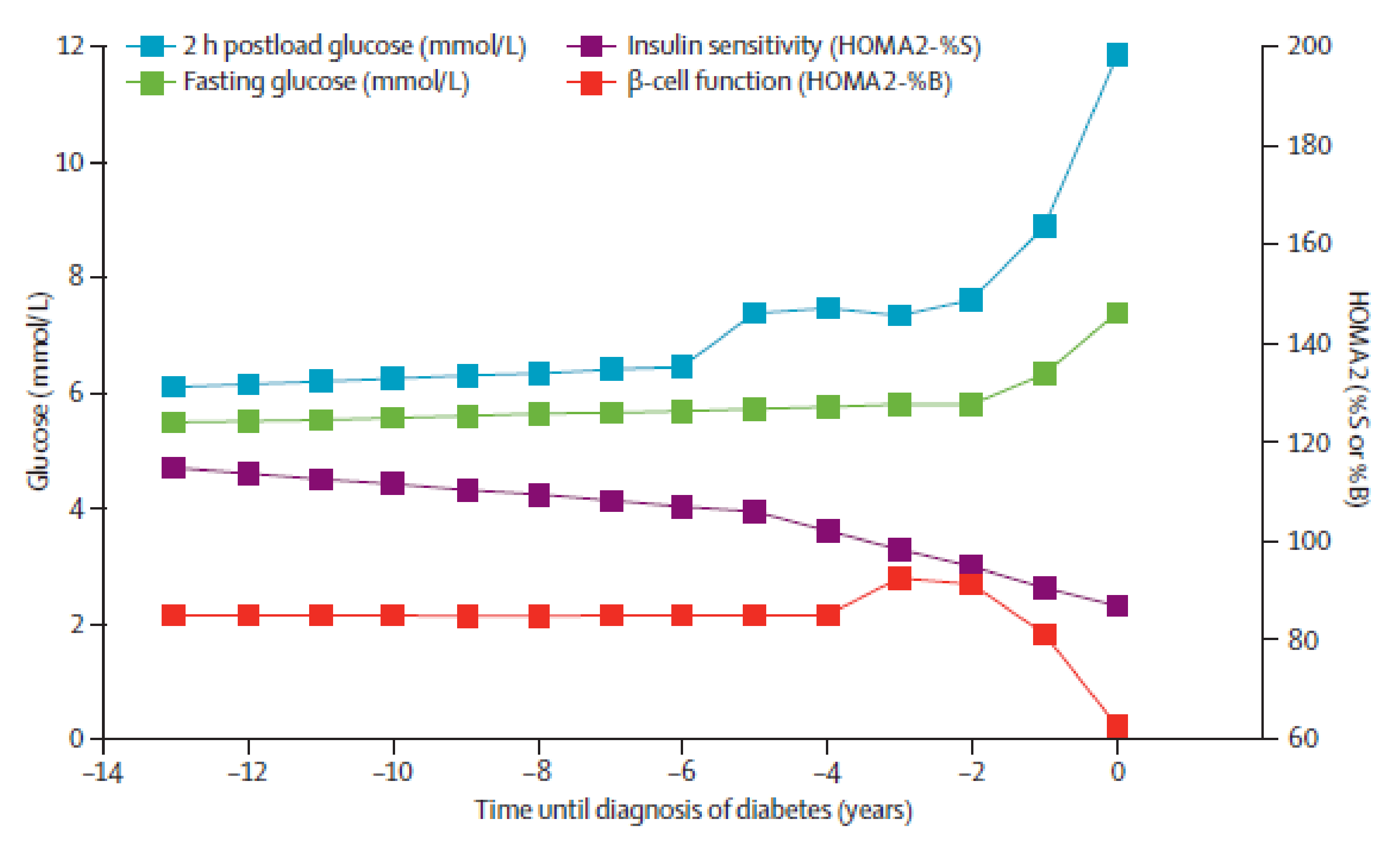
2.1.1. From Correct Glycaemia Through Prediabetes to Diabetes
2.1.2. IFG and/vs. IGT
2.1.3. The Mechanism Behind Complications
2.2. Microvascular Complications
2.2.1. Diabetic Retinopathy
2.2.2. Diabetic Kidney Disease
2.2.3. Diabetic Neuropathy
3. Future Prospects
4. Conclusions and Clinical Implications
Author Contributions
Funding
Conflicts of Interest
References
- Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999; Available online: https://apps.who.int/iris/handle/10665/66040 (accessed on 13 October 2020).
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: http://www.diabetesatlas.org (accessed on 13 October 2020).
- Brannick, B.; Wynn, A.; Dagogo-Jack, S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp. Biol. Med. 2016, 241, 1323–1331. [Google Scholar] [CrossRef]
- Hostalek, U. Global epidemiology of prediabetes—Present and future perspectives. Clin. Diabetes Endocrinol. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Štěpánek, L.; Horakova, D.; Nakládalová, M.; Cibickova, L.; Karasek, D.; Zadrazil, J. Significance of prediabetes as a nosological entity. Biomed. Pap. 2018, 162, 249–257. [Google Scholar] [CrossRef] [PubMed]
- WHO. Diabetes mellitus. Report of a WHO expert committee. World Health Organ. Tech. Rep. Ser. 1965, 310, 1–44. [Google Scholar]
- WHO. WHO Expert Committee on Diabetes Mellitus: Second report. World Health Organ. Tech. Rep. Ser. 1980, 646, 1–80. [Google Scholar]
- WHO Study Group on Diabetes Mellitus; World Health Organization. Diabetes Mellitus: Report of a WHO Study Group; World Health Organization: Geneva, Switzerland, 1985; Available online: https://apps.who.int/iris/handle/10665/39592 (accessed on 13 October 2020).
- World Health Organization; International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006; Available online: https://apps.who.int/iris/handle/10665/43588 (accessed on 13 October 2020).
