Oxygenation before Endoscopic Sedation Reduces the Hypoxic Event during Endoscopy in Elderly Patients: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Overview
2.2. Subjects
- (1)
- Type of research: Superiority test (Fisher’s exact test)
- (2)
- Alpha: 0.05; Beta: 0.20; Power: 0.80; Two-tailed test; Allocation ratio N2/N1: 1
- (3)
- Proportion p1: (0%), proportion p2: 0.3 (30%)
2.3. Study Process and Measurement Variables
- (1)
- Collect patient characteristics such as age, sex, underlying disease, medication history, and previous history of endoscopic sedation and measure their vital signs and oxygen saturation before sedation.
- (2)
- Administer oxygen at 2 L/min via nasal prongs 1 min before endoscopy in the pre-oxygenation group.
- (3)
- Inject midazolam or propofol (+/− pethidine) at 1–2-min intervals until moderate sedation is achieved.
- (4)
- Check oxygen saturation every 1 min during endoscopic sedation. In case of hypoxia below 90% oxygen saturation, follow the procedures below.
- (5)
- Check vital signs such as consciousness level, blood pressure, and oxygen saturation until the end of the endoscopic procedure.
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cohen, L.B.; DeLegge, M.H.; Aisenberg, J.; Brill, J.V.; Inadomi, J.M.; Kochman, M.L.; Piorkowski, J.D. AGA Institute Review of Endoscopic Sedation. Gastroenterology 2007, 133, 675–701. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, K.R.; Laine, L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest. Endosc. 2008, 67, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Kaoutzanis, C.; Tocco-Bradley, R.; Obear, J.; Welch, K.B.; Winter, S.; Cleary, R.K. Patients Prefer Propofol to Midazolam Plus Fentanyl for Sedation for Colonoscopy. Dis. Colon Rectum 2016, 59, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, J.K. Sedation in gastrointestinal endoscopy: Current issues. World J. Gastroenterol. 2013, 19, 463–481. [Google Scholar] [CrossRef]