- American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997, 20, 1183. [Google Scholar] [CrossRef]
- American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003, 26 (Suppl. 1), S5. [Google Scholar] [CrossRef]
- Standards of Medical Care in Diabetes—2010. Diabetes Care 2010, 33 (Suppl. 1), S11–S61. [CrossRef]
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can. J. Diabetes 2018, 42, S10–S15. [Google Scholar] [CrossRef]
- Bullard, K.M.; Saydah, S.H.; Imperatore, G.; Cowie, C.C.; Gregg, E.W.; Geiss, L.S.; Cheng, Y.J.; Rolka, D.B.; Williams, D.E.; Caspersen, C.J. Secular changes in U.S. prediabetes prevalence defined by hemoglobin a1c and fasting plasma glucose: National health and nutrition examination Surveys, 1999–2010. Diabetes Care 2013, 36, 2286–2293. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report; Centers for Disease Control and Prevention: Atlanta, GA, USA; U.S. Department of Health and Human Services: Washington, DC, USA, 2020; p. 32.
- Fonville, S.; Zandbergen, A.A.; Koudstaal, P.J.; Hertog, H.M.D. Prediabetes in patients with stroke or transient ischemic attack: Prevalence, risk and clinical management. Cerebrovasc. Dis. 2014, 37, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Tabák, Á.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- American Diabetes Association. Microvascular complications and foot care: Standards of medical care in diabetes−2020. Diabetes Care 2019, 43, S135–S151. [Google Scholar] [CrossRef]
- American Diabetes Association. Cardiovascular disease and risk management: Standards of medical care in diabetes—2020. Diabetes Care 2019, 43, S111–S134. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of -cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.H.; Stevens, G.A.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Ferrannini, E.; Gastaldelli, A.; Iozzo, P. Pathophysiology of prediabetes. Med. Clin. N. Am. 2011, 95, 327–339. [Google Scholar] [CrossRef]
- DeFronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Tabák, A.G.; Jokela, M.; Akbaraly, T.N.; Brunner, E.J.; Kivimäki, M.; Witte, D. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: An analysis from the Whitehall II study. Lancet 2009, 373, 2215–2221. [Google Scholar] [CrossRef]
- Sattar, N.; McConnachie, A.; Ford, I.; Gaw, A.; Cleland, S.J.; Forouhi, N.G.; McFarlane, P.; Shepherd, J.; Cobbe, S.; Packard, C. Serial metabolic measurements and conversion to type 2 diabetes in the west of Scotland coronary prevention study: Specific elevations in alanine aminotransferase and triglycerides suggest hepatic fat accumulation as a potential contributing factor. Diabetes 2007, 56, 984–991. [Google Scholar] [CrossRef]
- Mason, C.C.; Hanson, R.L.; Knowler, W.C. Progression to type 2 diabetes characterized by moderate then rapid glucose increases. Diabetes 2007, 56, 2054–2061. [Google Scholar] [CrossRef]
- Ferrannini, E.; Nannipieri, M.; Williams, K.; Gonzales, C.; Haffner, S.M.; Stern, M.P. Mode of onset of type 2 diabetes from normal or impaired glucose tolerance. Diabetes 2004, 53, 160–165. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Ferrannini, E.; Miyazaki, Y.; Matsuda, M.; DeFronzo, R.A. Beta-cell dysfunction and glucose intolerance: Results from the San Antonio metabolism (SAM) study. Diabetologia 2004, 47, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Weir, G.C.; Bonner-Weir, S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004, 53 (Suppl. 3), S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Kanat, M.; DeFronzo, R.A.; Abdul-Ghani, M.A. Treatment of prediabetes. World J. Diabetes 2015, 6, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Abdul-Ghani, M.A. Preservation of β-cell function: The key to diabetes prevention. J. Clin. Endocrinol. Metab. 2011, 96, 2354–2366. [Google Scholar] [CrossRef] [PubMed]
- Kanat, M.; Mari, A.; Norton, L.; Winnier, D.; DeFronzo, R.A.; Jenkinson, C.; Abdul-Ghani, M.A. Distinct β-cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes 2012, 61, 447–453. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; DeFronzo, R.A.; Jayyousi, A. Prediabetes and risk of diabetes and associated complications. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 394–399. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose. Diabetic Med. 2007, 24, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Wang, J.J.; Wong, T.Y.; Kayama, T.; Yamashita, H. Impaired glucose tolerance, but not impaired fasting glucose, is associated with retinopathy in Japanese population: The Funagata study. Diabetes Obes. Metab. 2008, 10, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Lamparter, J.; Raum, P.; Pfeiffer, N.; Peto, T.; Hoehn, R.; Elflein, H.; Wild, P.; Schulz, A.; Schneider, A.; Mirshahi, A. Prevalence and associations of diabetic retinopathy in a large cohort of prediabetic subjects: The gutenberg health study. J. Diabetes Complicat. 2014, 28, 482–487. [Google Scholar] [CrossRef]
- Wong, T.Y.; Liew, G.; Tapp, R.J.; Schmidt, M.I.; Wang, J.J.; Mitchell, P.; Klein, R.; Klein, B.E.K.; Zimmet, P.; Shaw, J. Relation between fasting glucose and retinopathy for diagnosis of diabetes: Three population-based cross-sectional studies. Lancet 2008, 371, 736–743. [Google Scholar] [CrossRef]
- Tyrberg, M.; Melander, A.; Lövestam-Adrian, M.; Lindblad, U.; Lvestam-Adrian, M. Retinopathy in subjects with impaired fasting glucose: The NANSY-Eye baseline report. Diabetes Obes. Metab. 2008, 10, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Azmi, S.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Alam, U.; Fadavi, H.; Asghar, O.; Marshall, A.; Atkinson, A.J.; Jones, W.T.; et al. Corneal confocal microscopy identifies small-fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care 2015, 38, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Gregg, E.W.; Geiss, L.S.; Imperatore, G.; Williams, D.E.; Zhang, X.; Albright, A.L.; Cowie, C.C.; Klein, R.; Saaddine, J.B. Association of A1C and Fasting Plasma Glucose Levels With Diabetic Retinopathy Prevalence in the U.S. Population: Implications for diabetes diagnostic thresholds. Diabetes Care 2009, 32, 2027–2032. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hsu, W.; Lai, C.; Chen, S.; Liang, C. The association between glycated albumin, glycohemoglobin, and glycated albumin to glycohemoglobin ratio in diabetic retinopathy of prediabetes. Kaohsiung J. Med. Sci. 2019, 35, 695–701. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wang, J.J.; Wong, T.Y. Retinal Vascular Changes in Pre-Diabetes and Prehypertension: New findings and their research and clinical implications. Diabetes Care 2007, 30, 2708–2715. [Google Scholar] [CrossRef]
- Zaleska-Żmijewska, A.; Piątkiewicz, P.; Śmigielska, B.; Sokołowska-Oracz, A.; Wawrzyniak, Z.; Romaniuk, D.; Szaflik, J.; Szaflik, J.P. Retinal photoreceptors and microvascular changes in prediabetes measured with adaptive optics (rtx1™): A case-control study. J. Diabetes Res. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Plantinga, L.C.; Crews, D.C.; Coresh, J.; Miller, E.R.; Saran, R.; Yee, J.; Hedgeman, E.; Pavkov, M.; Eberhardt, M.S.; Williams, D.E.; et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin. J. Am. Soc. Nephrol. 2010, 5, 673–682. [Google Scholar] [CrossRef]
- Fox, C.S.; Larson, M.G.; Leip, E.P.; Meigs, J.B.; Wilson, P.W.; Levy, D. Glycemic status and development of kidney disease: The framingham heart study. Diabetes Care 2005, 28, 2436–2440. [Google Scholar] [CrossRef]
- Kurella, M.; Lo, J.C.; Chertow, G.M. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J. Am. Soc. Nephrol. 2005, 16, 2134–2140. [Google Scholar] [CrossRef]
- Tozawa, M.; Iseki, C.; Tokashiki, K.; Chinen, S.; Kohagura, K.; Kinjo, K.; Takishita, S.; Iseki, K.; Tozawa, C.I.M. Metabolic syndrome and risk of developing chronic kidney disease in Japanese adults. Hypertens. Res. 2007, 30, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Lucove, J.; Vupputuri, S.; Heiss, G.; North, K.; Russell, M. Metabolic syndrome and the development of CKD in American Indians: The strong heart study. Am. J. Kidney Dis. 2008, 51, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Ghanbarian, A.; Azizi, F. Are patients who have metabolic syndrome without diabetes at risk for developing chronic kidney disease? Evidence based on data from a large cohort screening population. Clin. J. Am. Soc. Nephrol. 2007, 2, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Chang, Y.; Woo, H.Y.; Lee, K.B.; Kim, S.G.; Kim, D.I.; Kim, W.S.; Suh, B.S.; Jeong, C.; Yoon, K. Time-dependent association between metabolic syndrome and risk of CKD in Korean men without hypertension or diabetes. Am. J. Kidney Dis. 2009, 53, 59–69. [Google Scholar] [CrossRef]
- Sun, F.; Tao, Q.; Zhan, S. Metabolic syndrome and the development of chronic kidney disease among 118 924 non-diabetic Taiwanese in a retrospective cohort. Nephrology 2010, 15, 84–92. [Google Scholar] [CrossRef]
- Watanabe, H.; Obata, H.; Watanabe, T.; Sasaki, S.; Nagai, K.; Aizawa, Y. Metabolic syndrome and risk of development of chronic kidney disease: The Niigata preventive medicine study. Diabetes Metab. Res. Rev. 2010, 26, 26–32. [Google Scholar] [CrossRef]
- Schöttker, B.; Brenner, H.; Koenig, W.; Müller, H.; Rothenbacher, D. Prognostic association of HbA1c and fasting plasma glucose with reduced kidney function in subjects with and without diabetes mellitus. Results from a population-based cohort study from Germany. Prev. Med. 2013, 57, 596–600. [Google Scholar] [CrossRef]
- Jadhakhan, F.; Marshall, T.; Ryan, R.; Gill, P. Risk of chronic kidney disease in young adults with impaired glucose tolerance/impaired fasting glucose: A retrospective cohort study using electronic primary care records. BMC Nephrol. 2018, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koshi, T.; Sagesaka, H.; Sato, Y.; Hirabayashi, K.; Koike, H.; Yamauchi, K.; Nishimura, R.; Noda, M.; Yamashita, K.; Aizawa, T. Elevated haemoglobin A1c but not fasting plasma glucose conveys risk of chronic kidney disease in non-diabetic individuals. Diabetes Res. Clin. Pract. 2018, 146, 233–239. [Google Scholar] [CrossRef]