- Agostoni, M.; Fanti, L.; Gemma, M.; Pasculli, N.; Beretta, L.; Testoni, P.A. Adverse events during monitored anesthesia care for GI endoscopy: An 8-year experience. Gastrointest. Endosc. 2011, 74, 266–275. [Google Scholar] [CrossRef]
- Roh, W.-S.; Kim, D.K.; Jeon, Y.; Kang, W.-S.; Lee, S.C.; Ko, Y.-K.; Lee, Y.-C.; Lee, G.-H. Analysis of Anesthesia-related Medical Disputes in the 2009-2014 Period Using the Korean Society of Anesthesiologists Database. J. Korean Med. Sci. 2015, 30, 207–213. [Google Scholar] [CrossRef]
- Dumonceau, J.-M.; Riphaus, A.; Schreiber, F.; Vilmann, P.; Beilenhoff, U.; Aparicio, J.R.; Vargo, J.J.; Manolaraki, M.; Wientjes, C.; Rácz, I.; et al. Non-anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates Guideline–Updated June 2015. Endoscopy 2015, 47, 1175–1189. [Google Scholar] [CrossRef]
- Early, D.S.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Evans, J.A.; Fanelli, R.D.; Fisher, D.A.; Foley, K.Q.; Fonkalsrud, L.; et al. Modifications in endoscopic practice for the elderly. Gastrointest. Endosc. 2013, 78, 1–7. [Google Scholar] [CrossRef]
- Enestvedt, B.K.; Eisen, G.M.; Holub, J.; Lieberman, D.A. Is the American Society of Anesthesiologists classification useful in risk stratification for endoscopic procedures? Gastrointest. Endosc. 2013, 77, 464–471. [Google Scholar] [CrossRef]
- Early, D.S.; Lightdale, J.R.; Vargo, J.J.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Evans, J.A.; Fisher, D.A.; Fonkalsrud, L.; Hwang, J.H.; et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest. Endosc. 2018, 87, 327–337. [Google Scholar] [CrossRef]
- Miller’s Anesthesia, 7th ed.; Miller, R.D., Ed.; Churchill Livingstone Elsevier: Amsterdam, The Netherlands, 2009; pp. 719–768. [Google Scholar]