- Vieira, M.B.; Neves, J.S.; Leitão, L.; Baptista, R.B.; Magriço, R.; Dias, C.V.; Oliveira, A.; Carvalho, D.; Mc Causland, F.R. Impaired fasting glucose and chronic kidney disease, albuminuria, or worsening kidney function: A secondary analysis of the SPRINT. J. Clin. Endocrinol. Metab. 2019, 104, 4024–4032. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.S.; Oh, H.H.; Kim, S.H.; Kim, B.O.; Byun, Y.S. Association between prediabetes (defined by HbA1C, fasting plasma glucose, and impaired glucose tolerance) and the development of chronic kidney disease: A 9-year prospective cohort study. BMC Nephrol. 2019, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, A.; Jiang, J.; Liu, G.; Wang, M.; Li, D.; Wen, J.; Mu, Y.; Du, X.; Gaisano, H.; et al. Risk of chronic kidney disease defined by decreased estimated glomerular filtration rate in individuals with different prediabetic phenotypes: Results from a prospective cohort study in China. BMJ Open Diabetes Res. Care 2020, 8, e000955. [Google Scholar] [CrossRef] [PubMed]
- Melsom, T.; Schei, J.; Stefansson, V.T.N.; Solbu, M.D.; Jenssen, T.G.; Mathisen, U.D.; Wilsgaard, T.; Eriksen, B.O. Prediabetes and risk of glomerular hyperfiltration and albuminuria in the general nondiabetic population: A prospective cohort study. Am. J. Kidney Dis. 2016, 67, 841–850. [Google Scholar] [CrossRef]
- De Nicola, L.; Conte, G.; Minutolo, R. Prediabetes as a precursor to diabetic kidney disease. Am. J. Kidney Dis. 2016, 67, 817–819. [Google Scholar] [CrossRef]
- Neves, J.S.; Correa, S.; Baptista, R.B.; Vieira, M.B.; Waikar, S.S.; Mc Causland, F.R. Association of prediabetes with CKD progression and adverse cardiovascular outcomes: An analysis of the CRIC Study. J. Clin. Endocrinol. Metab. 2020, 105, 105. [Google Scholar] [CrossRef]
- Won, J.C.; Hong, J.W.; Kim, J.M.; Kim, T.N.; Noh, J.; Ko, K.S.; Rhee, B.D.; Kim, D.-J. Increased prevalence of albuminuria in individuals with higher range of impaired fasting glucose: The 2011 Korea National Health and Nutrition Examination Survey. J. Diabetes Complicat. 2015, 29, 50–54. [Google Scholar] [CrossRef]
- Thomas, G.; Sehgal, A.R.; Kashyap, S.R.; Srinivas, T.R.; Kirwan, J.P.; Navaneethan, S.D. Metabolic syndrome and kidney disease: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2011, 6, 2364–2373. [Google Scholar] [CrossRef]
- Curhan, G.C. Prediabetes, prehypertension … is it time for pre-CKD? Clin. J. Am. Soc. Nephrol. 2010, 5, 557–559. [Google Scholar] [CrossRef]
- Papanas, N.; Vinik, A.I.; Ziegler, D. Neuropathy in prediabetes: Does the clock start ticking early? Nat. Rev. Endocrinol. 2011, 7, 682–690. [Google Scholar] [CrossRef]
- Ziegler, D.; Rathmann, W.; Dickhaus, T.; Meisinger, C.; Mielck, A. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: The MONICA/KORA Augsburg surveys S2 and S3. Pain Med. 2009, 10, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.R.H.; Smith, B.E.; Ross, M.A.; Hernandez, J.; Bosch, E.P. Value of the oral glucose tolerance test in the evaluation of chronic idiopathic axonal polyneuropathy. Arch. Neurol. 2006, 63, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Nebuchennykh, M.; Lseth, S.; Jorde, R.; Mellgren, S.I.; Løseth, S. Idiopathic polyneuropathy and impaired glucose metabolism in a Norwegian patient series. Eur. J. Neurol. 2008, 15, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Sumner, C.; Sheth, S.; Griffin, J.; Cornblath, D.; Polydefkis, M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003, 60, 108–111. [Google Scholar] [CrossRef]
- Singleton, J.R.; Smith, A.G.; Bromberg, M.B. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001, 24, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Ramachandran, P.; Tripp, S.; Singleton, J.R. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology 2001, 57, 1701–1704. [Google Scholar] [CrossRef]
- Putz, Z.; Tabák, Á.G.; Tóth, N.; Istenes, I.; Németh, N.; Gandhi, R.A.; Hermányi, Z.; Keresztes, K.; Jermendy, G.; Tesfaye, S.; et al. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care 2009, 32, 181–183. [Google Scholar] [CrossRef]
- Ziegler, D.; Papanas, N.; Vinik, A.I.; Shaw, J.E. Epidemiology of polyneuropathy in diabetes and prediabetes. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 126, pp. 3–22. [Google Scholar] [CrossRef]
- Annuzzi, G.; Rivellese, A.; Vaccaro, O.; Ferrante, M.R.; Riccardi, G.; Mancini, M. The relationship between blood glucose concentration and beat-to-beat variation in asymptomatic subjects. Acta Diabetol. Lat. 1984, 21, 57–62. [Google Scholar] [CrossRef]
- Fujimoto, W.Y.; Leonetti, D.L.; Kinyoun, J.L.; Shuman, W.P.; Stolov, W.C.; Wahl, P.W. Prevalence of complications among second-generation Japanese-American men with diabetes, impaired glucose tolerance, or normal glucose tolerance. Diabetes 1987, 36, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Dekker, J.M.; TenVoorde, B.J.; Bertelsmann, F.W.; Kostense, P.J.; Stehouwer, C.D.A.; Heine, R.J.; Nijpels, G.; Heethaar, R.M.; Bouter, L.M. Glucose tolerance and other determinants of cardiovascular autonomic function: The Hoorn Study. Diabetologia 2000, 43, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Larson, M.G.; O’Donnell, C.J.; Wilson, P.F.; Tsuji, H.; Lloyd-Jones, D.M.; Levy, D. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am. J. Cardiol. 2000, 86, 309–312. [Google Scholar] [CrossRef]
- Schroeder, E.B.; Chambless, L.E.; Liao, D.; Prineas, R.J.; Evans, G.W.; Rosamond, W.D.; Heiss, G. Diabetes, glucose, insulin, and heart rate variability: The atherosclerosis risk in communities (ARIC) study. Diabetes Care 2005, 28, 668–674. [Google Scholar] [CrossRef]
- Perciaccante, A.; Fiorentini, A.; Paris, A.; Serra, P.; Tubani, L. Circadian rhythm of the autonomic nervous system in insulin resistant subjects with normoglycemia, impaired fasting glycemia, impaired glucose tolerance, type 2 diabetes mellitus. BMC Cardiovasc. Disord. 2006, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.K.; Barzilay, J.I.; Domitrovich, P.P.; Chaves, P.M.; Gottdiener, J.S.; Heckbert, S.R.; Kronmal, R.A. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: The cardiovascular health study. Diabet. Med. 2007, 24, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Lin, T.S.; Huang, Y.H.; Lu, F.H.; Yang, Y.C.; Chen, J.J.; Chang, C.J. Epidemiological evidence of altered cardiac autonomic function in subjects with impaired glucose tolerance but not isolated impaired fasting glucose. J. Clin. Endocrinol. Metab. 2007, 92, 3885–3889. [Google Scholar] [CrossRef]
- Isak, B.; Tanrıdağ, T.; Yitmen, I.; Us, O.; Oflazoğlu, B. Evaluation of peripheral and autonomic neuropathy among patients with newly diagnosed impaired glucose tolerance. Diabetes Metab. Res. Rev. 2008, 24, 563–569. [Google Scholar] [CrossRef]
- Laitinen, T.; Lindström, J.; Eriksson, J.G.; Ilanne-Parikka, P.; Aunola, S.; Keinänen-Kiukaanniemi, S.; Tuomilehto, J.; Uusitupa, M. Cardiovascular autonomic dysfunction is associated with central obesity in persons with impaired glucose tolerance. Diabet. Med. 2011, 28, 699–704. [Google Scholar] [CrossRef]
- Putz, Z.; Nemeth, N.; Istenes, I.; Martos, T.; Gandhi, R.A.; Körei, A.E.; Hermanyi, Z.; Szathmári, M.; Jermendy, G.; Tesfaye, S.; et al. Autonomic dysfunction and circadian blood pressure variations in people with impaired glucose tolerance. Diabet. Med. 2013, 30, 358–362. [Google Scholar] [CrossRef]
- Ziegler, D.; Voss, A.; Rathmann, W.; Strom, A.; Perz, S.; Roden, M.; Peters, A.; Meisinger, C. Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: The KORA S4 survey. Diabetologia 2015, 58, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Tiftikçioğlu, B.I.; Bilgin, S.; Duksal, T.; Kose, S.; Zorlu, Y. Autonomic neuropathy and endothelial dysfunction in patients with impaired glucose tolerance or type 2 diabetes mellitus. Medicine 2016, 95, e3340. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Tankova, T.; Guergueltcheva, V.; Tournev, I.; Chakarova, N.; Grozeva, G.; Dakovska, L. Risk factors for autonomic and somatic nerve dysfunction in different stages of glucose tolerance. J. Diabetes Complicat. 2017, 31, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Tankova, T.; Kirilov, G.; Chakarova, N.; Grozeva, G.; Dakovska, L. Endothelial and autonomic dysfunction at early stages of glucose intolerance and in metabolic syndrome. Horm. Metab. Res. 2020, 52, 39–48. [Google Scholar] [CrossRef]
- Coopmans, C.; Zhou, T.L.; Henry, R.M.; Heijman, J.; Schaper, N.C.; Koster, A.; Schram, M.T.; Van Der Kallen, C.J.; Wesselius, A.; Engelsman, R.J.D.; et al. Both prediabetes and type 2 diabetes are associated with lower heart rate variability: The maastricht study. Diabetes Care 2020, 43, 1126–1133. [Google Scholar] [CrossRef]
- Spallone, V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: What is defined, what is new, and what is unmet. Diabetes Metab. J. 2019, 43, 3–30. [Google Scholar] [CrossRef]
- Warren, B.; Pankow, J.S.; Matsushita, K.; Punjabi, N.M.; Daya, N.R.; Grams, M.; Woodward, M.; Selvin, E. Comparative prognostic performance of definitions of prediabetes: A prospective cohort analysis of the atherosclerosis risk in communities (ARIC) study. Lancet Diabetes Endocrinol. 2017, 5, 34–42. [Google Scholar] [CrossRef]
- Bergman, M.; Abdul-Ghani, M.; DeFronzo, R.A.; Manco, M.; Sesti, G.; Fiorentino, T.V.; Ceriello, A.; Rhee, M.; Phillips, L.S.; Chung, S.; et al. Review of methods for detecting glycemic disorders. Diabetes Res. Clin. Pract. 2020, 165, 108233. [Google Scholar] [CrossRef]
- Vaishya, S.; Sarwade, R.D.; Seshadri, V. MicroRNA, proteins, and metabolites as novel biomarkers for prediabetes, diabetes, and related complications. Front. Endocrinol. 2018, 9, 180. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Niemira, M.; Maliszewska, K.; Erol, A.; Bielska, A.; Szałkowska, A.; Adamska-Patruno, E.; Szczerbinski, L.; Gorska, M.; Krętowski, A. Circulating miRNAs as a predictive biomarker of the progression from prediabetes to diabetes: Outcomes of a 5-year prospective observational study. J. Clin. Med. 2020, 9, 2184. [Google Scholar] [CrossRef]
- Zhang, J.R.; Sun, H.J. Roles of circular RNAs in diabetic complications: From molecular mechanisms to therapeutic potential. Gene 2020, 763, 145066. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Gao, C.; Rao, L.; Hao, P.; Jian, D.; Li, W.; Tang, H.; Li, M. The diagnostic value of whole blood lncRNA ENST00000550337.1 for pre-diabetes and type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2017, 125, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, X.; Du, Y.; Dong, Z.; Qiao, C.; Li, T.; Chen, P.; Lou, P. Screening and functional studies of long noncoding RNA in subjects with prediabetes. Endocrine 2020, 68, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Bridges, K. Assessing Salivary Biomarkers for Risk Stratification in Metabolic Syndrome; Clinical Trial Registration Study/NCT01086137; National Library of Medicine: Maryland, MD, USA, 2013. Available online: Clinicaltrials.gov (accessed on 13 October 2020).