- Vargo, J.J. Minimizing Complications: Sedation and Monitoring. Gastrointest. Endosc. Clin. N. Am. 2007, 17, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Nimmagadda, U.; Chiravuri, S.D.; Salem, M.R.; Joseph, N.J.; Wafai, Y.; Crystal, G.J.; El-Orbany, M.I. Preoxygenation with Tidal Volume and Deep Breathing Techniques: The Impact of Duration of Breathing and Fresh Gas Flow. Anesthesia Analg. 2001, 92, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Ling, L.C.; Cardosa, M.S.; Wong, A.K.H.; Wong, N.W. Hypoxia during upper gastrointestinal endoscopy with and without sedation and the effect of pre-oxygenation on oxygen saturation. Anaesthesia 2000, 55, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Rozario, L.; Sloper, D.; Sheridan, M.J. Supplemental Oxygen During Moderate Sedation and the Occurrence of Clinically Significant Desaturation During Endoscopic Procedures. Gastroenterol. Nurs. 2008, 31, 281–285. [Google Scholar] [CrossRef]
- Patterson, K.W.; Noonan, N.; Keeling, N.W.; Kirkham, R.; Hogan, D.F. Hypoxemia during outpatient gastrointestinal endoscopy: The effects of sedation and supplemental oxygen. J. Clin. Anesth 1995, 7, 136–140. [Google Scholar] [CrossRef]
- Iwao, T.; Toyonaga, A.; Shigemori, H.; Sumino, M.; Oho, K.; Tanikawa, K. Supplemental oxygen during endoscopic variceal ligation: Effects on arterial oxygenation and cardiac arrhythmia. Am. J. Gastroenterol. 1995, 90, 2186–2190. [Google Scholar]
- Haines, D.J.; Bibbey, D.; Green, J.R. Does nasal oxygen reduce the cardiorespiratory problems experienced by elderly patients undergoing endoscopic retrograde cholangiopancreatography? Gut 1992, 33, 973–975. [Google Scholar] [CrossRef]
- Gross, J.B.; Long, W.B. Nasal oxygen alleviates hypoxemia in colonoscopy patients sedated with midazolam and meperidine. Gastrointest. Endosc. 1990, 36, 26–29. [Google Scholar] [CrossRef]