- Garg, V.; Kumar, M.; Mahapatra, H.S.; Chitkara, A.; Gadpayle, A.K.; Sekhar, V. Novel urinary biomarkers in pre-diabetic nephropathy. Clin. Exp. Nephrol. 2015, 19, 895–900. [Google Scholar] [CrossRef]
- Gu, D.; Chen, Y.; Masucci, M.; Xiong, C.; Zou, H.; Holthofer, H. Potential urine biomarkers for the diagnosis of prediabetes and early diabetic nephropathy based on ISN CKHDP program. Clin. Nephrol. 2020, 93, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Yan, Z.Q.; Han, L.P.; Yin, A.L.; Xu, J.Y.; Zhai, Y.R.; Hao, S.; Zhang, L.; Xie, Y. The Association between phosphorylated neurofilament heavy chain (pNF-H) and small fiber neuropathy (SFN) in patients with impaired glucose tolerance. Diabetes Ther. 2020, 11, 71–81. [Google Scholar] [CrossRef]
- Richdale, K.; Chao, C.; Hamilton, M. Eye care providers’ emerging roles in early detection of diabetes and management of diabetic changes to the ocular surface: A review. BMJ Open Diabetes Res. Care 2020, 8, e001094. [Google Scholar] [CrossRef]
- Greco, M.; Chiefari, E.; Accattato, F.; Corigliano, D.M.; Arcidiacono, B.; Mirabelli, M.; Liguori, R.; Brunetti, F.S.; Pullano, S.A.; Scorcia, V.; et al. MicroRNA-1281 as a novel circulating biomarker in patients with diabetic retinopathy. Front. Endocrinol. 2020, 11, 528. [Google Scholar] [CrossRef]
- American Diabetes Association 3. Prevention or delay of type 2 diabetes: Standards of medical care in diabetes—2020. Diabetes Care 2019, 43, S32–S36. [Google Scholar] [CrossRef]
- Gabriel, R.; Abdelkader, N.B.; Acosta, T.; Gilis-Januszewska, A.; Gómez-Huelgas, R.; Makrilakis, K.; Kamenov, Z.; Paulweber, B.; Satman, I.; Djordjevic, P.; et al. Early prevention of diabetes microvascular complications in people with hyperglycaemia in Europe. ePREDICE randomized trial. Study protocol, recruitment and selected baseline data. PLoS ONE 2020, 15, e0231196. [Google Scholar] [CrossRef]
- Papaetis, G.S. Incretin-based therapies in prediabetes: Current evidence and future perspectives. World J. Diabetes 2014, 5, 817–834. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Huang, L.; Zeng, H.; He, G.; Chen, L.; Ma, R.; Fu, W.; Ning, B. Anti-diabetic agents for prevention of type 2 diabetes mellitus in people with pre-diabetes: A systematic review and network meta-analysis protocol. BMJ Open 2019, 9, e029073. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar]
- Huang, Y.Q.; Liu, L.; Huang, C.; Yu, Y.L.; Lo, K.; Huang, J.Y.; Chen, C.L.; Zhou, Y.L.; Feng, Y.Q. Impacts of pre-diabetes or prehypertension on subsequent occurrence of cardiovascular and all-cause mortality among population without cardiovascular diseases. Diabetes Metab. Syndr. Obesity Targets Ther. 2020, 13, 1743–1752. [Google Scholar] [CrossRef] [PubMed]

| WHO | ADA | CDA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1965 [6] | 1980 [7] | 1985 [8] | 1999, 2006 [9] | 1997 [10] | 2003 [11] | 2010 [12] | 2018 [13] | ||
| Prediabetes | At least 1 of the conditions: | ||||||||
| FPG (mg/dL) | 120–139 | <140 | 110–125 a | 110–125 a | 100–125 a | 100–125 a | 110–125 a | ||
| 2-h PG OGTT (mg/dl) | 110–129 # | 140–200 | 140–200 | 140–199 b | 140–199 b | 140–199 b | 140–199 b | 140–199 b | |
| HbA1c (%) | 5.7–6.4% | 6.0–6.4% | |||||||
| Diabetes | At least 1 of the conditions: | ||||||||
| FPG (mg/dL) | ≥130 * | ≥140 | ≥140 | ≥126 | ≥126 | ≥126 | ≥126 | ≥126 | |
| 2-h PG OGTT (mg/dl) | ≥130 | ≥200 | ≥200 | ≥200 | ≥200 | ≥200 | ≥200 | ≥200 | |
| HbA1c (%) | ≥6.5% | ≥6.5% | |||||||
| Study and Year | Population | Number (n) | Ethnicity | Age (Years) | Criteria | Outcome Definition | Follow-Up (Years) | Number of Events | Effect Estimate: OR or RR (95% CI) | DKD Correlates |
|---|---|---|---|---|---|---|---|---|---|---|
| Fox et al. (2005) [47] | All | 2398 | Mainly white | 28–62 | IFG: FPG 100–125 mg/dL; IGT: 2-h PG 140–199 mg/dL | CKD (eGFR <60 mL/min/1.73 m2)—MDRD study equation | 7 | 167 | age, sex, baseline eGFR, SBP, AH treatment, smoking, BMI, TC, HDL, and prevalent MI or congestive HF | |
| NGT | 1502 | |||||||||
| IFG or IGT | 704 | 0.98 (0.67–1.45) | ||||||||
| n-DM | 82 | 1.71 (0.83–3.55) | ||||||||
| k-DM | 110 | 1.93 (1.06–3.49) | ||||||||
| Kurella et al. (2005) [48] | All | 10,096 | Mainly white, Black (20%) | 45–64 | IFG: FPG ≥ 110 mg/dl | CKD (eGFR <60 mL/min/1.73 m2)—MDRD study equation | 9 | 691 | 1.11 (0.87–1.40) | age, gender, race |
| IFG | 691 | |||||||||
| Tozawa et al. (2007) [49] | All | 6371 | Asian/Japanese | 19-84 | IFG: FPG ≥ 110 mg/dl | CKD defined as dipstick positive proteinuria (≥1+) or eGFR <60 mL/min/1.73 m2 using the MDRD study equation | 5 | 369 | 1.22 (0.89–1.67) | age, sex, current, cigarette smoking, and alcohol drinking habits |
| Lucove et al. (2008) [50] | All | 2420 | American Indians | 45–74 | IFG: FPG ≥ 110 mg/dl | CKD defined as ACR ≥30 mg/g or eGFR <60 ml/min/1.73 m2 using the MDRD study equation | 9 | 388 | 1.4 (1.1–1.8) | age, sex, study center, and cigarette smoking |
| IFG | 605 | |||||||||
| Rashidi et al. (2007) [51] | All | 4607 | Asian/Iranian | >18 | IFG: FPG ≥ 110 mg/dL | CKD (eGFR <60 mL/min/1.73 m2)—Cockroft–Gault study equation | 3 | 111 | 1.02 (1.00–1.04) | Not provided |
| Ryu et al. (2009) [52] | All | 10685 | Asian/Korean | 30–59 | IFG: FPG ≥ 110 mg/dl | CKD (eGFR <60 mL/min/1.73 m2)—MDRD study equation | 3.8 | 291 | 1.61 (0.95–2.73) | age, baseline eGFR, γ-glutamyl transpeptidase level, uric acid level, obesity, increased TG, low HDL, increased BP |
| Sun et al. (2010) [53] | All | 118,924 | Asian/Chinese | 20–74 | IFG: FPG ≥ 100 mg/dL | CKD defined as dipstick positive proteinuria (≥ 1+) or eGFR <60 mL/min/1.73 m2 using the MDRD study equation | 3.7 | 12648 | 0.97 (0.93–1.01) | age, sex, check-up centers and current smoking |
| IFG | 28,827 | |||||||||
| Watanabe et al. (2012) [54] | All | 27,359 | Asian/Japanese | ≥20 | IFG: FPG ≥ 110 mg/dL | CKD (eGFR < 60 mL/min/1.73 m2)—MDRD study equation | 5.8 | 513 | 1.84 (1.42–2.38) | age and sex |
| Schöttker et al. (2013) [55] | All | 3538 | Caucasian | 50–74 | IFG: FPG ≥ 110 mg/dL; eHbA1c: 39−47 mmol/mol | CKD (eGFR < 60 mL/min/1.73 m2)—CKD EPI study equation | 8 | 0.97 (0.75–1.25) | age, sex, baseline eGFR, BMI, SBP (continuous), TC, use of antihypertensive drugs, use of statins, smoking status and history of self-reported cardiovascular disease (MI or stroke) | |
| normal FPG | 2076 | 364 | 0.97 (0.75–1.25) | |||||||
| IFG | 516 | 87 | ||||||||
| normal HbA1c | 2014 | 321 | 1.03 (0.86–1.23) | |||||||
| eHbA1c | 1034 | 213 | ||||||||
| normal FPG and HbA1c | 1471 | 235 | 1.06 (0.71–1.32) | |||||||
| both IFG and eHbA1c | 258 | 55 | ||||||||
| Jadhakhan et al. (2018) [56] | All | 40,092 | Mainly white and Asian | 18–40 | IFG: FPG ≥ 110 mg/dL; IGT: 2-h gc ≥ 140 mg/dL ≤ 199 mg/dL; eHbA1c: 42–47 mmol/mol | CKD (eGFR < 60 ml/min/1.73 m2)—CKD EPI study equation | 2 | 2.6 (2.0–3.4) | age, sex, ethnic group, deprivation quintile, BMI, CVD, HF, AF, AH, and steroid use | |
| NGT | 29,531 | 126 | ||||||||
| IFG and IGT | 10,561 | 182 | ||||||||
| Koshi et al. (2018) [57] | All | 25,109 | Asian/Japanese | ≥18 | Prediabetes 1: FPG 100–125 mg/dl and/or eHbA1c 39−47 mmol/mol; Prediabetes 2: FPG 110–125 mg/dL and/or eHbA1c: 42–47 mmol/mol | eGFR <60 mL/min/1.73 m2 and/or dipstick positive proteinuria—IDMS-MDRD study equation | 5.3 | 2483 | sex, age, insulin sensitivity, SBP, baseline eGFR, serum alanine aminotransferase level | |
| PrediabetesADA | 10,367 | 1.21 (1.12–1.32) | ||||||||
| PrediabetesWHO | 2561 | 1.31 (1.16–1.48) | ||||||||
| Vieira et al. (2019) [58] | All | 9361 | Main white (55%), Black (32%), Hispanic (10%) | ≥50 | IFG: FPG ≥ 100 mg/dL | Worsening kidney function a or albuminuria | 3.3 | 193 | 1.02 (0.75–1.37) | age, sex, race, smoking status, SBP, prior CVD, BMI, statin use, aspirin use, and trial treatment arm |
| NGT | 5424 | 114 | ||||||||
| IFG | 3897 | 79 | ||||||||
| Kim et al. (2020) [59] | All | 7728 | Asian/Korean | ≥18 | IFG: 110–125 mg/dL; IGT: 2-h gc ≥ 140 mg/dL ≤ 199 mg/dL; eHbA1c: 39–47 mmol/mol | CKD (eGFR < 60 mL/min/1.73 m2)—CKD EPI study equation | 8.7 | 871 | age >65, sex, AH, obesity, regular activity, baseline eGFR, and metabolic syndrome | |
| IFG | 652 | 84 | 0.92 (0.72–1.17) | |||||||
| IGT | 1882 | 297 | 1.14 (1.18–1.31) | |||||||
| eHbA1c | 2461 | 425 | 1.39 (1.21–1.60) | |||||||
| Li et al. (2020) [60] | All | 6446 | Asian/Chinese | 40–84 | IFG: FPG 100–125 mg/dL; IGT: 2-h gc ≥ 140 mg/dL ≤ 199 mg/dL; eHbA1c: 39−47 mmol/mo | CKD (eGFR < 60 mL/min/1.73 m2)—CKD EPI study equation | 3 | 88 | age, sex, BMI, TC, TG, HDL, LDL, SBP, DBP, baseline eGFR, smoking and drinking status | |
| NGT | 1836 | 10 | ||||||||
| Prediabetes | 4610 | 78 | 2.45 (1.26–4.81) | |||||||
| IFG | 1954 | 38 | 2.51 (1.21–5.20) | |||||||
| IGT | 1696 | 36 | 2.39 (1.14–5.03) | |||||||
| eHBA1c | 3848 | 65 | 2.60 (1.31-5.16) |
| Study and Year | Population | Number (n) | Criteria | Test (s) Used | Prevalence | Differences vs. NGT | CAN Correlates |
|---|---|---|---|---|---|---|---|
| Annuzzi et al. (1983) [76] | All | 186 | EASD 1979 | DB | Not provided | No difference | Age, BMI |
| NGT | 124 | ||||||
| IGT | 62 | ||||||
| Fujimoto et al. (1987) [77] | All | 151 | WHO 1980 | DB | Not provided | No difference | Fasting glucose concentration |
| NGT | 79 | ||||||
| IGT | 72 | ||||||
| k-DM | 78 | ||||||
| Gerritsen et al. (2000) [78] | All | 631 | WHO 1985 | Short-term HRV, BRS, DB, LS, OH | Not provided | ↓SDNN | Age, antihypertensive drugs, presence of diabetes |
| NGT | 288 | ||||||
| IGT | 169 | ||||||
| n-DM | 95 | ||||||
| k-DM2 | 79 | ||||||