- Crantock, L.; Cowen, A.; Ward, M.; Roberts, R. Supplemental low flow oxygen prevents hypoxia during endoscopic cholangiopancreatography. Gastrointest. Endosc. 1992, 38, 418–420. [Google Scholar] [CrossRef]
- Bowling, T.E.; Hadjiminas, C.L.; Polson, R.J.; Baron, J.H.; Foale, R.A. Effects of supplemental oxygen on cardiac rhythm during upper gastrointestinal endoscopy: A randomised controlled double blind trial. Gut 1993, 34, 1492–1497. [Google Scholar] [CrossRef]
- Bell, G.D.; Quine, A.; Hugh, J.; Antrobus, L.; Morden, A.; Burridge, S.M.; Lee, J.; Coady, T.J. Upper gastrointestinal endoscopy: A prospective randomized study comparing continuous supplemental oxygen via the nasal or oral route. Gastrointest. Endosc. 1992, 38, 319–325. [Google Scholar] [CrossRef]
- Sharma, V.K.; Nguyen, C.C.; Crowell, M.D.; Lieberman, D.A.; De Garmo, P.; Fleischer, D.E. A national study of cardiopulmonary unplanned events after GI endoscopy. Gastrointest. Endosc. 2007, 66, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Heuss, L.T.; Froehlich, F.; Beglinger, C. Nonanesthesiologist-administered propofol sedation: From the exception to standard practice. Sedation and monitoring trends over 20 years. Endoscopy 2012, 44, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Dong, S.H.; Kim, E.S.; Moon, S.-H.; Park, H.J.; Yang, D.-H.; Yoo, Y.C.; Lee, T.H.; Kil, L.S.; Hyun, J.J.; et al. Room for Quality Improvement in Endoscopist-Directed Sedation: Results from the First Nationwide Survey in Korea. Gut Liver 2016, 10, 83–94. [Google Scholar] [CrossRef]
| Oxygenated Group (n = 35) | Non-Oxygenated Group (n = 35) | |
|---|---|---|
| Sex (male, %) | 12 (34.3%) | 16 (45.7%) |
| Age (years, mean ± SD) | 75.8 ± 6.5 | 71.8 ± 5.3 |
| Height (cm, mean ± SD) | 156.8 ± 8.6 | 157.7 ± 9.7 |
| Weight (kg, mean ± SD) | 60.4 ± 10.8 | 61.3 ± 11.4 |
| BMI (mean ± SD) | 24.6 ± 4.0 | 24.6 ± 3.5 |
| BMI > 25 (n, %) | 15 (42.9%) | 14 (40.0%) |
| Smoking (%) | 3 (8.6%) | 3 (8.6%) |
| Alcohol (%) | 2 (5.7%) | 2 (5.7%) |
| Medical history | ||
| Hypertension (%) | 17 (48.6%) | 17 (48.6%) |
| Diabetes (%) | 8 (22.9%) | 7 (20.0%) |
| Heart disease (%) | 8 (22.9%) | 4 (11.4%) |
| Pulmonary disease (%) | 2 (5.7%) | 5 (14.3%) |
| Cerebrovascular disease (%) | 2 (5.7%) | 1 (2.9%) |
| Renal disease (%) | 2 (5.7%) | 3 (8.6%) |
| Mallampati score | ||
| 1 | 22 (62.9%) | 19 (54.3%) |
| 2 | 8 (22.9%) | 10 (28.6%) |
| 3 | 3 (8.6%) | 4 (11.4%) |
| 4 | 2 (5.7%) | 2 (5.7%) |
| Midazolam (mg, mean ± SD) | 2.7 ± 0.5 | 2.7 ± 0.8 |
| Propofol (mg, mean ± SD) | 14.6 ± 11.5 | 12.6 ± 12.7 |
| Pethidine (mg, mean ± SD) | 12.9 ± 22.2 | 11.4 ± 21.3 |
| Pre-Oxygenated Group (n = 35) | Non-Oxygenated Group (n = 35) | p Value | |
|---|---|---|---|
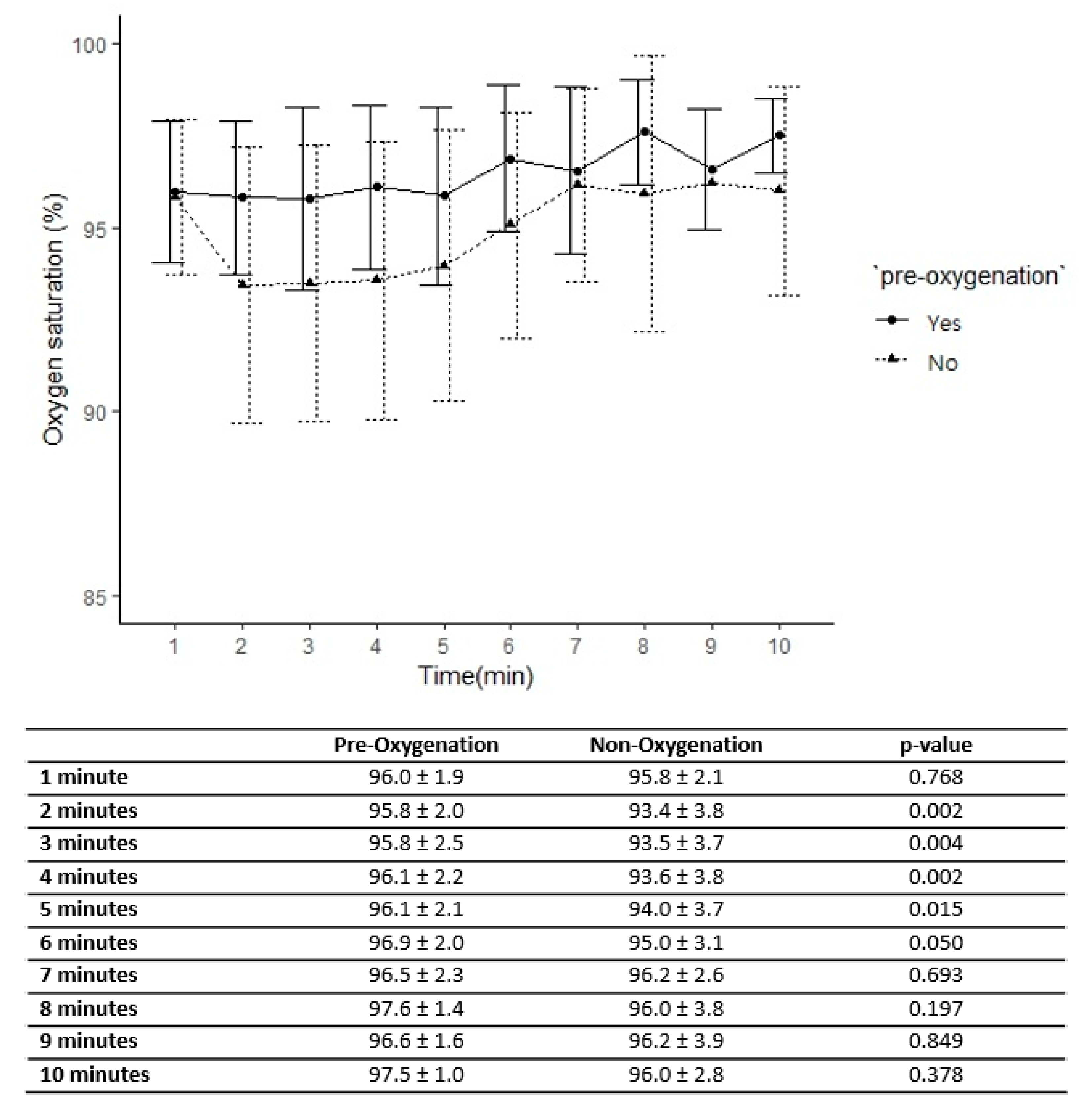
| Hypoxia before procedure (no., %) | 0 (0%) | 1 (2.9%) | 0.314 |
| SaO2 before procedure (mean ± SD) | 95 ± 2 | 96 ± 2 | 0.726 |
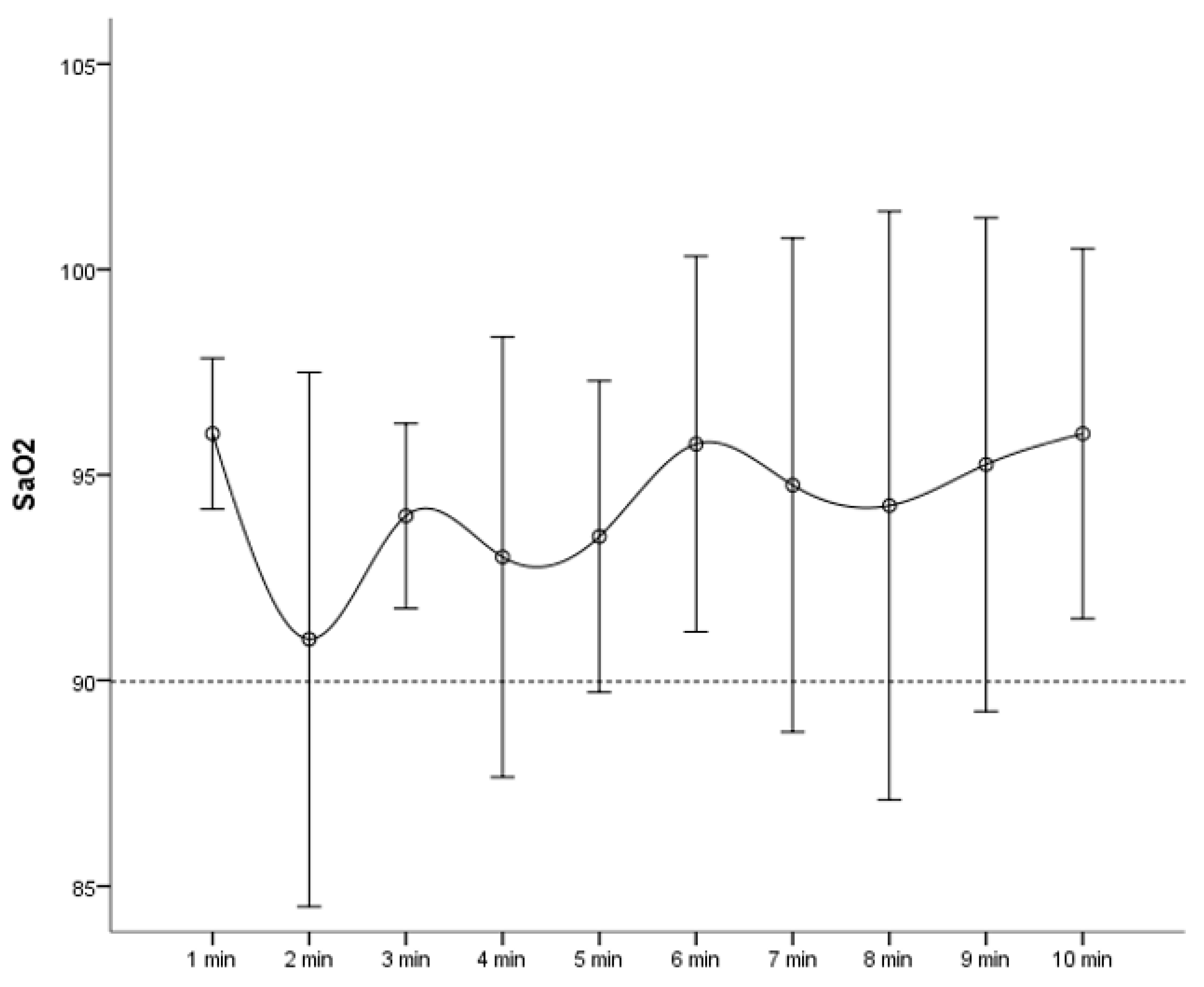
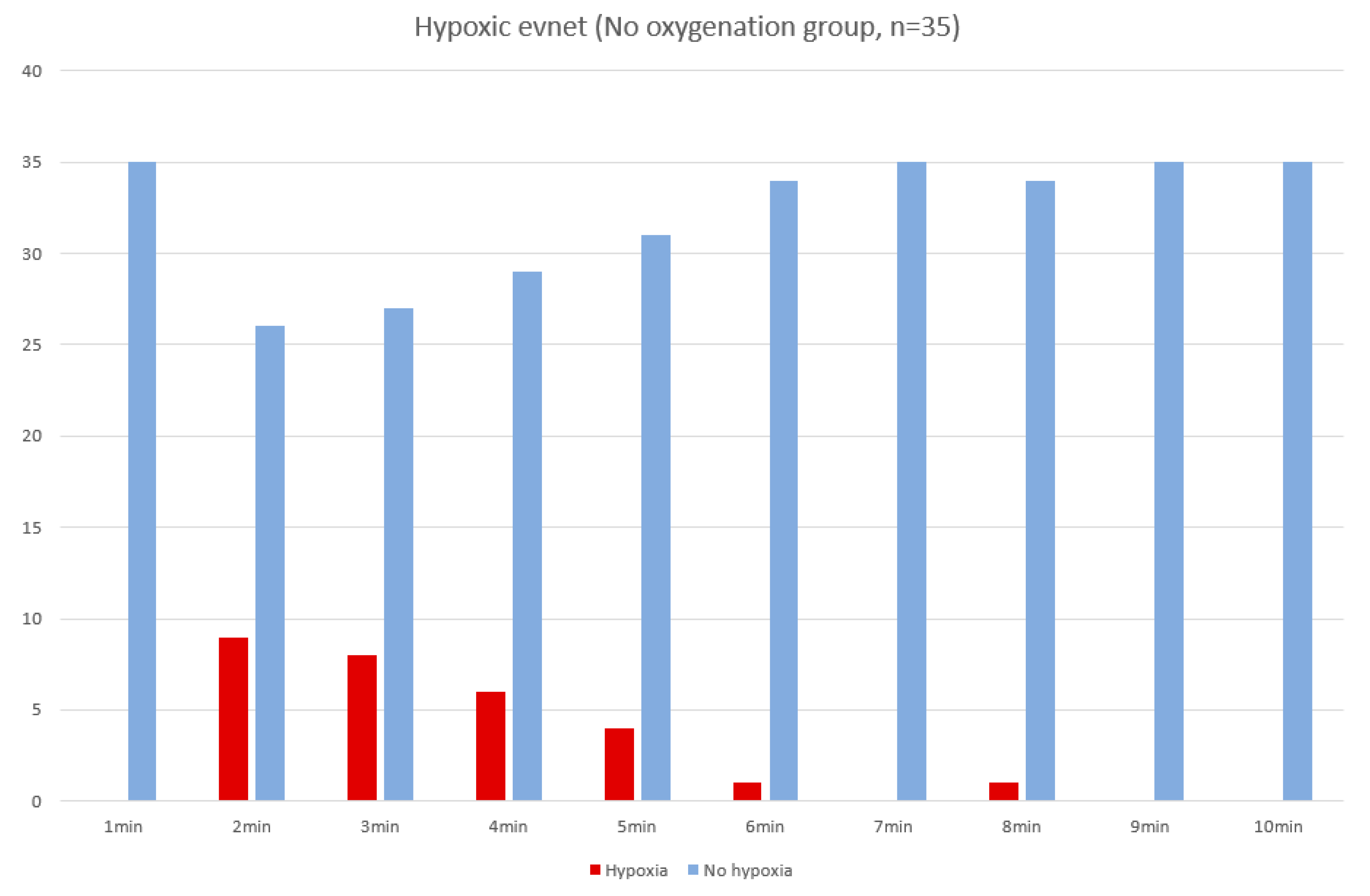
| Hypoxia during procedure (no., %) | 0 (0%) | 28 (80%) | <0.001 |
| Outcome (Hypoxia) | Incidence Cases, n (%) | Crude OR (95% CI) |
|---|---|---|
| Pre-oxygenated group | 28/35 (80.0) | 1.00 (reference) |
| Non-oxygenated group | 0/35 (0.0) | 13.12 (12.96–13.29) |
| Hypoxia Group (n = 28) | Non-Hypoxia Group (n = 7) | p Value | |
|---|---|---|---|
| Sex (male, %) | 14 (50.0%) | 2 (28.6%) | 0.415 * |
| Age (years, mean ± SD) | 71.3 ± 5.4 | 73.9 ± 4.7 | 0.260 |
| Height (cm, mean ± SD) | 158.9 ± 9.8 | 153.1 ± 8.2 | 0.164 |
| Weight (kg, mean ± SD) | 62.5 ± 11.8 | 57.0 ± 8.8 | 0.266 |
| BMI (mean ± SD) | 24.6 ± 3.5 | 24.4 ± 3.7 | 0.867 |
| BMI > 25 (n, %) | 13 (46.4%) | 1 (14.3%) | 0.203 * |
| Smoking (%) | 3 (10.7%) | 0 (0%) | >0.999 * |
| Alcohol (%) | 2 (7.1%) | 0 (0%) | >0.999 * |