| Singh et al. (2000) [79] | All | 1919 | WHO 1985 | Short-term HRV | Not provided | ↓SDNN, HF, and LF in IFG and DM | Fasting glucose |
| NFG | 1779 | ||||||
| IFG | 56 | ||||||
| DM | 84 | ||||||
| Schroeder et al. (2005) [80] | All | 9940 | ADA 2003 | Short-term HRV | Not provided | ↓RR interval and rMSSD in IFG at baseline | Fasting glucose and HRV (weak association at baseline) in nondiabetic subjects |
| NFG | 5410 | ||||||
| IFG | 3561 | ↓SDNN, rMSSD, and R-R interval in DM at baseline | |||||
| DM | 969 | ||||||
| Perciaccante et al. (2006) [81] | All | 100 | ADA 2003 | 24-h HRV | Not provided | ↓SDNN, low TP, and ↑LFnu in DM, IFG, and IGT | HOMA-I |
| NGT | 20 | ||||||
| IFG | 20 | ↑LFnu in IFG and IGT than in DM and NGT | |||||
| IGT | 20 | ||||||
| DM2 | 20 | ↑LFnu in NGT than IFG | |||||
| NGT without insulin resistance | 20 | ↓HF in NGR and IGT than IFG and DM | |||||
| Stein et al. (2007) [82] | All | 1259 | ADA 2003 | 24-h HRV | Not provided | ↓RR interval, SDNN and TP in IFG-2 and DM | Fasting glucose, metabolic syndrome components |
| NFG | 536 | ||||||
| IFG-1 | 363 | ||||||
| IFG-2 | 182 | ||||||
| DM | 178 | ||||||
| Wu et al. (2007) [83] | All | 1440 | ADA 2003 | Short-term HRV, DB, LS | Not provided | ↓DB in DM than IGT | Not provided |
| NGT | 983 | ↓SDNN and DB in IFG | |||||
| i-IFG | 163 | ↓SDNN, LS, HF power and DB, but ↑LF:HF ratio in DM and IGF | |||||
| IGT | 188 | ||||||
| DM | 106 | ||||||
| Isak et al. (2008) [84] | All | 50 | ADA 2003 | DB, LS, VM, OH, Handgrip, Sudomotor function | Not provided | No differences apart from in sympathetic skin response | Not provided |
| NGT | 25 | ||||||
| IGT | 25 | ||||||
| Laitinen et al. (2011) [85] | All | 268 | ADA 2003 | DB, OH | 25% abnormal DB | Not provided (no control group) | Age, weight, BMI, waist, SBP, triglycerides, sex (male) in DB |
| IGT | 268 | 6% abnormal OH | SBP in OH | ||||
| Putz et al. (2013) [86] | All | 115 | ADA 2003 | DB, LS, VM, OH, Handgrip test, Triangle index | IGT: 57.5% one abnormal test | ↓DB, Valsalva ratio, OH, handgrip test, and triangle index | Not provided |
| NFG | 40 | ||||||
| IGT | 75 | ||||||
| Ziegler et al. (2015) [87] | All | 1332 | ADA 2003 | 4 Out of 120 short-term HRV indices | 4 in i-IFG and 6 in IFG-IGT and n-DM HRV measures more frequently abnormal | HR, BMI, hypertension, smoking, creatinine, sex, age, HbA1c, physical activity, drugs suppressing HRV as predictors of diminished HRV | |
| NGT | 565 | 4.5% | |||||
| i-IFG | 336 | 8.1% | |||||
| i-IGT | 72 | 5.9% | |||||
| IFG-IGT | 151 | 11.4% | |||||
| n-DM | 78 | 11.7% | |||||
| k-DM | 130 | 17.5% | |||||
| Tiftikcioglu et al. (2016) [88] | All | 80 | ADA 2003 | short-term HRV, Sudomotor function | Not provided | ↓SDNN, CV, TP, LF, LF: HF in IGT and DM | Not provided |
| NGT | 30 | ||||||
| IGT | 25 | ||||||
| DM | 25 | ||||||
| Dimova et al. (2016) [89] | All | 478 | WHO 2006 | short-term HRV indices | ↓Sympathetic and parasympathetic tone in IFG, iIGT and DM | Age, QTc-i, waist for sympathetic and parasympathetic indices | |
| NGT | 130 | 12.3% | |||||
| preDM | 227 | 19.8% | |||||
| IFG | 125 | 13.2% | |||||
| iIGT | 102 | 20.6% | DBP, 2-h BG for sympathetic indices | ||||
| NDT1DM | 121 | 32.2% | |||||
| Dimova et al. (2019) [90] | All | 87 | WHO 2006 | short-term HRV indices, DB, VM, LS | ↓parasympathetic activity at rest in n-DM | Age, hypertension, waist, BMI, visceral fat, total body fat | |
| NGT | 35 | 5.7% | |||||
| Prediabetes | 35 | 8.6% | ↑HR at rest in n-DM | ||||
| n-DM | 17 | 23.5% | |||||
| Coopmans et al. (2020) [91] | All | 2107 | WHO 2006 | 24 h HRV | Not provided | ↓time and frequency domain HRV in prediabetes and DM | Age, female sex, BMI, HDL-cholesterol, alcohol use, smoking behavior, HbA1c, FPG, 2-h BG |
| NGT | 1226 | ||||||
| Prediabetes | 331 | ||||||
| DM | 550 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowska-Jurkun, A.; Matuszewski, W.; Bandurska-Stankiewicz, E. Chronic Microvascular Complications in Prediabetic States—An Overview. J. Clin. Med. 2020, 9, 3289. https://doi.org/10.3390/jcm9103289
Baranowska-Jurkun A, Matuszewski W, Bandurska-Stankiewicz E. Chronic Microvascular Complications in Prediabetic States—An Overview. Journal of Clinical Medicine. 2020; 9(10):3289. https://doi.org/10.3390/jcm9103289
Chicago/Turabian StyleBaranowska-Jurkun, Angelika, Wojciech Matuszewski, and Elżbieta Bandurska-Stankiewicz. 2020. "Chronic Microvascular Complications in Prediabetic States—An Overview" Journal of Clinical Medicine 9, no. 10: 3289. https://doi.org/10.3390/jcm9103289
APA StyleBaranowska-Jurkun, A., Matuszewski, W., & Bandurska-Stankiewicz, E. (2020). Chronic Microvascular Complications in Prediabetic States—An Overview. Journal of Clinical Medicine, 9(10), 3289. https://doi.org/10.3390/jcm9103289