| Medical history | |||
| Hypertension (%) | 11 (39.3%) | 6 (85.7%) | 0.041 * |
| Diabetes (%) | 7 (25.0%) | 0 (0%) | 0.301 * |
| Cardiovascular disease (%) | 2 (7.1%) | 2 (28.6%) | 0.171 * |
| Pulmonary disease (%) | 4 (14.3%) | 1 (14.3%) | >0.999 * |
| Cerebrovascular disease (%) | 1 (3.6%) | 0 (0%) | >0.999 * |
| Renal disease (%) | 3 (10.7%) | 0 (0%) | >0.999 * |
| Mallampati score 1,2 vs. 3,4 | 0.311 | ||
| 1 | 15 (53.6%) | 4 (57.1%) | |
| 2 | 7 (25.0%) | 3 (42.9%) | |
| 3 | 4 (14.3%) | 0 (0%) | |
| 4 | 2 (7.1%) | 0 (0%) | |
| Midazolam (mg, mean ± SD) | 2.9 ± 0.6 | 2.1 ± 1.1 | 0.022 |
| Propofol (mg, mean ± SD) | 11.8 ± 11.2 | 15.7 ± 18.1 | 0.472 |
| Pethidine (mg, mean ± SD) | 14.3 ± 23.0 | 0 | 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Hyun, J.N.; Lee, K.J.; Kim, H.-S.; Park, H.J. Oxygenation before Endoscopic Sedation Reduces the Hypoxic Event during Endoscopy in Elderly Patients: A Randomized Controlled Trial. J. Clin. Med. 2020, 9, 3282. https://doi.org/10.3390/jcm9103282
Kim H, Hyun JN, Lee KJ, Kim H-S, Park HJ. Oxygenation before Endoscopic Sedation Reduces the Hypoxic Event during Endoscopy in Elderly Patients: A Randomized Controlled Trial. Journal of Clinical Medicine. 2020; 9(10):3282. https://doi.org/10.3390/jcm9103282
Chicago/Turabian StyleKim, Hyunil, Jeong Nam Hyun, Kyong Joo Lee, Hyun-Soo Kim, and Hong Jun Park. 2020. "Oxygenation before Endoscopic Sedation Reduces the Hypoxic Event during Endoscopy in Elderly Patients: A Randomized Controlled Trial" Journal of Clinical Medicine 9, no. 10: 3282. https://doi.org/10.3390/jcm9103282
APA StyleKim, H., Hyun, J. N., Lee, K. J., Kim, H.-S., & Park, H. J. (2020). Oxygenation before Endoscopic Sedation Reduces the Hypoxic Event during Endoscopy in Elderly Patients: A Randomized Controlled Trial. Journal of Clinical Medicine, 9(10), 3282. https://doi.org/10.3390/jcm9103282





